Solid-State Fermentation with Rhizopus oryzae: Enhancing Antioxidant and Phenolic Content in Pigmented Corn
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Physicochemical Characterization of Blue Corn
2.3. Microorganism
2.4. Effect of Different Factors on the Release of TPC
2.5. Effect of SSF on the Physicochemical Composition of Blue Corn
2.6. Analytical Analysis
2.6.1. Determination of Phenolic Content
2.6.2. Determination of Antioxidant Capacity
ABTS Antioxidant Assay
DPPH Antioxidant Assay
Ferric Reducing Antioxidant Power (FRAP) Assay
2.6.3. Identification of Phenolic Compounds by HPLC-MS
2.7. Statistical Analysis
3. Results
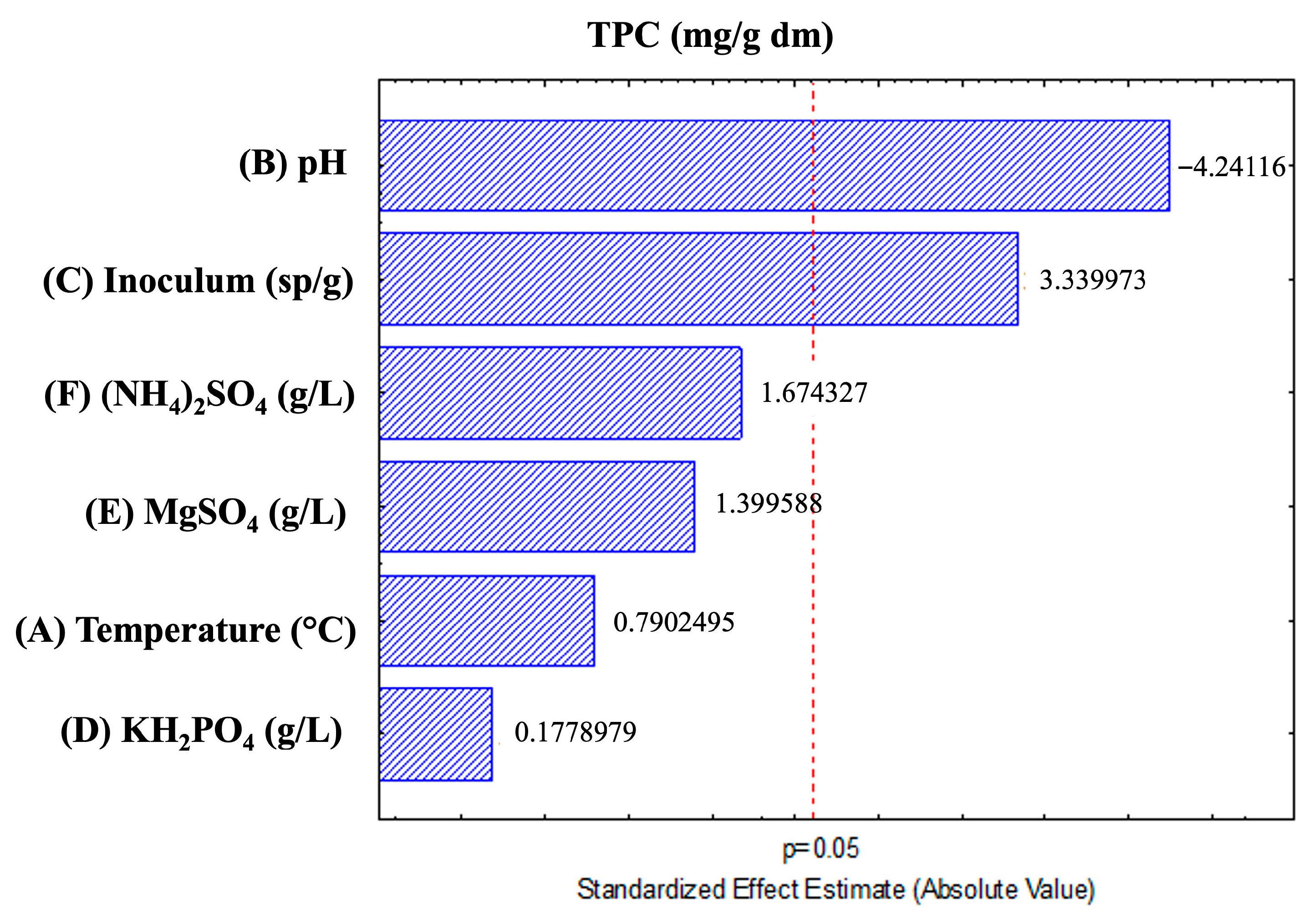
3.1. Effect of Different Factors on the Release of TPC
3.2. Effect of SSF on the Physicochemical Composition of Blue Corn
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuevas Mejía, J.d.J. Maíz: Alimento fundamental en las tradiciones y costumbres mexicanas. PASOS 2014, 12, 425–432. [Google Scholar] [CrossRef]
- Bello-Pérez, L.A.; Camelo-Mendez, G.A.; Agama-Acevedo, E.; Utrilla-Coello, R.G. Aspecto nutracéuticos de los maíces pigmentados: Digestibilidad de los carbohidratos y antocianinas. Agrociencia 2016, 50, 1041–1063. [Google Scholar]
- Rouf Shah, T.; Prasad, K.; Kumar, P. Maize—A potential source of human nutrition and health: A review. Cogent Food Agric. 2016, 2, 1166995. [Google Scholar] [CrossRef]
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and Functional Foods: The Foods for the Future World. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, B.; Song, D. Co-microbiological regulation of phenolic release through solid-state fermentation of corn kernels (Zea mays L.) to improve their antioxidant activity. LWT 2021, 142, 111003. [Google Scholar] [CrossRef]
- Streimikyte, P.; Viskelis, P.; Viskelis, J. Enzymes-Assisted Extraction of Plants for Sustainable and Functional Applications. Int. J. Mol. Sci. 2022, 23, 2359. [Google Scholar] [CrossRef] [PubMed]
- Rabanal-Atalaya, M.; Medina-Hoyos, A. Análisis de antocianinas en el maíz morado (Zea mays L.) del Perú y sus propiedades antioxidantes. Terra Latinoam. 2021, 39, 1–12. [Google Scholar] [CrossRef]
- Huynh, N.T.; Van Camp, J.; Smagghe, G.; Raes, K. Improved Release and Metabolism of Flavonoids by Steered Fermentation Processes: A Review. Int. J. Mol. Sci. 2014, 15, 19369–19388. [Google Scholar] [CrossRef]
- Alves Magro, A.E.; de Castro, R.J.S. Effects of solid-state fermentation and extraction solvents on the antioxidant properties of lentils. Biocatal. Agric. Biotechnol. 2020, 28, 101753. [Google Scholar] [CrossRef]
