Abstract
Preparations of the Cymbopogon citratus leaves are used in folk medicine for the treatment of inflammatory processes. The present study investigated the proposed anti-inflammatory properties of C. citratus essential oil (EOCC) in human skin in vivo using the methylnicotinate (MN) microinflammation skin model. Skin exposure to MN causes a disturbance that triggers the production of reactive oxygen species and evokes a short duration microinflammatory reaction that might be explored to meet this objective. Fourteen participants of both sexes were selected after providing informed consent. Three areas (3 cm × 3 cm) were drawn on both forearms. One randomly chosen area was treated for 14 days, twice a day, with a polyacrylic acid gel containing 5% EOCC. Remaining areas were used as controls. Results revealed a clear protective effect at the EOCC-treated site. The MN reaction showed significantly lower transepidermal water loss, blood perfusion, erythema, and edema when compared with the other areas. Furthermore, the methodology here proposed is an innovative approach to study the clinical impact of these substances on human skin, contributing to an evidence-based support regarding the interest of using these products in human health.
1. Introduction
Cymbopogon citratus (C. citratus), commonly known as lemongrass, originally from Southeast Asia, is now widely found and cultivated in tropical and subtropical regions around the world [1,2]. C. citratus is commonly used in food preparations such as soups and curries, or served as an accompaniment to poultry, fish, beef, or seafood. It is also consumed as an infusion beverage (tea). Aromatic essential oils extracted from C. citratus leaves are used in the chemical and pharmaceutical industries in fragrances, flavors, perfumery, cosmetics, detergents, and medicines [3]. These essential oils consist of a complex mixture of hydrophobic and volatile compounds, normally monoterpenes and sesquiterpenes, with a pleasant smell that justifies its organoleptic applications. This essential oil from C. citratus (EOCC) is generally recognized as safe (GRAS) in accordance with criteria of the Food and Drug Administration (FDA) [3,4,5]. These essential oils are also well known from ethnopharmacology, where they are often applied in traditional medicine to reduce stress, fever, stomach disorders, constipation, pain, and anxiety [3,4].
The main component of C. citratus oil is the monoterpene citral, which gives it the characteristic citrus aroma. Other components can also be isolated in lower concentrations from this essential oil, such as nerol, geraniol, citronellal, terpinolene, geranyl acetate, myrecene, and terpinol methylheptenone [3,5,6]. These chemical substances have long been used in medicine to treat various adverse conditions, with emphasis on the treatment of inflammatory processes [3]. Our group recently confirmed its good safety profile and identified several beneficial effects of C. citratus essential oil (EOCC) in human skin regarding epidermal hydration, barrier integrity, and skin biomechanical properties [7].
Previous studies have shown that the essential oil and the infusion obtained from C. citratus significantly reduced the inflammatory process in both oral and topical administrations, reinforcing its potential use in the treatment of inflammatory skin disorders [5,6,7,8]. It has been suggested that these anti-inflammatory effects might be related to the activity of EOCC components, particularly citral, through multiple ways, including to act as a COX-2 suppressor and a PPARα and γ activator [9,10,11]. Studies focusing this EOCC anti-inflammatory activity were developed in vitro or using in vivo animal (rats and mice) models; hence, a direct extrapolation to human skin was not possible.
The present study aimed to develop an experimental human model designed to test and evaluate the anti-inflammatory properties of a formulation containing EOCC in the human skin using methyl nicotinate as a challenge agent.
2. Materials and Methods
2.1. Formulation and Essential Oil Characterization
The essential oil obtained from Cymbopogon citratus aerial components was obtained from a portuguese supplier, the Cantinho das Aromáticas Viveiros Lda. (V.N Gaia, Portugal). Gas Chromatography–Mass Spectrometry (GC-MS) (Clarus 600T, Perkin Elmer, Shelton, CT, USA) confirmed that EOCC is mainly composed of citral, with 42.3% occurring in the geranial (trans-citral) form and 33.2% in the form of neral (cis-citral) form, which corresponds to a total of 75.5% of the sample constituents. Twenty three minority compounds were also identified. The formulation preparation and chemical characterization of the EOCC were performed as recently described [7]. The quantitative composition of the EOCC contained in gel is shown in Table 1.

