1. Introduction
The greater burdock plant (
Arctium lappa L.) is an herbaceous plant that has been used as a therapeutic globally and in traditional Chinese medicine (TCM) for hundreds of years [
1]. Burdock extracts have been studied extensively and show a wide range of benefits including anti-inflammatory, anticancer, antidiabetic, antimicrobial, and antiviral activities [
1,
2]. Parts of the burdock plant include the root, stem, leaves, seed, and a whole fruit which consists of a round, purple flower head with modified prickly leaves called bracts, and achenes which contain seeds that encompass the fruit pericarp that are surrounded by hook-like burrs [
3,
4]. While there have been numerous published studies on the production and pharmacological potential of burdock extracts, most of the research has focused on burdock root and whole fruit, which has left the seed alone underutilized and understudied. Additionally, the lignans of primary interest in burdock, arctiin and arctigenin (ATG), are abundant in the seeds and their extracts have shown higher antioxidant activity and polyphenolic content compared to root and leaf extracts [
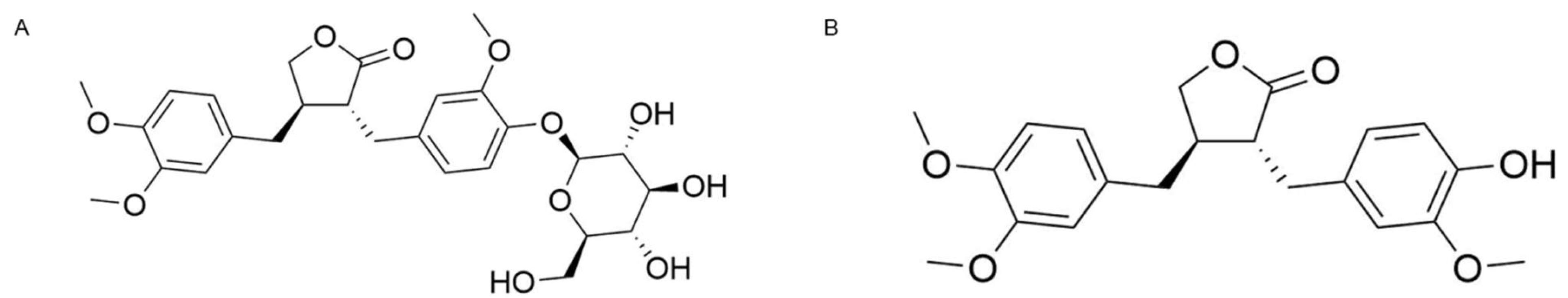
5]. Chemical structures of arctiin and ATG are shown in
Figure 1. Polyphenol arctigenin, a phenylpropanoid dizbenzyl butyrolactone lignan, has also been shown to possess extensive anti-inflammatory properties by multiple pathways including suppression of nitric oxide (NO) production, modulation of pro-inflammatory cytokines, and inactivation of NF-κB [
6]. Moreover, burdock fruit and leaf extracts have been reported to inhibit tyrosinase in a biochemical assay and decrease melanin content in mouse melanoma cells; however, there are little-to-no studies in more advanced tissue models or in human subjects exploring this activity [
7,
8].
Hyperpigmentation and skin tone unevenness are common dermatologic concerns that have a significant impact on quality of life [
9]. Common types of hyperpigmentation include solar lentigo, melasma, and post inflammatory hyperpigmentation [
10]. Hydroquinone and topical steroids have been successfully used to combat hyperpigmentation clinically, but both are associated with adverse side effects and safety concerns including exogenous ochronosis, skin irritation, and contact dermatitis [
11,
12]. Additionally, hydroquinone is banned for cosmetic use in Europe, Japan, Australia, and other countries because of these safety concerns. With consumers becoming more informed about the contents of their skincare products, there is an increased desire for natural, safe, and effective cosmetic ingredients. This demand has created a focus on cosmeceuticals, which are skincare ingredients that encompass the aesthetic properties of a cosmetic while also harnessing drug-like efficacy with data-backed claims [
13]. In Japan specifically, development of a cosmeceutical with skin brightening or skin lightening claims falls under the “quasi-drug” designation, which requires approval by the Japanese Ministry of Health, Labor, and Welfare [
14] to ensure safety and activity. Developing skin brightening ingredients that meet these demands and standards is essential to provide consumers with safe, effective products.