- Londoño-Hernández, L.; Ramírez-Toro, C.; Ruiz, H.A.; Ascacio-Valdés, J.A.; Aguilar-Gonzalez, M.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Rhizopus oryzae—Ancient microbial resource with importance in modern food industry. Int. J. Food Microbiol. 2017, 257, 110–127. [Google Scholar] [CrossRef]
- Aoki, H.; Chuma, S.; Iba, Y.; Tashiro, H.; Watanabe, N.; Oyama, H. Comparison of Bioactive Components in Tempeh Produced by Three Different Rhizopus Starters and Immunomodulatory Effect of Tempeh on Atopic Dermatitis Mice. Food Sci. Technol. Res. 2020, 26, 665–672. [Google Scholar] [CrossRef]
- Ramirez-Esparza, U.; De La Rosa-Esteban, A.K.; Baeza-Jiménez, R.; Martínez-Ávila, G.; Ascacio-Valdés, J.A.; Buenrostro Figueroa, J.J. Chapter 11—Recent advances in the extraction of phenolic compounds using biotechnological processes. In Enzymatic Processes for Food Valorization; González, M.L.C., Buenrostro Figueroa, J.J., Verma, D.K., Aguilar, C.N., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 157–172. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.-H.; Pintea, A. Phenolic compounds, flavonoids, lipids and antioxidant potential of apricot (Prunus armeniaca L.) pomace fermented by two filamentous fungal strains in solid state system. Chem. Cent. J. 2017, 11, 92. [Google Scholar] [CrossRef]
- Leite, P.; Silva, C.; Salgado, J.M.; Belo, I. Simultaneous production of lignocellulolytic enzymes and extraction of antioxidant compounds by solid-state fermentation of agro-industrial wastes. Ind. Crops Prod. 2019, 137, 315–322. [Google Scholar] [CrossRef]
- Othman, B.; Sebo, N.H. Utilization of Some Agro Wests for the Production of Acid Protease by Aspergillus niger. J. Surv. Fish. Sci. 2023, 10, 4319–4331. [Google Scholar]
- Cámpora, M.C. Alimentos funcionales: Tecnología que hace la diferencia. RIA. Rev. De Investig. Agropecu. 2016, 42, 131–137. [Google Scholar]
- Martirosyan, D.; Kanya, H.; Nadalet, C. Can functional foods reduce the risk of disease? Advancement of functional food definition and steps to create functional food products. Funct. Foods Health Dis. 2021, 11, 213–221. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC INTERNATIONAL, 19th ed.; AOAC International, Ed.; AOAC INTERNATIONAL: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Wong-Paz, J.E.; Muñiz-Márquez, D.B.; Aguilar-Zárate, P.; Rodríguez-Herrera, R.; Aguilar, C.N. Microplate quantification of total phenolic content from plant extracts obtained by conventional and ultrasound methods. Phytochem. Anal. 2014, 25, 439–444. [Google Scholar] [CrossRef]