Table 1.
Chemical composition of the essential oil obtained from the Cymbopogon citratus aerial components and analyzed by gas chromatography–mass spectrometry (GC-MS).
2.2. Experimental
This study was conducted in a convenience sample of fourteen healthy participants (nine women and five men, mean age 32.9 ± 17.6 years old) recruited within our university center. All procedures respected the principles of good clinical practice stated in the Declaration of Helsinki and its subsequent amendments [12]. Informed written consent was obtained from all subjects involved in the study, and the experimental protocol was previously approved by the Ethics Commission of the University Health Sciences School (approval number 04/13).
Preliminary selection criteria required for all participants: (i) the absence of any cutaneous lesions at the application sites; (ii) no past or present history of atopy or any other dermatological disease; (iii) no application of any cosmetics in the test areas 48 h prior to the study and during the study development as well; (iv) any pharmacological treatment that might interfere with measurements; and (v) no direct sun exposure or solarium use prior to the study.
The formulation safety profile was previously confirmed by our research group using a modified open (epicutaneous) test version involving a single application and follow-up for the detection of any skin reaction within the 48 h following application [7,13].
The intervention trial was designed for 14 days (D0–D14) with daily applications of two formulations (0.1 mL using a syringe), one containing EOCC and another without EOCC, containing only the vehicle gel. Three areas (3 cm × 3 cm) were drawn on each participant forearm and application sites were randomly chosen for each participant using the Latin square procedure. The intervention was single-blinded, with only the principal investigator (PI) aware of the formulations’ identification and content. The empty site corresponded to the negative control. The first application took place at the lab to illustrate the procedure. Each volunteer also received a simplified graphical representations of the application sequence to respect for each application. The PI explained all the steps, recommending applications twice a day (morning and evening) at home. The PI regularly confirmed the participants’ compliance by phone call. During the study period, participants were instructed not to apply detergents, emollients, moisturizers, or any cosmetics in the tested sites on both forearms.
To test the formulation’s protective effects, we developed an adapted version of the microinflammatory methyl nicotinate (MN) exposure model previously published [14]. Skin responses were measured by non-invasive technologies. These included local perfusion, using laser doppler flowmetry (LDF, Perimed AB, Järfälla, Sweden), erythema measured by the Mexameter MX18® (Courage and Khazaka Electronics, Cologne, Germany) [15], and transepidermal water loss (TEWL) using the Tewameter TM300® system (Courage and Khazaka Electronics, Cologne, Germany) [16]. These variables were measured at the inclusion day (D0) and after two weeks of the regular application of the formulation (D14). On this day, after control measurements, a 0.5% methyl nicotinate aqueous solution was applied in contact with the skin for 1 min in all the tested sites. High-resolution sonography images (HRS, DermaScan C Cortex Twechnology, Aalborg, Denmark) were also obtained before and after the induction of microinflammatory reaction. The color images were converted into a greyscale image for further analysis, processed by the software ImageJ® (NIH, Bethesda, MD, USA) [17].
All measurements were performed in the same lab, by the same technician, in a temperature- and humidity-controlled environment (humidity ~50%, temperature 21 ± 2 °C). Participants were allowed to acclimatize to the laboratory conditions for at least 20–30 min before starting the procedures.
2.3. Statistics
Data were reported as mean ± standard deviation (SD) and compared by one-way ANOVA followed by Bonferroni Test using GraphPad Prism 5® software (GraphPad Software, San Diego, CA, USA). A p-value < 0.05 was considered significant.
3. Results
At D0, all skin variables were measured at the testing sites as described in the experimental section. No differences between sites were found, confirming that our participants were similar for these experimental conditions. At D14 after the intervention but before MN exposure, a significant decrease in TEWL (p < 0.01) and a significant increase in the superficial and deep epidermal hydration (p < 0.05) are noted, as well at the site treated with this formulation containing EOCC when compared with D0 (Table 2).

Table 2.
Epidermal water balance expressed by transepidermal water loss (TEWL), stratum corneum (SC) hydration, and deep epidermis (D) hydration measured in the control site, vehicle site, and EOCC-treated site for 14 days.
