Abstract
We investigated plausible reuse for the dermocosmetic industry of byproducts from the winemaking process of red grapes (Vitis vinifera L. cv. C. Sauvignon) through the evaluation of one extract (grape pomace extract, GPE) and two fractions (one chloroform, GPE-CHF; one ethyl acetate, GPE-EAF). The samples were characterized analytically by liquid chromatography (HPLC) using a NIH 3T3 fibroblast cell culture to verify a cytosafety profile in normal and stressful environment (presence of H2O2), and by using it in a sunscreen system to observe improvements in the in vitro efficacy by diffuse reflectance spectrophotometry with an integrating sphere. The HPLC results for GPE-EAF and GPE-CHF samples with the best profile of syringic and p-coumaric acids, quercetin, and trans-resveratrol were used in the further assays. GPE-EAF and GPE-CHF, both at 30.00 µg/mL, maintained the cell viability in the absence of H2O2 (normal condition). In the sequence, GPE-EAF and GPE-CHF were evaluated against the oxidative stressor H2O2 in NIH 3T3 cells. A sharp drop in viability was only observed for GPE-CHF, and cytotoxicity of GPE-EAF was considered absent even in a hostile environment. Since GPE-EAF previously developed the best results, its potential performance was investigated in a sunscreen system. The in vitro sun protection factor of the phytoderivative-free formulation was 9.0 + 2.5; by adding GPE-EAF at 10.0%, its efficacy was elevated to 15.0 + 2.5. Both samples suffered a negative effect after artificial ultraviolet exposition (500 W/m2); however, the presence of GPE-EAF improved the photostability of the sunscreen system.
1. Introduction
Grapes were domesticated thousands of years ago and the Vitis vinifera L. grapevine is the most cultivated grape species in the world, being about 50–70% of the entire production used in the elaboration of fine wines. The Cabernet Sauvignon variety (V. vinifera L. cv. C. Sauvignon) is one of the most cultivated and widely distributed in almost all continents. In winemaking, 20–30% of solid waste is formed from skins, seeds, residual pulp, stalks, and fermentation yeasts, and the grape pomace (GP) is the main residue of the wine industry, which could be representative in terms of a byproduct volume. On average, 1 ton of wine produces 200 kg of GP, generally used for energy, fertilizer production, animal food, distilled beverages, or building construction. The inappropriate waste disposal in the environment has an impact on crop, living beings, and water and soil quality due to antinutritional substances, oxygen depletion, and greater resistance to microorganisms [,]. Thus, this scenario highlights the necessity to create mechanisms to improve the reuse and to reduce this kind of industry residue.
GP contains a variety of substances that include unsaturated fatty acids, monosaccharides, polysaccharides, proteins, fibers, minerals, and polyphenols. Compared to other agrofood residues, the red GP (peels and seeds) shows high amount of phenolics, possibly due to the presence of flavanols in the seeds [,]. The waste of polyphenols remaining in this pomace and the greater demand for natural products, mainly with antioxidant, antibacterial, and antiviral activities, represents a sustainable and ecofriendly opportunity to obtain these bioactive compounds in several biotechnological applications [,].
Nowadays, consumers are more aware of and interested in natural topical products with less harm to health and to the environment instead of synthetic substances []. Natural antioxidants provide biological and therapeutic activities, high tolerability, and, additionally, biodegradability. Owing to several properties of polyphenols from V. vinifera, the development of dermocosmetics is an interesting strategy to vehiculate such substances, thus providing better health skin conditions [,,]. For instance, specialized literature reported that GP extract was able to improve the in vivo sun protection factor (SPF) of a sunscreen system by about 20% [].
Aiming at to investigate a plausible reuse of byproducts from the winemaking process in the dermocosmetic industry, we obtained one extract and two fractions from the pomace of red grapes (V. vinifera cv. C. Sauvignon). The natural samples prepared from the winemaking waste were characterized analytically by liquid chromatography, evaluated in a cell culture to design a cytosafety profile in a normal and in a stressful environment, and the most successful sample indicated from these tests had its potential application challenged in a sunscreen system by diffuse reflectance spectrophotometry with an integrating sphere.