- Hernández, C.; Ascacio-Valdés, J.; De la Garza, H.; Wong-Paz, J.; Aguilar, C.N.; Martínez-Ávila, G.C.; Castro-López, C.; Aguilera-Carbó, A. Polyphenolic content, in vitro antioxidant activity and chemical composition of extract from Nephelium lappaceum L. (Mexican rambutan) husk. Asian Pac. J. Trop. Med. 2017, 10, 1201–1205. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzmán, N.; Ascacio-Valdés, J.; Serna-Cock, L.; dos Santos Correia, M.T.; Contreras-Esquivel, J.C.; Aguilar, C.N. Solid-state fermentation with Aspergillus niger to enhance the phenolic contents and antioxidative activity of Mexican mango seed: A promising source of natural antioxidants. LWT 2019, 112, 108236. [Google Scholar] [CrossRef]
- Melendez, N.P.; Nevárez-Moorillón, V.; Rodríguez-Herrera, R.; Espinoza, J.C.; Aguilar, C.N.; Obal, N. A microassay for quantification of 2, 2-diphenyl-1-picrylhydracyl (DPPH) free radical scavenging. Afr. J. Biochem. Res. 2014, 8, 14–18. [Google Scholar] [CrossRef]
- López-Cárdenas, F.; Ochoa-Reyes, E.; Baeza-Jiménez, R.; Tafolla-Arellano, J.C.; Ascacio-Valdés, J.A.; Buenrostro-Figueroa, J.J. Solid-State Fermentation as a Sustainable Tool for Extracting Phenolic Compounds from Cascalote Pods. Fermentation 2023, 9, 823. [Google Scholar] [CrossRef]
- Cerda-Cejudo, N.D.; Buenrostro-Figueroa, J.J.; Sepúlveda, L.; Estrada-Gil, L.E.; Torres-León, C.; Chávez-González, M.L.; Aguilar, C.N.; Ascacio-Valdés, J.A. Enhancing the Release of Ellagic Acid from Mexican Rambutan Peel Using Solid-State Fermentation. Biomass 2024, 4, 1005–1016. [Google Scholar] [CrossRef]
- Krishna, C. Solid-State Fermentation Systems—An Overview. Crit. Rev. Biotechnol. 2005, 25, 1–30. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Ramteke, P.W.; Mishra, P.K. Chapter 23—Solid-State Fermentation Strategy for Microbial Metabolites Production: An Overview. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 345–354. [Google Scholar] [CrossRef]
- Das, S.; Nadar, S.S.; Rathod, V.K. Integrated strategies for enzyme assisted extraction of bioactive molecules: A review. Int. J. Biol. Macromol. 2021, 191, 899–917. [Google Scholar] [CrossRef]
- Egbune, E.O.; Orhonigbe, I.; Adheigu, R.O.; Oniyan, U.P.; Tonukari, N.J. Effect of inoculum size on solid state fermentation of pearl millet (Pennisetum glaucum) by Rhizopus oligosporus. Niger. J. Sci. Environ. 2022, 19, 1–9. [Google Scholar]
- Fan, M.; Sun, X.; Qian, Y.; Xu, Y.; Wang, D.; Cao, Y. Effects of metal ions in tea polysaccharides on their in vitro antioxidant activity and hypoglycemic activity. Int. J. Biol. Macromol. 2018, 113, 418–426. [Google Scholar] [CrossRef]
- Holzberg, M.; Artis, W.M. Hydroxamate siderophore production by opportunistic and systemic fungal pathogens. Infect. Immun. 1983, 40, 1134–1139. [Google Scholar] [CrossRef]