Regarding epidermal barrier function, measured by TEWL, erythema, and local perfusion after contact with the MN solution, we noted a dramatic increase in each of these variables in all exposed sites. However, this increase was much more discrete in the EOCC-pretreated site when compared with the other sites (Table 3). As shown, TEWL and erythema were significantly lower (p < 0.01) in the EOCC treated area as compared with the other sites. The same was observed for this MN related microinflammation perfusion increase—almost 4× fold in the control and vehicle sites after MN contact (Table 3). In fact, perfusion increased 302.69% in the control and 249.8% in the vehicle sites while in the EOCC-treated area it increased only 51.71%. Therefore, the local perfusion increase after contact with MN in the EOCC-treated site was significantly lower than values measured at the control (p < 0.01) and the vehicle (p < 0.05) sites, respectively.

Table 3.
Selected micro-inflammatory related variables (transepidermal water loss TEWL, erythema, LDF skin perfusion, and edema) measured at control, vehicle, and EOCC-treated sites before and 30 min after contact with a 0.5% methyl nicotinate solution following a 14-day pretreatment period.
Edema, a most revealing sign of inflammation was also quantified by high-resolution sonography through echogenicity analysis based on the poor echogenic character of water.
Table 3 shows the lowest echo-range (dark grey to black) evolution detected in dermis after contact with the MN solution. As also illustrated in Figure 1, the low-range echogenicity increased globally at sites after exposure to MN. However, the EOCC-treated site showed that a significantly lower increase in water within dermis was reduced in the EOCC-treated site (p < 0.01) when compared with the other two sites. We consider these results to be in line with previous observations regarding other related variables.
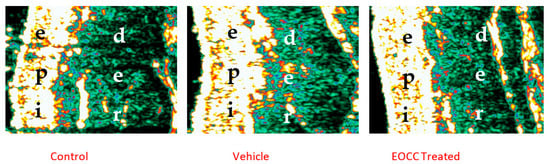
Figure 1.
Illustrative ultrasound images of the effects of EOCC formulation on the skin 30 min after MN application. Observed are an increase in epidermal echogenicity area (bright area) and a decrease in edema (black area) in EOCC-treated site; “epi” (epidermal layer) while in the “der” (dermis).
4. Discussion
The biological activity of essential oils is being progressively documented, following their classification as herbal medicines by the European Medicines Agency (EMA), mainly based on their traditional use as herbal medicinal products [18]. Permanent challenges such as drug resistance and the need for new antimicrobials elevated natural products and essential oils to a new level of interest in research. As examples of these interests, new application areas such as oncology [19,20] or metabolic diseases (e.g., obesity) [3] are currently being tested in vitro as well as in vivo. The traditional antimicrobial effects of essential oils are well documented and are not restricted to bacteria [3,21,22,23]. Antifungal [24], antiparasitic [25], and even antiviral [26,27] activities have been previously reported. Regarding the often mentioned anti-inflammatory, antioxidant, and healing properties, there is sufficient evidence that many essential oils contain different bioactives capable of interfering with the arachidonic metabolism, altering the production of inflammation mediators, and affecting pro-inflammatory gene expression [28,29], but only a few studies explored these mechanistic questions.
The beneficial activities of C. citratus have been recognized in ethnopharmacology [3,30,31], although some grey zones still exist [3,32]. In particular, citral (3,7-dimethyl-2,6-octadienal), the main monoterpene in C. citratus essential oils, has been studied for its anti-inflammatory capacities involving multiple pathways. In lipopolysaccharide-stimulated peritoneal macrophages from normal mice, this essential oil was able to suppress the pro- inflammatory cytokines IL-1β and IL-6 [3,4,28]. It has been shown to be a potent inhibitor of acetaldehyde oxidation by the low-KM mitochondrial form of aldehyde dehydrogenases [33]. In addition, it has shown interesting effects on lipopolysaccharide-induced inflammation in human endothelial cells [11]. Preclinical studies in mice have revealed a significant anti-inflammatory effect of C. citratus essential oil. Using the carrageenan-induced paw edema model, edema was reduced by 82.7% at ninety minutes after oral administration of a 10 mg/kg dosage, while a reduction of 86.2% was observed with a 100 mg/kg dosage. The anti-inflammatory activity of lemongrass oil was compared to the standard diclofenac, a well-established anti-inflammatory non-steroidal medicine showing similar edema reduction efficacy (86.2%) from a 50 mg/kg dosage per os [5].