2. Materials and Methods
2.1. Materials
Dulbecco MEM (DMEM) supplemented with L-glutamine, sodium bicarbonate, antibiotic, antimycotic, fetal bovine serum (FBS), tripsin, and MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) were from Cultilab® (Campinas, Brazil). Dimethyl sulfoxide (DMSO) was from Amresco® (São Paulo, Brazil) and ethyl alcohol was bought in Ciclo Farma Fabbe® (Serrana, Brazil). Phosphate-buffered saline was from Synth® (Diadema, São Paulo). Potassium chloride P.A., anhydrous monobasic potassium phosphate, and anhydrous bibasic sodium phosphate were purchased from Inlab® (São Paulo, Brazil). Hydrogen peroxide 35% was from Scharlab® (São Paulo, Brazil). Acetic acid, quercetin (≥98% purity), p-coumaric acid (≥98% purity), and syringic acid (≥95% purity) were from Sigma-Aldrich® (Cotia, Brazil) and trans-resveratrol (≥98% purity) was from Active Farmacêutica® (Palhoça, Brazil). Acetonitrile was from Honeywell® (Itajubá, Brazil). NIH 3T3 fibroblast cells (P6, 18th January 2010) were kindly donated by the Clinical Cytopathology Laboratory, Faculty of Pharmaceutical Sciences, University of São Paulo (São Paulo, Brazil). Ultrapure water was used in all experiments.
2.2. Agroindustrial Vinification Byproduct
Grape pomace byproduct (skins, seeds, stalks) from the winemaking industry was kindly provided by Vinícola Beraldo di Cale located in Jundiaí, state of São Paulo, Brazil. Red grapes (V. vinifera cv. C. Sauvignon) were collected during the end of summer in Caxias do Sul vineyard, state of Rio Grande do Sul, Brazil (29°13′29″ S, 51°9′31″ W and altitude 470 m). A voucher specimen (Hübner, A.1) was deposited in the herbarium (SPF) at the Institute of Bioscience, IB/University of São Paulo.
2.2.1. Dry Whole Grape Pomace Extract (GPE)
The vinification residue from the manufacture of wine (grape pomace byproduct) was dried in an air circulation oven (Fabbe Center®, São Paulo, Brazil) at 40 °C for five days until constant mass. A sample of the grape pomace weighing 4.65 kg was ground in a knife and hammer mill (Thomas Scientific®, Swedesboro, NJ, USA), passing through a 1 mm sieve. The fine dried powder (20 mesh) was extracted with ethanol:water (7:3 v/v) at 25 °C using the percolation method. The leachate was concentrated at reduced pressure around 65 °C in an ascending film evaporator, homogenized, and the aqueous solution freeze dried (lyophilizer model Liotop L202, Liobras®, São Carlos, Brazil). The GPE was stored in tight, light-resistant container.
2.2.2. Sample Fractions
GPE (20 g) was dissolved in 200 mL of ultrapure water and placed on a magnetic stirrer for 30 min. The formed aqueous solution was submitted to consecutive liquid–liquid extraction using chloroform or ethyl acetate []. First, the aqueous solution was extracted with 60 mL of chloroform by shaking (5 min) it at room temperature. The procedure was repeated three times until reaching a total volume of 200 mL, combined and evaporated to dryness under reduced pressure at 40 °C. The solid residue was denominated GPE-CHF. Forthwith, the same procedure was performed using ethyl acetate. The solid residue obtained after ethyl acetate evaporation was called GPE-EAF.
2.3. HPLC Analysis of Polyphenols
The quantification of polyphenols in the GPE, GPE-CHF, and GPE-EAF fractions were based on the comparison of retention times and maximum absorbance values of the peak areas detected in the samples with those obtained with the markers (p-coumaric acid, syringic acid, quercetin, and resveratrol) [,]. High performance liquid chromatography (HPLC) quantifications were performed with a Shimadzu® chromatograph and C18 Shim-pack VP-ODS column (4.3 × 250 mm, 5 µm), oven temperature at 35 °C, flow of 1.0 mL/min. The solvent system was (A) purified water (Milli-Q) and 0.1% acetic acid, and (B) acetonitrile. Gradient conditions were 0–10 min (98A/2B); 10–80 min (60A/40B); 80–85 min (2A/98B). GPE and GPE-EAF/GPE-CHF fractions were at 14.70 and 5.00 mg/mL, respectively, and the injection volume was 30 µL. The samples were evaluated using the linear regression equation for the analytical curve of the markers (equivalent in µmol/L extract). The result of phenolics was expressed as mean ± standard deviation (n = 9). Linear ranges of p-coumaric acid (347 nm), syringic acid (275 nm), and quercetin (309 nm) were between 1.00 and 100.00, and trans-resveratrol (309 nm), from 0.10 to 10.00 µg/mL. The coefficients of determination (R2) were all at least 0.996. Phenolic contents (mg/mL) were calculated according to Equation (1).