- Echegaray, N.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M.; Chabani, Z.; Farag, M.A.; Domínguez, R. Measurement of Antioxidant Capacity of Meat and Meat Products: Methods and Applications. Molecules 2021, 26, 3880. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Modi, H. Comparative study of DPPH, ABTS and FRAP assays for determination of antioxidant activity. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 636–641. [Google Scholar]
- Kupski, L.; de Carvalho Silvello, M.A.; Fontes, M.R.V.; Lima, T.S.; Treichel, H.; Badiale Furlong, E. Rhizopus oryzae Cellulases: A New Approach to Degrading Lignocellulosic Material. J. Food Biochem. 2015, 39, 129–138. [Google Scholar] [CrossRef]
- Denardi de Souza, T.; Leal, C.A.; Massarolo, K.C.; Badiale-Fulong, E. Profile of Phenolic Compounds Released from Rice Bran by Rhizopus oryzae and Trichoderma reesei: Their Relation with Hydrolases Activity. J. Food Sci. 2019, 84, 1382–1389. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.J.; Velázquez, M.; Flores-Ortega, O.; Ascacio-Valdés, J.A.; Huerta-Ochoa, S.; Aguilar, C.N.; Prado-Barragán, L.A. Solid state fermentation of fig (Ficus carica L.) by-products using fungi to obtain phenolic compounds with antioxidant activity and qualitative evaluation of phenolics obtained. Process Biochem. 2017, 62, 16–23. [Google Scholar] [CrossRef]
- Liao, W.; Liu, Y.; Frear, C.; Chen, S. A new approach of pellet formation of a filamentous fungus—Rhizopus oryzae. Bioresour. Technol. 2007, 98, 3415–3423. [Google Scholar] [CrossRef]
- Sukma, A.; Oktavianty, H.; Sumardiono, S. Optimization of solid-state fermentation condition for crude protein enrichment of rice bran using Rhizopus oryzae in tray bioreactor. Indones. J. Biotechnol. 2021, 26, 33–40. [Google Scholar] [CrossRef]
- Kupski, L.; Cipolatti, E.; da Rocha, M.; Oliveira, M.d.S.; Souza-Soares, L.d.A.; Badiale-Furlong, E. Solid-state fermentation for the enrichment and extraction of proteins and antioxidant compounds in rice bran by Rhizopus oryzae. Braz. Arch. Biol. Technol. 2012, 55, 937–942. [Google Scholar] [CrossRef]
- Olukomaiya, O.O.; Adiamo, O.Q.; Fernando, W.C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Effect of solid-state fermentation on proximate composition, anti-nutritional factor, microbiological and functional properties of lupin flour. Food Chem. 2020, 315, 126238. [Google Scholar] [CrossRef]
- Denardi-Souza, T.; Massarolo, K.C.; Tralamazza, S.M.; Badiale-Furlong, E. Monitoring of fungal biomass changed by Rhizopus oryzae in relation to amino acid and essential fatty acids profile in soybean meal, wheat and rice. CyTA-J. Food 2018, 16, 156–164. [Google Scholar] [CrossRef]
- López-Fernández, J.; Benaiges, M.D.; Valero, F. Rhizopus oryzae Lipase, a Promising Industrial Enzyme: Biochemical Characteristics, Production and Biocatalytic Applications. Catalysts 2020, 10, 1277. [Google Scholar] [CrossRef]