Here, we present for the first time the development of a novel, natural burdock seed extract, ATG-enriched burdock seed oil (ABSO). We report the successful extraction and enrichment of ATG to produce ABSO, which contains ~5-times higher levels of ATG than its initial extract burdock seed oil (BSO). We aim to demonstrate the melanin reduction capacity of ABSO compared to kojic acid, BSO, and synthetic ATG tested alone in vitro. Additionally, topically applied 1% ABSO is evaluated clinically to determine its tolerability and skin brightening potential in human subjects. Lastly, we look to establish that ABSO adheres to the criteria of the Japanese Standards of Quasi-Drug Ingredients [
15] for BSO.
2. Materials and Methods
2.1. Reagents
All chemicals, including kojic acid and arctigenin, were obtained from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA), ThermoFisher (Waltham, MA, USA), Accel Pharmtech (East Brunswick, NJ, USA), Protameen Chemical (Totowa, NJ, USA), Gattefossé (Paramus, NJ, USA), or PerkinElmer (Waltham, MA, USA). Burdock seed was sourced from Pharma Resources International (Altamonte Springs, FL, USA).
2.2. ABSO Extraction
ABSO (patent application JP 2022161418) was produced by suspending double-ground burdock seeds in reagent grade ethanol (5:1 v:w) and heating at 40 °C with stirring for 18 h. The suspension was allowed to cool and settle, followed by the filtrate being decanted and filtered over a pad of silica gel and Celite (2:1). The resulting solution was then treated with sulfuric acid and stirred at 70 °C for 18 h. The reaction mixture was monitored for arctiin conversion to arctigenin by high-performance liquid chromatography (HPLC). Amberlite® free-base resin (Sigma-Aldrich Co., Ltd.; St. Louis, MO, USA) was prepared and added to the reaction mixture. The pH was monitored until the reaction reached 5–6. NORIT® SA2 decolorizing charcoal was added and stirred for 18 h. The reaction was filtered over a Celite pad and washed with ethanol. The solution was concentrated to dryness on a rotary evaporator in a 30 °C water bath. The resultant bi-phasic mixture was allowed to settle overnight in a 22 °C oven. The next day, the clarified oil was decanted from the solid layer and dried overnight in a 22 °C vacuum oven.
2.3. HPLC Analysis
HPLC analysis was performed using an Agilent 1200 series (Agilent Technologies, Inc.; Santa Clara, CA, USA) equipped with a micro vacuum degasser, quaternary pump, autosampler, thermostatted column compartment, diode array and multiple wavelength detector SL. The analytical separations were performed with a Luna 5 µm C18(2) 100 Å 250 mm × 4.6 mm column equipped with a C18 guard column. The solvent system used was water with 0.05% trifluoroacetic acid (A) and acetonitrile with 0.05% trifluororacetic acid (B) with a flow rate of 1 mL/min. The method developed was 95% solvent A for 1 min, 95–5% solvent A over 12 min, then 5% solvent A for 3 min. Peak detection was done at 280 nm. Standard curves were produced using synthetic arctiin and arctigenin (Sigma-Aldrich Co., Ltd.; St. Louis, MO, USA) and were utilized for quantification.
2.4. Three-Dimensional Reconstructed Human Tissue Culture
EpiDerm™ skin cultures consisting of differentiated, human-derived epidermal keratinocytes were purchased as preserved culture inserts from MatTek Corp. (Ashland, MA, USA). Tissues were acclimated at the air liquid interface at 37 °C and 5% CO2 for 1 h in 6-well plates, then given fresh media and incubated overnight. Treatments were administered once by topical application (25 µL) and incubated for 48 h at 37 °C and 5% CO2.
MelanoDerm™ skin cultures consisting of differentiated co-cultures of keratinocytes and melanocytes of human origin from darkly pigmented donors were purchased as preserved culture inserts from MatTek Corp. (Ashland, MA, USA). Tissues were acclimated at the air liquid interface at 37 °C and 5% CO2 for 1 h in 6-well plates. Treatments were administered by topical application (25 µL) every 48 h for 14 days (day 1, 3, 5, 7, 9, 11, 13) beginning on day 1.
For both tissue models, treatments were formulated as w/w% lotions containing active ingredient (kojic acid, ATG, BSO, or ABSO) mixed with ethoxydiglycol (Gattefossé), polysorbate 80 (Protameen Chemicals), and water. Untreated tissues had nothing applied.
2.5. Skin Irritation Assay
EpiDerm™ skin cultures were used to determine the skin irritation potential of lotions containing different concentrations of ABSO. After 48 h of incubation with test materials, tissues were washed with 1× Dulbecco’s Phosphate Buffered Saline (DPBS) and blotted dry. Next, tissues were incubated with MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) at 37 °C and 5% CO
2 for 3 h, and then MTT was extracted with isopropanol. Resultant extracts were transferred to a 96-well plate where the absorbance was measured at 570 nm using a 2104 EnVision plate reader (PerkinElmer Inc., Waltham, MA, USA). The EpiDerm™ tissue model has previously been developed and validated as a suitable assay for in vitro determination of skin irritation of cosmetic ingredients [
16].