Phenolic [mg/g] = concentration (µg/mL) × 1 g/sample concentration (g/mL)/1000
2.4. Cytotoxicity Establishment
Solvents were sterilized by using sterile membrane with 0.20 µm pore size (Minisart®, Sartorius Stedim Biotech, Goettingen, Germany). A 10 µL aliquot of each sample solution derived from V. vinifera was added to a single well containing cells in 90 µL of culture medium (DMEM + 10% FBS) to reach the final concentration of 30.00 µg/mL. The mixture of DMSO (0.25%) and EtOH (0.75%) was used in this assay. Water was the negative control. Hydrogen peroxide 1:50.000 (H2O2, 206 µM) [,] was tested as the oxidative-process inducer to provide a hostile environment to further determine cytosafety profile.
2.4.1. NIH 3T3 Fibroblast Cell Viability
NIH 3T3 cells (3rd passage) were cultivated in monolayers in 25 cm2 flasks containing DMEM and 10% FBS, at 37 °C in a humidified atmosphere with 5% CO2 (Thermo Fisher Scientific®, Waltham, MA, USA) until reaching, approximately, 80% confluence. The fibroblasts were trypsinized and cell concentration was adjusted to 2.38 × 104 cells/well in culture medium. A 90 µL volume of this cell suspension was distributed in each well and cultured for 24 h before interaction with the samples at 37 °C for 48 h []. Immediately afterward, an aliquot of 100 µL of the 0.5 mg/mL MTT aqueous solution was added to each well and incubated for 3 h. Then, 100 µL of DMSO was added to each well and incubated for 1 h. The absorbance reading was performed in a multimode microplate reader (Infinite® M200 PRO, Tecan, Männedorf, Switzerland) at 570 nm. Cell viability of the samples (n = 8) was calculated by the ratio of the mean absorbance (Am) of the treated and control group, according to Equation (2).
Cell viability (%) = Am treated/Am control × 100
2.4.2. Hostile Environment by H2O2 Treatment
NIH 3T3 cells (2.38 × 104 cells/well) were cultured in a 96-well microplate (Kasvi®, São José dos Pinhais, PR, Brazil) for 24 h and followed by interaction with samples at 30 μg/mL, 37 °C for 48 h. After this period, 10 µL of H2O2 (206 µM) was added to each well and incubated at 37 °C and 5% CO2 for 3 h. Afterward, 10 μL of MTT (5.0 mg/mL) was added to each well and incubated for 3 h. The supernatants were removed from the wells individually and then 100 µL of DMSO was added to solubilize the blue formazan crystals from living cells, shaken gently, and incubated at 37 °C for 1 h []. The absorbance reading followed the same as described earlier.
2.5. In Vitro Sun Protection Factor (SPF) and Photostability Establishment
In vitro SPF of the sample with the best performance from the previous assays was determined using a diffuse reflectance spectrophotometer with an integrating sphere (Labsphere UV-2000S® Transmittance Analyzer) [,,], illustrated in Figure S1 (Supplementary Materials) as “diffuse reflectance spectrophotometer with integrating sphere from de Laboratory of Cosmetology, Faculty of Pharmaceutical Sciences, University of São Paulo []”. For the photostability test, we used a chamber with artificial UV ray emission (Suntest® CPS Atlas, Linsengericht, Germany). A sample amount of 0.75 mg/cm2 was applied uniformly over polymethylmethacrylate (PMMA) plates (HelioScreen® Helioplate HD 6, North Sutton, NH, USA) (n = 3). Right after sample application, plates were kept at in the dark at room temperature for 15 min. After 0, 1, and 2 h of exposure to UV radiation at the dose of 500 W/m2, the in vitro SPF was obtained through the UV2000 software. The sunscreen system was investigated earlier by our Research Group with its composition described by Hübner and coworkers, 2020 []; however, Table 1 describes the abovementioned system in terms of active ingredients. The best V. vinifera-derived sample from previous tests was used at 10.0% (w/w).

Table 1.
Qualitative and quantitative of the sunscreen-system active composition.
2.6. Statistical Analysis
Data were expressed as mean ± standard deviation. Analysis of variance test (ANOVA) followed by Tukey’s test were performed to statistical analyze and verify differences between groups. Minitab 21 (Minitab Inc., LLC, State College, PA, USA), Statistic Program 13.5 (TIBCO Software Inc., Palo Alto, CA, USA) and MS Excel (Microsoft Corp., Redmond, WA, USA) were used; p-values < 0.05 were considered significant.