- Sukma, A.; Anwar, H.; Ikhsanudin, A. Effect of Rhizopus oryzae fermentation on proximate composition, anti-nutrient contents, and functional properties of banana peel flour. Int. Food Res. J. 2022, 29, 1205–1214. [Google Scholar] [CrossRef]
- Olugosi, O.; Agbede, J.; Adebayo, I.; Onibi, G.; Ayeni, O. Nutritional enhancement of cocoa pod husk meal through fermentation using Rhizopus stolonifer. Afr. J. Biotechnol. 2019, 18, 901–908. [Google Scholar] [CrossRef]
- Pandey, A.K.; Negi, S. Enhanced cellulase recovery in SSF from Rhizopus oryzae SN5 and immobilization for multi-batch saccharification of carboxymethylcellulose. Biocatal. Agric. Biotechnol. 2020, 26, 101656. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.J.; Nevárez-Moorillón, G.V.; Chávez-González, M.L.; Sepúlveda, L.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Pedroza-Islas, R.; Huerta-Ochoa, S.; Arely Prado-Barragán, L. Improved Extraction of High Value-Added Polyphenols from Pomegranate Peel by Solid-State Fermentation. Fermentation 2023, 9, 530. [Google Scholar] [CrossRef]
- Janarny, G.; Gunathilake, K.D.P.P. Changes in rice bran bioactives, their bioactivity, bioaccessibility and bioavailability with solid-state fermentation by Rhizopus oryzae. Biocatal. Agric. Biotechnol. 2020, 23, 101510. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Socaciu, C. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 2016, 209, 27–36. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef] [PubMed]
- Bounegru, A.V.; Apetrei, C. Chapter 78—Caffeic and chlorogenic acid in coffee and methods for their detection. In Coffee in Health and Disease Prevention, 2nd ed.; Preedy, V.R., Patel, V.B., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 893–904. [Google Scholar] [CrossRef]
- Nasimi Shad, A.; Akhlaghipour, I.; Babazadeh Baghan, A.; Askari, V.R.; Baradaran Rahimi, V. Caffeic acid and its derivative caffeic acid phenethyl ester as potential therapeutic compounds for cardiovascular diseases: A systematic review. Arch. Pharm. 2024, 357, e2400240. [Google Scholar] [CrossRef] [PubMed]
- Chanda, J.; Mukherjee, P.K.; Kar, A.; Maitra, P.K.; Singha, S.; Halder, P.K.; Gajbhiye, R.; Vishnuvardh, R. LC–QTOF–MS-based metabolite profiling and evaluation of α-glucosidase inhibitory kinetics of fruit. Biomed. Chromatogr. 2020, 34, e4950. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Song, X.; Tan, L.; Guo, C.; Wang, M.; Liu, H.; Cao, Z.; Li, Y.; Peng, C. A Review of the Pharmacological Properties of Psoralen. Front. Pharmacol. 2020, 11, 571535. [Google Scholar] [CrossRef]
- NIH. PubChem Compound Summary for CID 69501207, Feruloyltartaric Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Feruloyltartaric-acid (accessed on 2 May 2025).