2.6. Melanin Assay
On Day 14, macroscopic assessment was performed from MelanoDerm™ tissues using a standard digital camera and a melanin colorimetric assay was performed. Briefly, tissue extract solutions were obtained by first washing tissues in 1× DPBS and then incubating in Solvable
TM solution (PerkinElmer, Inc.; Waltham, MA, USA) at 90 °C for 2 h. Samples were cooled, centrifuged, and measured at 490 nm using a 2104 EnVision plate reader (PerkinElmer Inc., Waltham, MA, USA). Melanin concentration of tissues were calculated using a standard curve of synthetic melanin (Sigma-Aldrich Co., Ltd.; St. Louis, MO, USA). The MelanoDerm™ tissue model has previously been validated as a suitable assay for screening and developing topically applied whitening/brightening products [
17].
2.7. Quasi-Drug Testing
To confirm ABSO met the Japanese Standards of Quasi-Drug Ingredients [
15] for burdock seed oil, ABSO was analyzed by a third-party company, Japan Food Research Laboratories (Tokyo, Japan). Test items were identification, acid value, saponification value, iodine value, unsaponifiable matter, heavy metals, and arsenic levels. Identification was determined by the liquid film method and the infrared absorption spectrum measurement method which measures the degree of absorption of infrared light as it passed through the sample at various wavelengths using a dispersive infrared spectrophotometer or a Fourier transform infrared spectrophotometer. Acid value was determined by measuring the number of milligrams of potassium hydroxide required to neutralize 1 g of ABSO sample. Saponification value is a method for measuring the amounts of ester and free acid contained in a sample and was determined by measuring the number of milligrams of potassium hydroxide required for saponification of ester and neutralization of free acids in 1 g of ABSO sample. Iodine value is a method for measuring the number of unsaturated groups in a sample and was determined by measuring the grams of iodine equivalent to the amount of halogen binding to 100 g of ABSO sample. Unsaponifiable matter was determined by identifying substances in the ABSO sample not saponifiable by hydroxylate alkali, soluble in organic solvents, and insoluble in water. Heavy metal content was determined by color change by sodium sulfide in acid. Arsenic level was determined by generator bottle method to measure the amount of diarsenic trioxide in the ABSO sample.
2.8. Clinical Study Design and Enrollment
A clinical study in healthy volunteers was conducted at Princeton Consumer Research (PCR; Winnipeg, MB, Canada) in accordance with the Declaration of Helsinki and PCR Standard Operating Procedures. This was an eight-week, double-blind, vehicle-controlled study with 49 subjects. Forty-five of the forty-nine enrolled subjects (
Table 1) completed the study. Of the four subjects (1% ABSO
n = 3; Vehicle
n = 1) who did not complete the study, two were discontinued due to non-compliance, one was lost to follow-up, and the other was discontinued due to a schedule conflict. Test articles supplied were formulated with ingredients that are safe and suitable for the product’s stated purpose.
The following inclusion criteria were used in this study: (1) Subjects of Asian (Chinese, Japanese, and Korean) decent, (2) Ages 30 through 60 years, (3) Fitzpatrick skin types II–IV, (4) Self-assessed uneven skin tone and/or age spots, (5) Skin brightness and tone evenness scores of 0–6 as determined by the evaluator at baseline, (6) Willingness to discontinue use of regular moisturizer and treatment products on the face and only use the test article twice daily during the study period with allowed use of regular cosmetics and/or shaving products, (7) Willingness to follow study instructions and availability to attend all study visits, (8) Willingness to withhold all facial treatments for the duration of the study including toxins, fillers, microdermabrasion, peels, facials, laser treatments, IPL, tightening treatments, or facial laser hair removal. The following exclusion criteria were used in this study: (1) Pregnant, breast feeding, or planning to become pregnant during the study. (2) Known allergy or hypersensitivity to facial serums or similar materials or their ingredients. (3) Current skin disease of any type on the test site or under the treatment of a doctor for any skin condition. (4) Current moderate to severe acne or any active inflammatory condition at the test site. (5) Any conditions on the test site that would interfere with evaluations (i.e., Tattoos, scars, open cuts, sunburn, piercings, excessive hair, etc.). (6) Insulin dependent diabetes. (7) Concurrent medication that might affect the response to the test articles including routine use of anti-inflammatory medications, antihistamines, and steroids. (8) Use of topical treatments such as over the counter acne medication or hydrocortisone on the test site in the last month. (9) Medical condition which, in the opinion of the Investigator, would compromise the safety of the subject or confound study results. (10) Facial chemical peel in the last 14 days. (11) Use of Isotretinone/Accutane, Retin-A, Retinol in the last three months. (12) Cosmetic medical procedures in the test area such as injectable anti-wrinkle products, facial cosmetic surgery, etc. in the last year.