3. Results
3.1. Analytical Characterization of the Natural Samples
The aspect of extract and fractions is illustrated in Figure 1. We noticed differences amongst GPE, GPE-CHF, and GPE-EAF (p < 0.05) regarding the presence and quantity of some natural compounds. Syringic acid concentration was the highest one (p < 0.0001). Syringic and p-coumaric acids and quercetin were distinct in all samples. Resveratrol concentration in GPE and GPE-CHF was considered similar (p > 0.05). The abovementioned results are shown in Figure 2. The GPE-EAF and GPE-CHF were the samples with the best profile of phytocompounds, which were further investigated in this research work.
Figure 1.
Aspect of the samples from the winemaking process using V. vinifera cv. C. Sauvignon.
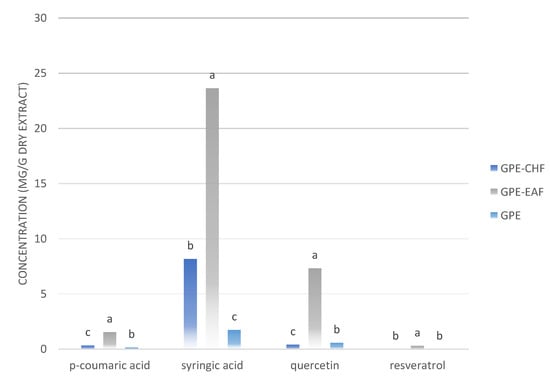
Figure 2.
Polyphenols in the grape pomace extract (GPE) and in the ethyl acetate (GPE-EAF) and chloroform (GPE-CHF) fractions. Results are expressed as mean ± standard deviation. In each group, different letters (a, b, and c) indicate significant differences (p < 0.05).
3.2. Cytotoxicity Establishment
GPE-EAF and GPE-CHF (in EtOH/DMSO solution) were tested using NIH 3T3 fibroblasts. The assay demonstrated that the concentration of the samples at 30.00 µg/mL maintained cell viability in the absence of H2O2, being V. vinifera derivatives cytobiocompatible with the fibroblast cells. There was no difference between the negative control and culture medium (p > 0.05), and we also observed that solvents (EtOH/DMSO) did not interfere with cell viability [,].
In the sequence, GPE-EAF and GPE-CHF were evaluated in vitro against the oxidative stressor H2O2 in NIH 3T3 cells, as shown in Table 2. A sharp drop in viability was only observed in the presence of GPE-CHF. GPE-EAF maintained the cell viability, being considered absent of cytotoxicity even in a hostile environment (presence of H2O2).

Table 2.
Effect of ethyl acetate (GPE-EAF) and the chloroform (GPE-CHF) fractions on viability of NIH 3T3 cells in the absence (normal condition) and presence (hostile conditions) of H2O2.
3.3. In Vitro SPF and Photostability
GPE-EAF developed the best results from the previous assays; therefore, its potential performance was investigated in a sunscreen system. The phytoderivative-free formulation developed in vitro SPF equal to 9.0 ± 2.5. By adding GPE-EAF at 10.0%, the sunscreen system had its efficacy parameter elevated to 15.0 ± 2.5. The photostability profiles are illustrated in Figure 3. Both samples suffered negative impact after 1 and 2 h of artificial UV exposition; however, the presence of GPE-EAF improved the photostability of the sunscreen system used, at least, for 1 h.
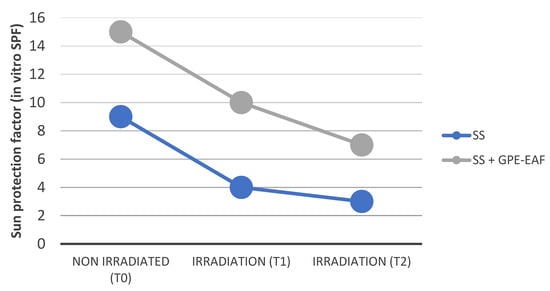
Figure 3.
Photostability profile of the sunscreen systems. SS = sunscreen system; SS + GPE-EAF = sunscreen system + 10.0% ethyl acetate fraction of grape pomace extract; T1 = 1 h of irradiation; T2 = 2 h of irradiation. According Dunnett’s multiple comparisons test, SS + GPE-EAF and SS had differences in the in vitro SPF in T0 and T1 (p < 0.001; p < 0.005, respectively).
4. Discussion
Valorization of natural compounds for use in the pharmaceutical, food, and cosmetic industries is a conscious measure of the entire production chain of agroindustrial resources, promoting sustainability and circular economy development. Circular economy aims to prolong the time of use of materials, products, and substances from waste, minimizing disposal and its consequences on the environment. Therefore, recovering an agroindustrial byproduct through its reuse and recycling make them new sources of raw materials for several sectors of the economy. During red wine vinification, there is production of organic residues such as stalks, pomace, and lees, but mainly grape pomace, composed of 25% seeds, 25% stalks, and 50% skins, and corresponding to approximately 20–25% of the weight of the processed grapes [,].