The topical anti-inflammatory effects of the EOCC have also been evaluated in mice using the croton oil-induced ear edema model. Topical pre-treatment with EOCC significantly reduced the ear edema, and appeared to be more effective than the diclofenac gel standard [6]. However, the anti-inflammatory mechanism of EOCC is still not completely understood.
Our group has been studying the safety and efficacy of topically applied essential oils on human skin [7,34]. Therefore, we proposed to develop a study model to quantify the anti-inflammatory capacity of this citral-based essential oil applied on human skin through this short-duration, controlled, microinflammatory process induced by MN [14]. In a recently published study to identify the impact of a topical formulation containing EOCC in human skin, we found significant improvements in epidermal water balance, firmness, and elasticity, confirming the protective capacity of the formulation under study, potentially attributable to the EOCC [7]. Similar benefits in skin physiology were clearly present in the current study. The continuous application of the EOCC formulation for two weeks resulted in a significant improvement in the epidermal barrier function, illustrated in terms of this TEWL reduction and epidermal water balance, associated with the epidermal (superficial and deep) water content increase (Table 2). These changes were not detected in the other sites (control and vehicle).
After controlled exposure to MN, a series of events was identified as an irritative response. Exposure to MN causes a disturbance that triggers the excessive production of reactive oxygen species in the skin, which in turn stimulates cyclooxygenase and prostaglandin synthesis [35,36]. These events evoke the production of endoperoxides that amplify the initial irritative response. This response is manifested as a micro-inflammatory process characterized by localized erythema due to the rapid vasodilation of peripheral blood capillaries located in the dermis, adjacent to the epidermis–dermis junction, and the disruption of the epidermal barrier. TEWL is recognized as a main indicator of the human epidermal barrier and is widely used to assess this function [37]. It is known that the epidermal barrier depends on the integrity of this layer; in particular, from the balance between lipids, mainly ceramides, cholesterol, and free fatty acids in the viable epidermis [38]. The skin response to MN contact peaks within thirty minutes and subsides in approximately two hours [14] and is amenable to monitoring by non-invasive technology. However, this micro-inflammatory response might also be monitored in terms of local perfusion changes, erythema, and edema [14,34,35,36].
As shown in Table 3, TEWL, erythema, and local perfusion increased dramatically at all sites as a direct consequence of this controlled exposure to MN. However, this increase was much more discrete at the site treated with the EOCC-containing topical formulation. This milder response can be interpreted as directly resulting from the protective ability, or anti-inflammatory capacity, of EOCC. In fact, this apparent protection seems to result directly from the repeated interaction of the essential oil components with the epidermis and, in particular, with its lipidic components [7]. The sonographic analyses obtained in this investigation depict an increase in the epidermis echogenicity and a reduced water content thein dermis. These two aspects seem to be related, since, as described before, essential oil components exert a “barrier” reinforcement effect at that level [7,39] (Table 3, Figure 1). Consequently, this reduced response to MN exposure in the EOCC-treated sites could result from the diminished production of free radicals and pro-inflammatory mediators, as previously suggested [3,4,14,28]. The antioxidant profile of EOCC has been regarded as fragile by some authors due to the low concentration of phenols [40]. However, other studies have shown EOCC with high antioxidant activity in vitro compared to standard antioxidants such as ascorbic acid [41], illustrating the need for more and accurate information on these themes.
In summary, our results provide clear evidence of a protective effect of topically applied EOCC in human skin and prompt the need to better define the antioxidant and/or anti-inflammatory capacities of EOCC when applied to healthy human skin.
Some limitations to our study should be considered, such as (a) the limited number of volunteers, not allowing extrapolation to wider populations, (b) the healthy state of the tested population, clearly different from a pathological cohort with a confirmed diagnosis of chronic inflammation or with an acute ongoing process, and (c) the recognized inter-individual variability of the response to the MN implied in this model. Clearly, more studies with different concentrations of EOCC are needed to further explore its bioactivity. Nevertheless, this methodology is reproducible and easy to apply, thus enabling new the generation of new evidence and further insights into these biological allegations involving essential oils.