Subjects reported to the testing facility for baseline screening at which time Informed Consent and demographics were obtained. Subject demographics are listed in
Table 1. Subjects were randomized to treatment groups 1% ABSO lotion (
n = 33) or vehicle (
n = 16), supplied with test articles, and instructed to apply two to three pumps of the test article to cover their entire face twice a day (once in morning and once in the evening) during the entirety of the eight-week treatment period. There was no wash out period other than what is specified in the exclusion criteria. Subjects were instructed to protect their face from excessive sun exposure and any form of tanning/sun burn for the duration of the study. The first application of test article was supervised at the testing facility after baseline measurements were taken. Subjects were instructed to continue use of their usual facial cleanser, shaving products, and cosmetic products that adhered to inclusion/exclusion criteria, and to not introduce any new products to their face other than the test article for the duration of the study.
2.9. Clinical Study Endpoints
Assessments and measurements were made at baseline, week 1, 4, and 8 of the study. Visual assessments of skin brightness, skin tone evenness, and objective and subjective tolerance were assessed by the blinded evaluator. Skin brightness and tone evenness were measured on 10-point scales (
Table S1) where 0 = dark, no brightness or extreme differences in skin tone over large areas, small areas of hyperpigmentation and 9 = high level of brightness, skin is too shiny or very uniform skin tone. To participate in the study, subjects needed a score of 0–6 in both categories. Tolerance was measured on a 4-point scale assigned by the evaluator (objective) or the subjects (subjective) where 0 = none, 1 = mild, 2 = moderate, and 3 = severe. Any scores of 2 or 3 were classified as an adverse event. Objective tolerance categories included erythema, edema, dryness, and peeling. Subjective tolerance categories included stinging, burning, itching, and tingling. There were no serious adverse events reported during the study, and only one non-serious adverse event of moderate severity reported in a vehicle-treated subject. Brightness was also measured by taking triplicate readings across each cheek using a Skin-Colorimeter CL 400 (Courage + Khazaka electronic GmbH, Köln, Germany). Colorimeter measurements utilized the L* value located on the white-black axis which is proportional to brightness. Digital images of subjects were captured using Visia
®-CW imaging booth (Canfield Scientific; Parsippany, NJ, USA). Upon completion of the eight-week study, subjects also completed a self-perception questionnaire (SPQ) in which subjects indicated their level of agreement to statements about the test article utilizing a five-point Likert scale (agree completely, agree somewhat, neither agree or disagree, disagree somewhat, and disagree completely).
2.10. Statistical Analysis
For all in vitro assays, statistical significance was determined by ANOVA followed by a Dunnett multiple comparisons test using p-values less than 0.05 as a significant difference using Microsoft Excel software. For melanin content analysis, samples were assayed in duplicate. For the clinical study, within treatment analyses for clinical and tolerance grading compared to baseline utilized Wilcoxon’s Signed Rank Test, and between-treatment analyses utilized Wilcoxon’s Rank Sum Test. Analysis of colorimeter data utilized t-tests on changes from baseline and between-treatments utilizing analysis of covariance. Analysis of SPQ responses utilized a chi-squared test and comparing ‘not top box’ responses (disagree, disagree somewhat, and disagree completely) to ‘top box’ responses (agree somewhat, and agree completely). All clinical study statistical tests used p-values less than 0.05 as a significant difference. Clinical study statistics were made using SAS analytic version 9.4 software.