The recovery of polyphenols from grape pomace depends on the plant material, extractive methods, suitable solvents, and harvest season []. Compared to other V. vinifera grape pomace extracts, the extractive condition adapted herein was efficient in obtaining active compounds, such as p-coumaric and syringic acids and quercetin. We found in the specialized literature similar values for syringic acid (1.74 ± 156.3 mg/g GPE DW) and quercetin (0.557 ± 83.9 mg/g GPE DW); a lower value for p-coumaric acid (63.6 ± 5.3 mg/g GPE DW); and a higher value for trans-resveratrol (36.0 ± 4.9 mg/g GPE DW) [,,]. The GPE-EAF sample had the highest concentrations of the markers, i.e., syringic acid and p-coumaric acids, quercetin, and resveratrol (23.64 ±4.63; 1.54 ± 0.29; 7.33 ± 3.13; and 0.29 ± 0.16 mg/g dry extract, respectively). C. Sauvignon pomace can present variation in its qualitative and quantitative phenolic profiles depending on the cultivation region and extraction procedure. V. vinifera is a species rich in polyphenols such as flavonoids in the skins, seeds, and stalks, and nonflavonoids in the fruit pulp. Biologically, phenolic compounds can act by sequestering and inactivating reactive oxygen species (ROS), for instance, thus protecting DNA. Additional to the antioxidant action, the oral and topical use of flavonoids are associated with anti-inflammatory, antimutagenic, anticarcinogenic, and enzyme-modulating properties [,,,]. Flavonoids may prevent oxidative stress and, consequently, cellular damage. In grapes and red wine, the main groups capable of exerting antioxidant behavior are flavonols (quercetin, kaempferol, myricetin, epicatechin, and rutin), flavones (luteolin, apigenin, and tangeritin), flavanones or dihydroflavones (hesperitin, naringenin, and eriodictyol), flavanols or flavan-3-ols (catechins), and anthocyanins (cyanidin, delphinidin, malvidin, pelargonidin, and peonidin). The beneficial health effects of polyphenols are given according to the grape variety, place of cultivation, extractive process, and antioxidant content [,,].
Considering that GPE samples presented the lowest content of the markers, we continued the investigation with GPE-EAF and GPE-CHF. Regarding the H2O2-free group, both fractions were biocompatible with the cell line in the tested concentration. This cytosafety profile allowed us to stress the fibroblasts with H2O2 to observe how the fractions would interact with the cells in such unfavored condition and suggest a possible mechanism of action to use in dermocosmetics. Specialized literature reported that ROS formation after exposure to H2O2 could be suppressed by pretreatment with 30.00 µg/mL of some extracts rich in polyphenols []. H2O2, is not as reactive as superoxide (O2•-) and hydroxyl (•OH) radicals; however, in high concentrations in the biological systems it results in cellular and molecular damage. All cells, mitochondria, and certain endogenous enzymes produce H2O2. The relationship between the effect of redox and the human aging process has aroused the interest of the scientific community to the use of antioxidants []. Studies have evaluated the antioxidant activity of grape pomace in preventing premature aging in skin cell cultures; however, to the best of our knowledge, little is known about the interactions and biological effects of the winemaking byproduct fractions, more specifically their use in sunscreen systems [,]. The extract richest in phytochemicals (GPE-EAF) showed better safety profile compared to GPE-CHF in cells damaged by H2O2. The most nonpolar sample, GPE-CHF, showed the strongest interaction with H2O2 stress, maximizing the oxidative effect (synergistic effect of H2O2 and GPE-CHF fraction induced loss of cell viability), which justified the use only of GPE-EAF in the in vitro establishment of photoprotection efficacy.
Since the GPE-EAF sample developed the best results from the aforementioned assays, i.e., more content of some phytocompounds and safety in the fibroblast culture in the absence and in the presence of H2O2, we further investigated its potential as an adjuvant for use in sunscreens, as a performance booster and/or photostabilizer agent. The GPE-CHF negative behavior in the fibroblasts treated with H2O2 must be considered in the context of the cutaneous photoprotection, since the skin in contact with the UV radiation can suffer augmentation of reactive species [] that could be once more amplified by the presence of chloroform fraction.