- Liu, G.; Zhu, W.; Li, S.; Zhou, W.; Zhang, H.; Wang, J.; Liu, X.; Zhang, J.; Liang, L.; Xu, X. Antioxidant capacity and interaction of endogenous phenolic compounds from tea seed oil. Food Chem. 2022, 376, 131940. [Google Scholar] [CrossRef]
- Starzyńska-Janiszewska, A.; Stodolak, B.; Mickowska, B.; Socha, R. Fermentation with edible Rhizopus strains as a beneficial alternative method in wheat germ cake processing. J. Cereal Sci. 2021, 102, 103309. [Google Scholar] [CrossRef]

| Treatment | A | B | C | D | E | F | |
|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 1 | 1 | 1 | |
| 2 | 1 | −1 | −1 | −1 | −1 | 1 | |
| 3 | −1 | 1 | −1 | −1 | 1 | −1 | |
| 4 | 1 | 1 | −1 | 1 | −1 | −1 | |
| 5 | −1 | −1 | 1 | 1 | −1 | −1 | |
| 6 | 1 | −1 | 1 | −1 | 1 | −1 | |
| 7 | −1 | 1 | 1 | −1 | −1 | 1 | |
| 8 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Code | Factor | Low level (−1) | High level (1) | ||||
| A | Temperature (°C) | 30 | 40 | ||||
| B | pH | 5 | 6 | ||||
| C | Inoculum (sp/g) | 1 × 106 | 1 × 107 | ||||
| D | KH2PO4 (g/L) | 1 | 2 | ||||
| E | MgSO4·7H2O (g/L) | 1 | 2 | ||||
| F | (NH4)2SO4 (g/L) | 4 | 8 | ||||
| Factor | TPC | ABTS | DPPH | FRAP |
|---|---|---|---|---|
| Temperature (°C) | NS | NS | NS | NS |
| pH | − | − | − | − |
| Inoculum (sp/g) | + | NS | NS | NS |
| KH2PO4 (g/L) | NS | + | NS | NS |
| MgSO4·7H2O (g/L) | NS | NS | + | NS |
| (NH4)2SO4 (g/L) | NS | NS | NS | − |
| Treatment | A | B | C | D | E | F | TPC (mg/g dm) |
|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 1 | 1 | 1 | 4.99 ± 0.24 b |
| 2 | 1 | −1 | −1 | −1 | −1 | 1 | 3.89 ± 0.25 c |
| 3 | −1 | 1 | −1 | −1 | 1 | −1 | 2.58 ± 0.11 e |
| 4 | 1 | 1 | −1 | 1 | −1 | −1 | 3.37 ± 0.26 d |
| 5 | −1 | −1 | 1 | 1 | −1 | −1 | 4.15 ± 0.14 c |
| 6 | 1 | −1 | 1 | −1 | 1 | −1 | 5.55 ± 0.04 a |
| 7 | −1 | 1 | 1 | −1 | −1 | 1 | 4.37 ± 0.38 c |
| 8 | 1 | 1 | 1 | 1 | 1 | 1 | 4.06 ± 0.57 c |
| Code | Factor | (−1) | (1) | ||||
| A | Temperature (°C) | 30 | 40 | ||||
| B | pH | 5 | 6 | ||||
| C | Inoculum (spo/g) | 1 × 106 | 1 × 107 | ||||
| D | KH2PO4 (g/L) | 1 | 2 | ||||
| E | MgSO4·7H2O (g/L) | 1 | 2 | ||||
| F | (NH4)2SO4 (g/L) | 4 | 8 | ||||
| Parameter | Control | Fermented |
|---|---|---|
| Protein | 9.03 ± 0.11 b | 9.94 ± 0.24 a |
| Total fat | 2.34 ± 0.16 c | 4.35 ± 0.16 a |
| Ash | 1.32 ± 0.09 b | 1.69 ± 0.06 a |
| Crude fiber | 2.55 ± 0.28 a | 2.35 ± 0.05 b |
| Total carb. | 84.38 ± 0.16 a | 82.03 ± 0.32 b |
| Lignin | 0.15 ± 0.01 a | 0.15 ± 0.03 a |
| TPC (mg/g dm) | 2.40 ± 0.30 b | 5.55 ± 0.04 a |