4. Discussion
Traditionally, burdock fruit (
Fructus arctii) was prepared for therapeutic use by stir-frying to induce chemical changes [
18]. Scientific advancement in detection methods such as HPLC and mass spectrometry have shown that traditional preparation methods produce a therapeutic burdock extract by significantly inducing the thermal conversion of arctiin to ATG [
18]. With most burdock studies reporting ATG as the most active burdock component [
1,
2,
6], research has expanded on traditional burdock preparation and new ways to isolate and convert arctiin to ATG have been examined. For example, Cai et al. [
19] and Liu et al. [
20] both use
Fructus arctii as a source to convert arctiin to ATG via enzymatic hydrolysis using β-glucosidase with the goal to isolate and purify ATG. Additionally, Kim et al. [
21] produced a burdock fruit extract via β-glucosidase hydrolysis with peak conversion at 24 h producing an extract with 9.6 mM ATG. Burdock extracts have historical therapeutic use in many dermatological conditions such as rashes, eczema, acne, and psoriasis [
2]. Extracts of burdock fruit and leaves have also been found to possess a potent tyrosinase inhibitory effect and decrease melanin content in mouse melanoma cells [
7,
8]. While these assays show promise for burdock extracts as skin brightening actives, they also limit the full examination of its activity as there are other mechanisms that can contribute to decreasing melanin production such as antioxidant pathways, inhibition of melanosome transfer, and increasing epidermal turnover [
22]. Despite these reported activities in skin and advancements in extraction methods, burdock’s role and activity in melanin-related skin conditions has not been extensively studied. Moreover, because most reported burdock activity comes from the main lignan component ATG, it is surprising that burdock seed alone has not been further explored as a starting material for extracts given it is particularly rich in lignans [
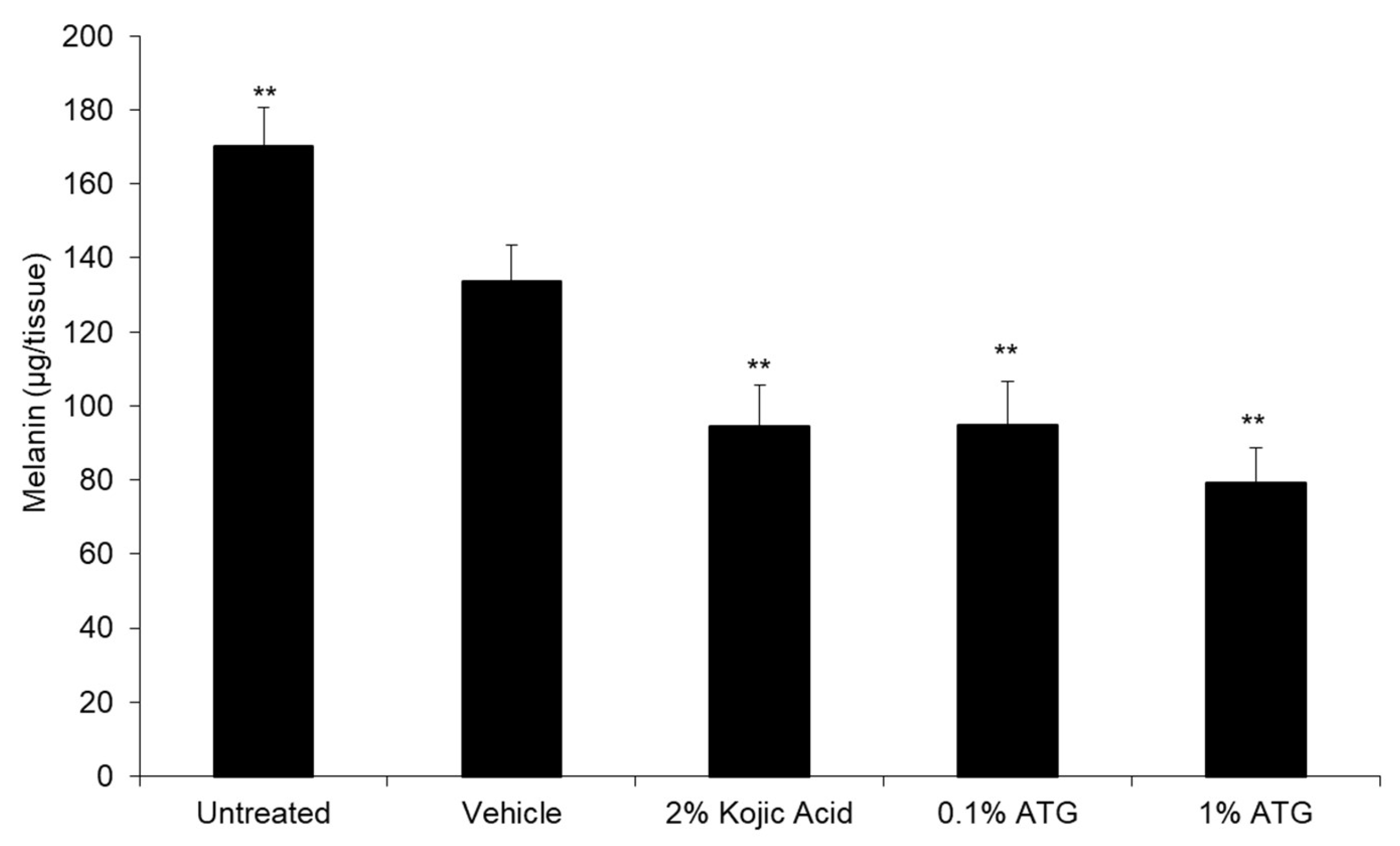
5]. With ATG being the primary component of interest in burdock, we first sought to determine its melanin reducing capacity in an in vitro 3D tissue model. Synthetic ATG tested at 0.1% and 1% produced significant melanin reduction similar to positive control kojic acid tested at 2% (
Figure 2). These results demonstrate ATG’s efficacy against an industry standard for skin brightening, as well as confirming that the lignans previously reported tyrosinase and mouse melanoma melanin reduction activities can translate to a human tissue model.