The addition of GPE-EAF at 10.0% improved the sunscreen-system efficacy in more than 60% (in vitro SPF from 9 to 15). Since our natural sample was recovered from the waste of the winemaking industry, such favorable response highlights a real source of potential adjuvants and/or active ingredients to be considered in the cosmetic industry. We have been investigating the behavior of bioactive sunscreens since 2008 and several natural compounds or substances, inspired by their nature, were studied with distinct performance profiles [,,]. Herein, GPE-EAF, rich in syringic acid and quercetin (greater content) but also presenting, in low concentration, p-coumarinic acid and trans-resveratrol, was able to elevate the sample’s action against UVB radiation by improving the SPF in vitro. Additionally, we noticed an interesting behavior of our fraction as a photostabilizer for at least 1 h of artificial irradiation. Even though the in vitro SPF decayed in function of the irradiation time exposition, GPE-EAF maintained the best results of this efficacy parameter (for 1 h), suggesting that the phytochemical interaction [] with the sunscreen system favored its functional stability.
Considering some limitations from this study, although our investigation has real potential use in the dermocosmetic industry, possible impacts on regional, agronomic (soil, grape quality, strain, and others), and/or climatic variation (radiation, precipitation, wind speed, and others) may affect the grape composition. For example, phenolic compounds are sensitive to variations in soil and climate [,]. Another important point is the clonal variability, observed in wine grape V. vinifera cv. Kalecik Karasi. Results showed approximately 40% phenotypic variation in grape berry clones and significant differences in the number of phenolic compounds (protocatechuic acid and gallic acid) []. Therefore, further studies with other grape strains and with V. vinifera from different regions of the world may better elucidate the chemical variables involved in the cytosafe and cytotoxic effects.
5. Conclusions
From the V. vinifera waste of the winemaking process, we obtained three natural derivatives (one extract and two fractions). GPE-CHF and GPE-EAF fractions were the ones with the highest content of syringic and p-coumaric acids, quercetin, and trans-resveratrol. Both fractions were cytobiocompatible with fibroblasts in normal condition culture; however, GPE-CHF developed cytotoxicity when the cells were treated with H2O2 (stressful environment). Since GPE-EAF had more content of the evaluated phytocompounds and developed a protective profile in the fibroblast culture in the H2O2 presence, its potential as an adjuvant for sunscreens was investigated. GPE-EAF elevated the in vitro SPF, along with its photostability for at least 1 h, highlighting that winemaking waste could be considered as a plausible source for dermocosmetic adjuvant and/or active ingredients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics10010002/s1, Figure S1: Diffuse reflectance spectrophotometer with integrating sphere from de Laboratory of Cosmetology, Faculty of Pharmaceutical Sciences, University of São Paulo [].
Author Contributions
Conceptualization, I.S.K., A.R.B. and E.M.B.; methodology, I.S.K. and A.A.H.; formal analysis, I.S.K., D.P.D., F.R.L., A.A.H. and A.R.B.; data curation, I.S.K., D.P.D., F.R.L. and A.R.B.; writing—original draft preparation, I.S.K., D.P.D., A.A.H. and A.R.B.; writing—review and editing, I.S.K., D.P.D., C.R. and A.R.B.; supervision, I.S.K., E.M.B. and A.R.B.; funding acquisition, C.R. and A.R.B. All authors have read and agreed to the published version of the manuscript.
Funding
A.R.B. is highly grateful to CNPq-Brazil (305250/2019-1) and FAPESP (2019/16169-0). A.A.H. deeply acknowledges CAPES (Finance Code 001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Venkitasamy, C.; Zhao, L.; Zhang, R.; Pan, Z. Grapes. In Integrated Processing Technologies for Food and Agricultural By-Products; Pan, Z., Zhang, R., Zicari, S., Eds.; Elsevier: Amsterdan, The Netherlands, 2019; pp. 133–163. [Google Scholar]
- Abbasi-Parizad, P.; de Nisi, P.; Scaglia, B.; Scarafoni, A.; Pilu, S.; Adani, F. Recovery of Phenolic Compounds from Agro-Industrial by-Products: Evaluating Antiradical Activities and Immunomodulatory Properties. Food Bioprod. Process. 2021, 127, 338–348. [Google Scholar] [CrossRef]