5. Conclusions
This methodology proposes several clinical outcomes that contribute to evidence of the clinical impact of essential oils as anti-inflammatory in vivo bioactive agents in human skin. The procedure is safe with regard for the participant’s comfort and security, easy to apply, and fully reproducible, suggesting that this approach might be further developed with other biomarkers with the aim to gain further mechanistic insights.
Author Contributions
Experimental planning S.F.d.A. and L.M.R.; formulation technology and chemistry C.P.-L. and M.d.C.C.; experimental and data curation E.J.P., C.R., S.F.d.A.; text drafting S.F.d.A., and L.M.R.; review and final approval L.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work is funded by national funds through FCT—Foundation for Science and Technology, I.P., under projects UIDB/04567/2020, UIDP/04567/2020 and UIDP/50017/2020 + UID B/50017/2020 + LA/P/0094/2020.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and previously approved by the Lusofona University Ethics Committee (protocol code ECTS 04/13).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge Ana Cristina Figueiredo from CESAM—Environment and Sea Research Center (Lisboa) for the chromatography analysis contribution, and FCT—the Foundation for Science and Technology (Portugal), and Lusofona University for financial support.
Conflicts of Interest
All authors have stated that there are no financial and/or personal relationships that could represent a potential conflict of interest.
References
- Ekpenyong, C.E.; Akpan, E.; Nyoh, A. Ethnopharmacology, phytochemistry, and biological activities of Cymbopogon citratus (DC.) Stapf extracts. Chin. J. Nat. Med. 2015, 13, 321–337. [Google Scholar] [PubMed]
- Adeneye, A.A.; Agbaje, E.O. Hypoglycemic and hypolipidemic effects of fresh leaf aqueous extract of Cymbopogon citratus Stapf. in rats. J. Ethnopharmacol. 2007, 112, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Oladeji, O.S.; Adelowo, F.E.; Ayodele, D.T.; Odelade, K.A. Phytochemistry and pharmacological activities of Cymbopogon citratus: A review. Sci. Afr. 2019, 6, e00137. [Google Scholar] [CrossRef]
- Shah, G.; Shri, R.; Panchal, V.; Sharma, N.; Singh, B.; Mann, A. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass). J. Adv. Pharm. Technol. Res. 2011, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Majewska, E.; Kozłowska, M.; Gruczyńska-Sękowska, E.; Kowalska, D.; Tarnowska, K. Lemongrass (Cymbopogon citratus) Essential Oil: Extraction, Composition, Bioactivity and Uses for Food Preservation–a Review. Pol. J. Food Nutr. Sci. 2019, 69, 327–341. [Google Scholar]
- Boukhatem, M.N.; Ferhat, M.A.; Kameli, A.; Saidi, F.; Kebir, H.T. Lemon grass (Cymbopogon citratus) essential oil as a potent anti-inflammatory and antifungal drugs. Libyan J. Med. 2014, 9, 25431. [Google Scholar] [CrossRef]
- Andrade, S.F.; Pinheiro, E.J.; Figueiredo, A.C.; Pereira-Leite, C.; Costa, M.d.C.; Monteiro-Rodrigues, L. Cymbopogon citratus (DC.) Stapf essential oil: Unraveling potential benefits on human skin. Biomed. Biopharm. Res. 2022, 19, 168–180. [Google Scholar]
- Costa, G.; Ferreira, J.P.; Vitorino, C.; Pina, M.E.; Sousa, J.J.; Figueiredo, I.V.; Batista, M.T. Polyphenols from Cymbopogon citratus leaves as topical anti-inflammatory agents. J. Ethnopharmacol. 2016, 178, 222–228. [Google Scholar] [CrossRef]
- Costa, G.; Garcia, R.; Ferreira, J.P.; Santos, T.; Branco, F.; Caramona, M.; Carvalho, R.d.; Dinis, A.M.; Batista, M.T.; Branco, M.C.; et al. Evaluation of Anti-inflammatory and Analgesic Activities of Cymbopogon citratus In vivo-Polyphenols Contribution. Res. J. Med. Plant 2015, 9, 1–13. [Google Scholar] [CrossRef]