Given the robust data and extensive research on burdock fruit extracts, the further development of a burdock fruit extract for dermatological use seemed advantageous; however, burdock fruit is not listed as an approved quasi-drug ingredient in The Japanese Standards of Quasi-Drug Ingredients [
15]. Of all the components of burdock, only the root and seed oil extracts are approved for use as a quasi-drug. Of the two approved burdock extract starting components, seeds have been reported to have a higher amount of lignans present than the root and are higher in total phenolic content and antioxidant activity [
5]. Numerous polyphenols and flavonoids have been reported to have melanin inhibition activity [
12,
22] and anti-aging capacity due to their antioxidant and anti-inflammatory nature [
23,
24]. Higher amounts of these phenolic compounds could provide additional brightening efficacy and anti-aging benefits to a skincare product, thus making the development of a burdock seed extract more advantageous. Given that burdock seeds have previously been reported to contain a relatively low quantity of ATG compared to its glucoside arctiin [
6,
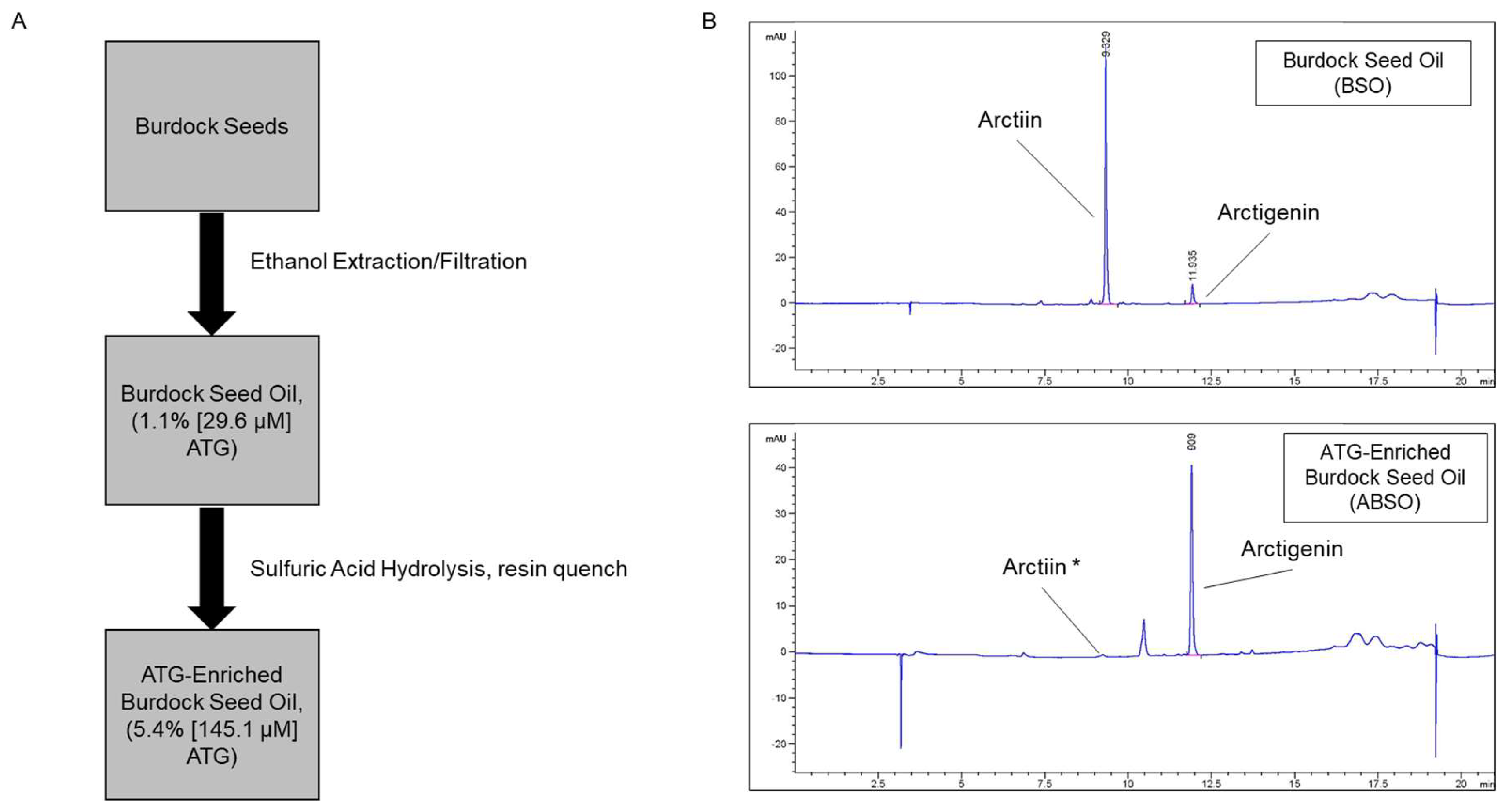
21], we developed a two-step extraction process (