- Iqbal, A.; Schulz, P.; Rizvi, S.S.H. Valorization of Bioactive Compounds in Fruit Pomace from Agro-Fruit Industries: Present Insights and Future Challenges. Food Biosci. 2021, 44, 101384. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic Content and in Vitro Antioxidant Characteristics of Wine Industry and Other Agri-Food Solid Waste Extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Reboredo-Rodriguez, P.; Cianciosi, D.; Mezzetti, B.; Quiles, J.L.; Bompadre, S.; Battino, M.; Giampieri, F. Strawberry-Based Cosmetic Formulations Protect Human Dermal Fibroblasts against UVA-Induced Damage. Nutrients 2017, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin Photoprotection by Natural Polyphenols: Anti-Inflammatory, Antioxidant and DNA Repair Mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Hübner, A.; Sobreira, F.; Neto, A.V.; de Oliviera Pinto, C.A.S.; Dario, M.F.; Díaz, I.E.C.; Lourenço, F.R.; Rosado, C.; Baby, A.R.; Bacchi, E.M. The Synergistic Behavior of Antioxidant Phenolic Compounds Obtained from Winemaking Waste’s Valorization, Increased the Efficacy of a Sunscreen System. Antioxidants 2019, 8, 530. [Google Scholar] [CrossRef]
- Hübner, A.A.; Sarruf, F.D.; Oliveira, C.A.; Neto, A.V.; Fischer, D.C.H.; Kato, E.T.M.; Lourenço, F.R.; Baby, A.R.; Bacchi, E.M. Safety and Photoprotective Efficacy of a Sunscreen System Based on Grape Pomace (Vitis vinifera L.) Phenolics from Winemaking. Pharmaceutics 2020, 12, 1148. [Google Scholar] [CrossRef]
- Wagner, H.; Bladt, S. Plant Drug Analysis; Springe: Berlin/Heidelberg, Germany, 1996; ISBN 978-3-540-58676-0. [Google Scholar]
- Fontana, A.; Antoniolli, A.; D’Amario Fernández, M.A.; Bottini, R. Phenolics Profiling of Pomace Extracts from Different Grape Varieties Cultivated in Argentina. RSC Adv. 2017, 7, 29446–29457. [Google Scholar] [CrossRef]
- Antoniolli, A.; Fontana, A.R.; Piccoli, P.; Bottini, R. Characterization of Polyphenols and Evaluation of Antioxidant Capacity in Grape Pomace of the Cv. Malbec. Food Chem. 2015, 178, 172–178. [Google Scholar] [CrossRef]
- Perra, M.; Lozano-Sánchez, J.; Leyva-Jiménez, F.J.; Segura-Carretero, A.; Pedraz, J.L.; Bacchetta, G.; Muntoni, A.; de Gioannis, G.; Manca, M.L.; Manconi, M. Extraction of the Antioxidant Phytocomplex from Wine-Making by-Products and Sustainable Loading in Phospholipid Vesicles Specifically Tailored for Skin Protection. Biomed. Pharmacother. 2021, 142, 111959. [Google Scholar] [CrossRef]
- Zhang, J.; Melton, L.D.; Adaim, A.; Skinner, M.A. Cytoprotective Effects of Polyphenolics on H2O2-Induced Cell Death in SH-SY5Y Cells in Relation to Their Antioxidant Activities. Eur. Food Res. Technol. 2008, 228, 123–131. [Google Scholar] [CrossRef]
- Mendoza, L.; Navarro, F.; Melo, R.; Báez, F.; Cotoras, M. Characterization of Polyphenol Profile of Extracts Obtained from Grape Pomace and Synergistic Effect of These Extracts and Fungicides against Botrytis Cinerea. J. Chil. Chem. Soc. 2019, 64, 4607–4609. [Google Scholar] [CrossRef]
- Wróblewska, K.B.; Baby, A.R.; Grombone Guaratini, M.T.; Moreno, P.R.H. In Vitro Antioxidant and Photoprotective Activity of Five Native Brazilian Bamboo Species. Ind. Crop. Prod. 2019, 130, 208–215. [Google Scholar] [CrossRef]
- Cândido, T.M.; Ariede, M.B.; de Oliviera Pinto, C.A.S.; Lourenço, F.R.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. Prospecting In Vitro Antioxidant and Photoprotective Properties of Rosmarinic Acid in a Sunscreen System Developed by QbD Containing Octyl P-Methoxycinnamate and Bemotrizinol. Cosmetics 2022, 9, 29. [Google Scholar] [CrossRef]
- Velasco, M.V.R.; Sarruf, F.D.; Salgado-Santos, I.M.N.; Haroutiounian-Filho, C.A.; Kaneko, T.M.; Baby, A.R. Broad Spectrum Bioactive Sunscreens. Int. J. Pharm. 2008, 363, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.G.E.; Sarruf, F.D.; de Oliveira, L.C.D.; Arêas, E.P.G.; Kaneko, T.M.; Consiglieri, V.O.; Velasco, M.V.R.; Baby, A.R. Influence of Particle Size on Appearance and in Vitro Efficacy of Sunscreens. Braz. J. Pharm. Sci. 2013, 49, 251–261. [Google Scholar] [CrossRef]
- Pinteus, S.; Lemos, M.F.L.; Silva, J.; Alves, C.; Neugebauer, A.; Freitas, R.; Duarte, A.; Pedrosa, R. An Insight into Sargassum Muticum Cytoprotective Mechanisms against Oxidative Stress on a Human Cell in Vitro Model. Mar. Drugs 2017, 15, 353. [Google Scholar] [CrossRef]
- Hoss, I.; Rajha, H.N.; el Khoury, R.; Youssef, S.; Manca, M.L.; Manconi, M.; Louka, N.; Maroun, R.G. Valorization of Wine-Making By-Products’ Extracts in Cosmetics. Cosmetics 2021, 8, 109. [Google Scholar] [CrossRef]
- United Nations Industrial Development Organization Circular Economy and Agrobusiness Development. Available online: https://www.unido.org/sites/default/files/files/2021-07/CE4ABD.pdf (accessed on 23 October 2022).