- Francisco, V.; Costa, G.; Figueirinha, A.; Marques, C.; Pereira, P.; Miguel Neves, B.; Celeste Lopes, M.; García-Rodríguez, C.; Teresa Cruz, M.; Teresa Batista, M. Anti-inflammatory activity of Cymbopogon citratus leaves infusion via proteasome and nuclear factor-κB pathway inhibition: Contribution of chlorogenic acid. J. Ethnopharmacol. 2013; 148, 126–134. [Google Scholar]
- Song, Y.; Zhao, H.; Liu, J.; Fang, C.; Miao, R. Effects of Citral on Lipopolysaccharide-Induced Inflammation in Human Umbilical Vein Endothelial Cells. Inflammation 2015, 39, 663–671. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Meloni, M.; Berardesca, E. The Impact of COLIPA Guidelines for Assessment of Skin Compatibility on the Development of Cosmetic Products. Am. J. Clin. Dermatol. 2001, 2, 65–68. [Google Scholar] [CrossRef]
- Monteiro Rodrigues, L.; Faloni de Andrade, S.; Rocha, C. Topically applied methyl nicotinate evokes a temporary inflammation on human skin. Biomed. Biopharm. Res. 2021, 18, 38–47. [Google Scholar] [CrossRef]
- Qian, C.Y.; Yuan, C.; Tan, Y.M.; Liu, X.P.; Dong, Y.Q.; Yang, L.J.; Wu, P.L.; Wang, X.M. Comparing performance of Chromameter®, Mexameter® and full-field laser perfusion imaging for measurement of ultraviolet B light-induced erythema. Clin. Exp. Dermatol. 2015, 40, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Pinnagoda, J.; Tupkek, R.A.; Agner, T.; Serup, J. Guidelines for transepidermal water loss (TEWL) measurement. Contact Dermat. 1990, 22, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Seidenari, S.; Nakijo, A.D.; Pepe, P.; Giannetti, A. Ultrasound B scanning with image analysis for assessment of allergic patch test reactions. Contact Dermat. 1991, 24, 216–222. [Google Scholar]
- Quality of Essential Oils as Active Substances in Herbal Medicinal Products/Traditional Products-Scientific Guideline European Medicines Agency. (s.d.). European Medicines Agency. Available online: http://www.ema.europa.eu/en/quality-essential-oils-active-substances-herbal-medicinal-products-traditional-herbal-medicinal (accessed on 12 March 2022).
- Liu, K.; Deng, W.; Hu, W.; Cao, S.; Zhong, B.; Chun, J. Extraction of ‘Gannanzao’ Orange Peel Essential Oil by Response Surface Methodology and its Effect on Cancer Cell Proliferation and Migration. Molecules 2019, 24, 499. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.; Alves, R.; D´Elia, G.; Anunciação, T.; Silva, V.; Santos, L.; Soares, M.; Cardozo, N.; Costa, E.; Silva, F.; et al. Antitumor Effect of the Essential Oil from the Leaves of Croton matourensis Aubl. (Euphorbiaceae). Molecules 2018, 23, 2974. [Google Scholar] [CrossRef]
- Van de Vel, E.; Sampers, I.; Raes, K. A review on influencing factors on the minimum inhibitory concentration of essential oils. Crit. Rev. Food Sci. Nutr. 2017, 59, 357–378. [Google Scholar]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Yang, S.K.; Yusoff, K.; Thomas, W.; Akseer, R.; Alhosani, M.S.; Abushelaibi, A.; Lim, S.H.E.; Lai, K.S. Lavender essential oil induces oxidative stress which modifies the bacterial membrane permeability of carbapenemase producing Klebsiella Pneumoniae. Sci. Rep. 2020, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, L.; Chen, J.; Sui, J.; Yi, G.; Wu, J.; Ma, Y. Correlation between Chemical Composition and Antifungal Activity of Clausena lansium Essential Oil against Candida spp. Molecules 2019, 24, 1394. [Google Scholar] [CrossRef] [PubMed]