Figure 3A) to extract and enrich for ATG. HPLC analysis demonstrated the successful extraction of glucoside arctiin (19.32%, 361.1 µM) and ATG (1.1%, 29.6 µM) from ethanol-extracted burdock seed oil, and subsequent acid hydrolysis for conversion of arctiin to ATG (
Figure 3B). This process produced ABSO extract with arctiin below the limit of detection and ATG at 5.4% (145.1 µM), which is ~5-times the amount of ATG than its first step product BSO. Given the extensive benefits attributed to ATG, this enrichment provides a novel, natural source of ATG with many potential uses in the cosmeceutical space.
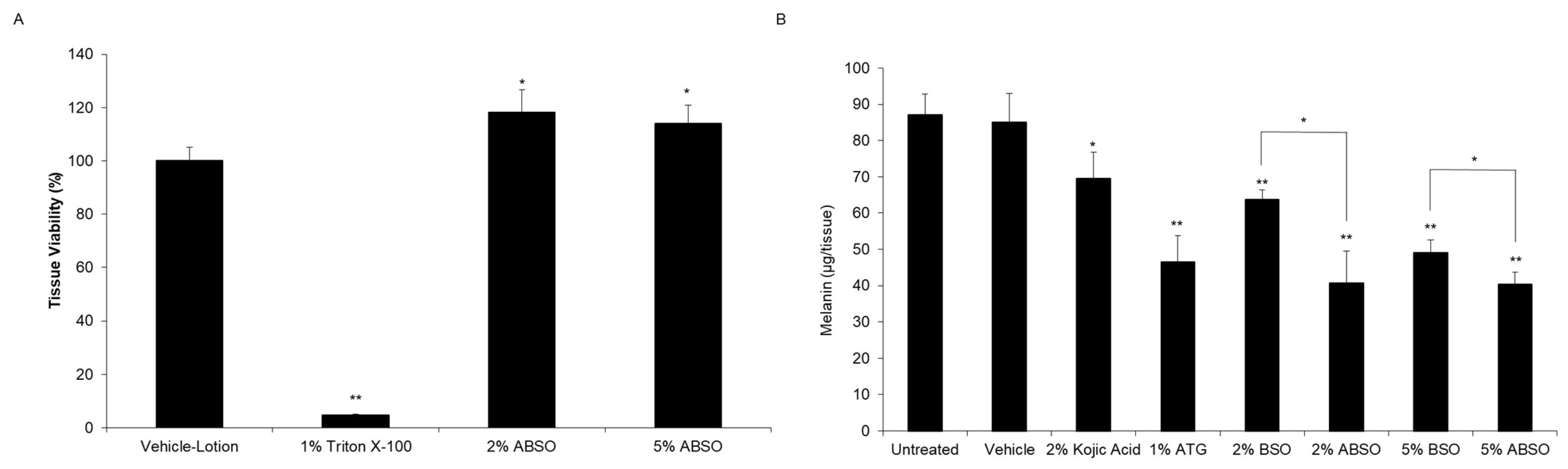
Considering that ATG possessed melanin reducing capacity, our next step was to determine if ABSO retained the same activity, and if our process produced a more efficacious extract compared to its non-enriched counterpart BSO. Interestingly, when tested in the MelanoDerm
TM tissue model, ABSO retained the activity of its enriched component, ATG, tested alone and reduced melanin content significantly better than BSO (
Figure 4). This data demonstrates that our two-step extraction process produces a burdock seed extract with superior melanin reduction activity, while offering a natural alternative to synthetic ATG without losing efficaciousness.