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent Selection for Efficient Extraction of Bioactive Compounds from Grape Pomace. Ind. Crop. Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef]
- de la Cerda-Carrasco, A.; López-Solís, R.; Nuñez-Kalasic, H.; Peña-Neira, Á.; Obreque-Slier, E. Phenolic Composition and Antioxidant Capacity of Pomaces from Four Grape Varieties (Vitis vinifera L.). J. Sci. Food Agric. 2015, 95, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Cotoras, M.; Vivanco, H.; Melo, R.; Aguirre, M.; Silva, E.; Mendoza, L. In Vitro and in Vivo Evaluation of the Antioxidant and Prooxidant Activity of Phenolic Compounds Obtained from Grape (Vitis vinifera) Pomace. Molecules 2014, 19, 21154–21167. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Y.I.; Kosir, V.; Yin, X.; Ross, K.; Diarra, M.S. Grape Pomace as a Promising Antimicrobial Alternative in Feed: A Critical Review. J. Agric. Food Chem. 2019, 67, 9705–9718. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Warraich, U.-A.; Hussain, F.; Kayani, H.U.R. Aging—Oxidative Stress, Antioxidants and Computational Modeling. Heliyon 2020, 6, e04107. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione Peroxidase-1 in Health and Disease: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Gough, D.R.; Cotter, T.G. Hydrogen Peroxide: A Jekyll and Hyde Signalling Molecule. Cell Death Dis. 2011, 2, e213. [Google Scholar] [CrossRef]
- Reyes, E.; Vitale, M.A. Avances En Fotoprotección. Mecanismos Moleculares Implicados. Piel 2013, 28, 235–247. [Google Scholar] [CrossRef]
- Ruscinc, N.; Morocho-Jácome, A.L.; Martinez, R.M.; Magalhães, W.V.; Escudeiro, C.C.; Giarolla, J.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. Vaccinium Myrtillus L. Extract Associated with Octocrylene, Bisoctrizole, and Titanium Dioxide: In Vitro and in Vivo Tests to Evaluate Safety and Efficacy. J. Cosmet. Dermatol. 2022, 21, 4765–4774. [Google Scholar] [CrossRef] [PubMed]
- Morocho-Jácome, A.L.; Freire, T.B.; de Oliveira, A.C.; de Almeida, T.S.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. In Vivo SPF from Multifunctional Sunscreen Systems Developed with Natural Compounds—A Review. J. Cosmet. Dermatol. 2020, 20, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Tomazelli, L.C.; de Assis Ramos, M.M.; Sauce, R.; Cândido, T.M.; Sarruf, F.D.; de Oliveira Pinto, C.A.S.; de Oliveira, C.A.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. SPF Enhancement Provided by Rutin in a Multifunctional Sunscreen. Int. J. Pharm. 2018, 552, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Pereira, A.C.; Wurlitzer, N.J.; Dionisio, A.P.; Lacerda Soares, M.V.; do Scorgo Rocha Bastos, M.; Elesbão Alves, R.; Montenegro Brasil, I. Synergistic, Additive and Antagonistic Effects of Fruit Mixtures on Total Antioxidant Capacities and Bioactive Compounds in Tropical Fruit Juices. Arch. Latinoam. Nutr. 2015, 65, 119–127. [Google Scholar]
- Keskin, N.; Kunter, B.; Celik, H.; Kaya, O.; Keskin, S. Evaluation of Clonal Variability of Berry Phenolics in Vitis vinifera L. Cv. Kalecik Karası. Erwerbs-Obstbau 2022, 64 (Suppl 1), 65–72. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).