- Rottini, M.M.; Amaral, A.C.F.; Ferreira, J.L.P.; Oliveira, E.S.C.; Silva, J.R.D.A.; Taniwaki, N.N.; dos Santos, A.R.; Almeida-Souza, F.; de Souza, C.D.S.F.; Calabrese, K.D.S. Endlicheria bracteolata (Meisn.) Essential Oil as a Weapon Against Leishmania amazonensis: In Vitro Assay. Molecules 2019, 24, 2525. [Google Scholar] [PubMed]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [PubMed]
- Schnitzler, P. Essential Oils for the Treatment of Herpes Simplex Virus Infections. Chemotherapy 2019, 64, 1–7. [Google Scholar] [PubMed]
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252–9287. [Google Scholar] [PubMed]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Essential Oils and Health. Yale J. Biol. Med. 2020, 93, 291–305. [Google Scholar]
- Novais, M.H.; Santos, I.; Mendes, S.; Pinto-Gomes, C. Studies on pharmaceutical ethnobotany in Arrabida Natural Park (Portugal). J. Ethnopharmacol. 2004, 93, 183–195. [Google Scholar] [CrossRef]
- Avoseh, O.; Oyedeji, O.; Rungqu, P.; Nkeh-Chungag, B.; Oyedeji, A. Cymbopogon Species; Ethnopharmacology, Phytochemistry and the Pharmacological Importance. Molecules 2015, 20, 7438–7453. [Google Scholar]
- Capetti, F.; Tacchini, M.; Marengo, A.; Cagliero, C.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Citral-Containing Essential Oils as Potential Tyrosinase Inhibitors: A Bio-Guided Fractionation Approach. Plants 2021, 10, 969. [Google Scholar]
- Boyer, C.S.; Petersen, D.R. The metabolism of 3,7-dimethyl-2,6-octadienal (citral) in rat hepatic mitochondrial and cytosolic fractions. Interactions with aldehyde and alcohol dehydrogenases. Drug Metab. Dispos. 1991, 19, 81–86. [Google Scholar] [PubMed]
- Silva, H.; Rosado, C.; Antunes, J.; Monteiro Rodrigues, L. Exploring human in vivo microcirculation with methylnicotinate in different perfusion conditions. J. Biomed. Biopharm. Res. 2014, 11, 207–214. [Google Scholar]
- Elawa, S.; Mirdell, R.; Tesselaar, E.; Farnebo, S. The microvascular response in the skin to topical application of methylnicotinate: Effect of concentration and variation between skin sites. Microvasc. Res. 2019, 124, 54–60. [Google Scholar] [CrossRef]
- Vertuani, S.; Ziosi, P.; Solaroli, N.; Buzzoni, V.; Carli, M.; Lucchi, E.; Valgimigli, L.; Baratto, G.; Manfredini, S. Determination of antioxidant efficacy of cosmetic formulations by non-invasive measurements. Ski. Res. Technol. 2003, 9, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Ratz-lyko, A.; Arct, J.; Pytkowska, K. Moisturizing and antiinflammatory properties of cosmetic formulations containing Centella asiatica extract. Indian J. Pharm. Sci. 2016, 78, 27. [Google Scholar] [PubMed]
- Bouwstra, J.A.; Ponec, M. The skin barrier in healthy and diseased state. Biochim. Et Biophys. Acta (BBA) Biomembr. 2006, 1758, 2080–2095. [Google Scholar]
- de Andrade, S.F.; Rijo, P.; Rocha, C.; Zhu, L.; Rodrigues, L.M. Characterizing the Mechanism of Action of Essential Oils on Skin Homeostasis—Data from Sonographic Imaging, Epidermal Water Dynamics, and Skin Biomechanics. Cosmetics 2021, 8, 36. [Google Scholar] [CrossRef]
- Alarcón-Moyano, J.; Matiacevich, S. Active emulsions based on alginate and lemongrass/citral essential oils: Effect of encapsulating agents on physical and antimicrobial properties. Internat. J. Food Prop. 2019, 22, 1952–1965. [Google Scholar]
- De Oliveira, E.; Silva, F.; Soares, J.C.M.; Valdez, A.; da Silva Ferreira, M.V.; da Silva Cecim, M. Cymbopogon citratus protects erythrocytes from lipid peroxidation in vitro. Cardiov. Hemat. Ag. Med. Chem. 2021, 19, 166–169. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).