Given ABSO’s promising in vitro melanin reduction activity, we then tested it clinically to determine its tolerability and skin brightening potential when applied topically in human subjects. Results from the 8-week clinical study show that 1% ABSO lotion applied twice a day was well-tolerated and significantly improved skin tone evenness and brightness compared to baseline (
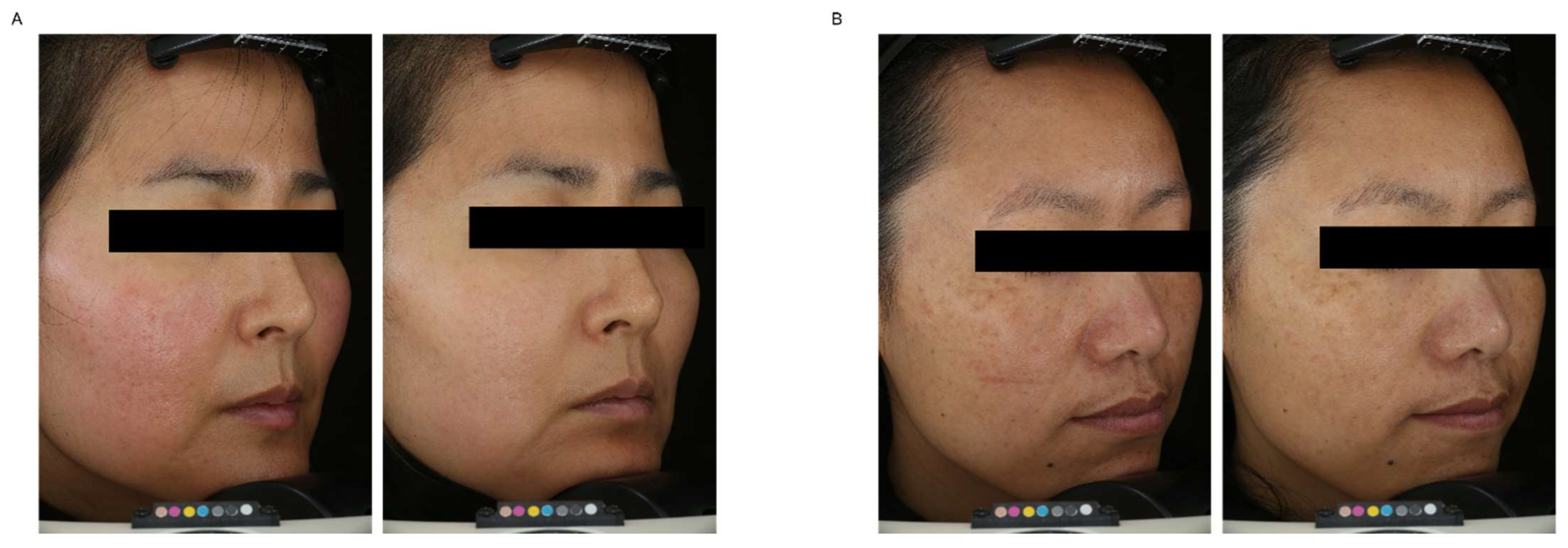
Table 3). Although the visual brightness endpoint at week 4 was the only statistically significant measurement when analyzed compared to vehicle, 1% ABSO consistently and statistically improved every endpoint at weeks 4 and 8 compared to baseline. Images captured at baseline and week 8 (
Figure 5) demonstrate a marked increase in skin tone evenness, brightness, and even a reduction in redness. While the primary goal of the study was to investigate the safety and tolerability of ABSO in a topical lotion formulation, the additional brightness and tone evenness endpoints show promising potential for ABSO as a skin brightening ingredient. Limitations of the study include the sample size and low diversity of subjects. In future studies we will look to increase the sample size and validate the formulation in other populations to confirm ABSO’s activity profile.
Altogether, this data supports ABSO as a novel, safe, and robust skin brightening ingredient. To our knowledge, if successful, this will be the first quasi-drug product developed for skin brightening utilizing burdock seed oil. Our future goals are to investigate and elucidate the mechanism(s) that ABSO modulates to decrease melanin content, further characterize ABSO’s activity profile in skin, and to develop a quasi-drug approved ABSO skin brightening product to test in a larger human clinical study with primary skin brightening and anti-aging endpoints.