| ABTS (mg TE/g dm) | 1.08 ± 0.10 b | 1.70 ± 0.04 a |
| DPPH (mg TE/g dm) | 0.61 ± 0.05 b | 0.93 ± 0.10 a |
| FRAP (mg Fe2+ /g dm) | 1.58 ± 0.13 b | 2.40 ± 0.02 a |
| RT m (Min) | [M–H]¯ | Phenolic Compounds | Family | UF | F |
|---|---|---|---|---|---|
| m/z | |||||
| 8.97 | 340.9 | Caffeic acid 4-O-glucoside | Hydroxycinnamic acids | x | x |
| 14.64 | 344.9 | Rosmanol | Phenolic terpenes | x | x |
| 40.12 | 488.9 | Quercetin 3-O-acetyl-rhamnoside | Flavonols | x | x |
| 42.75 | 472.9 | p-Coumaroyl tartaric acid glucosidic ester | Hydroxycinnamic acids | x | x |
| 44.32 | 436.1 | Phloridzin | Dihydrochalcones | x | |
| 47.78 | 185.1 | Psoralen | Furanocoumarins | x | |
| 49.87 | 713.3 | Not identified | x | x | |
| 51.77 | 617 | 2-S-Glutathionyl caftaric acid | Hydroxycinnamic acids | x | x |
| 52.39 | 633.1 | Galloyl-HHDP-hexoside | Ellagitannins | x | |
| 56.49 | 439.1 | Not identified | x | x | |
| 58.75 | 161 | Umbelliferone | Hydroxycoumarins | x | x |
| 59.11 | 377 | Oleuropein-aglycone | Tyrosols | x | |
| 59.86 | 325.1 | Feruloyl tartaric acid | Methoxycinnamic acids | x |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Esparza, U.; Ordoñez-Cano, A.J.; López-Martínez, L.X.; Espinoza-Hicks, J.C.; Alvarado-González, M.; Ascacio-Valdés, J.A.; Buenrostro-Figueroa, J.J. Solid-State Fermentation with Rhizopus oryzae: Enhancing Antioxidant and Phenolic Content in Pigmented Corn. Resources 2025, 14, 158. https://doi.org/10.3390/resources14100158
Ramírez-Esparza U, Ordoñez-Cano AJ, López-Martínez LX, Espinoza-Hicks JC, Alvarado-González M, Ascacio-Valdés JA, Buenrostro-Figueroa JJ. Solid-State Fermentation with Rhizopus oryzae: Enhancing Antioxidant and Phenolic Content in Pigmented Corn. Resources. 2025; 14(10):158. https://doi.org/10.3390/resources14100158
Chicago/Turabian StyleRamírez-Esparza, Ulises, Andrés J. Ordoñez-Cano, Leticia X. López-Martínez, José C. Espinoza-Hicks, Mónica Alvarado-González, Juan A. Ascacio-Valdés, and José Juan Buenrostro-Figueroa. 2025. "Solid-State Fermentation with Rhizopus oryzae: Enhancing Antioxidant and Phenolic Content in Pigmented Corn" Resources 14, no. 10: 158. https://doi.org/10.3390/resources14100158
APA StyleRamírez-Esparza, U., Ordoñez-Cano, A. J., López-Martínez, L. X., Espinoza-Hicks, J. C., Alvarado-González, M., Ascacio-Valdés, J. A., & Buenrostro-Figueroa, J. J. (2025). Solid-State Fermentation with Rhizopus oryzae: Enhancing Antioxidant and Phenolic Content in Pigmented Corn. Resources, 14(10), 158. https://doi.org/10.3390/resources14100158









