N-acetyl-L-cysteine Prevents Lactate-Mediated PGC1-alpha Expression in C2C12 Myotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Real-Time RT-PCR
2.3. Immunoblots
2.4. Statistics
3. Results
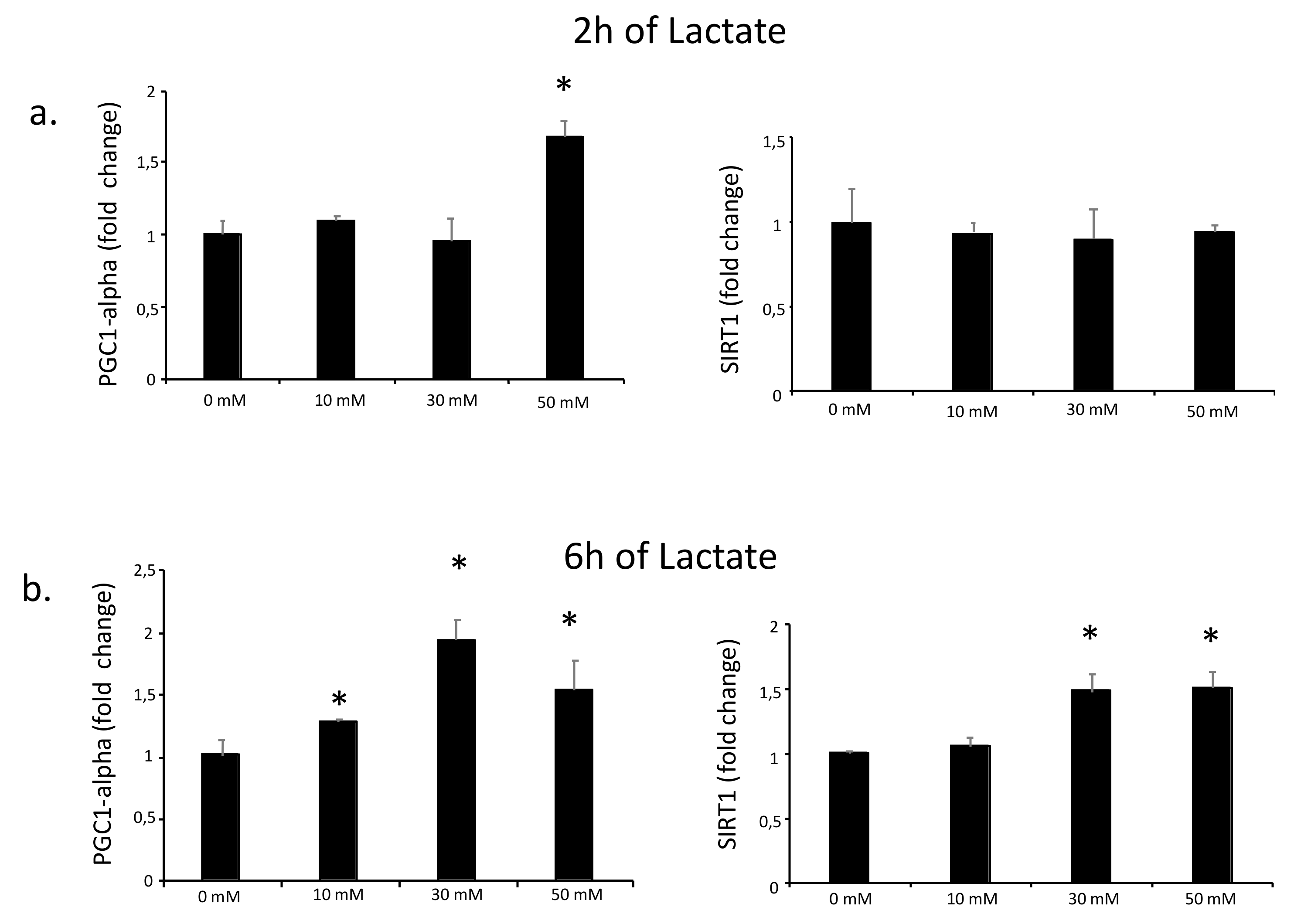
3.1. PGC1-alpha Expression Depends on Lactate Concentration and Exposure Time
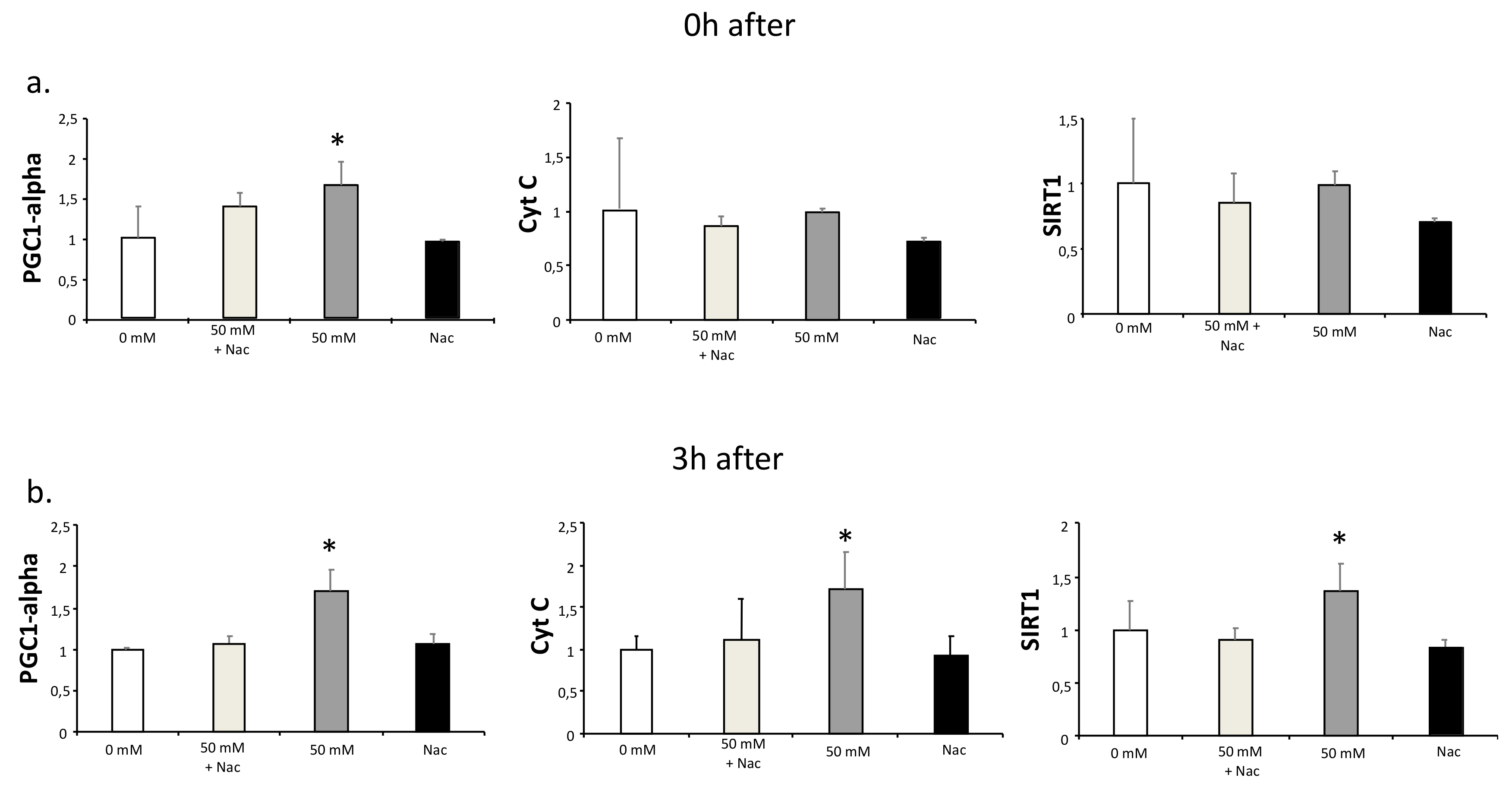
3.2. Lactate-Mediated Up-regulation of PGC1-alpha Is Blocked by N-acetyl-L-cysteine (Nac)
3.3. Lactate Intermittency Is an Efficient Way to Increase PGC1-alpha Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nalbandian, M.; Takeda, M. Lactate as a Signaling Molecule That Regulates Exercise-Induced Adaptations. Biology 2016, 5, 38. [Google Scholar] [CrossRef]
- Dankel, S.J.; Mattocks, K.T.; Jessee, M.B.; Buckner, S.L.; Mouser, J.G.; Loenneke, J.P. Do metabolites that are produced during resistance exercise enhance muscle hypertrophy? Eur. J. Appl. Physiol. 2017, 117, 2125–2135. [Google Scholar] [CrossRef]
- Mosienko, V.; Teschemacher, A.G.; Kasparov, S. Is L-lactate a novel signaling molecule in the brain? J. Cereb. Blood Flow Metab. 2015, 35, 1–7. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L.; Kane, D.A.; Rightmire, Z.; Gladden, L.B. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar] [CrossRef] [PubMed]
- Benton, C.R.; Yoshida, Y.; Lally, J.; Han, X.-X.; Hatta, H.; Bonen, A. PGC-1alpha increases skeletal muscle lactate uptake by increasing the expression of MCT1 but not MCT2 or MCT4. Physiol. Genom. 2008, 35, 45–54. [Google Scholar] [CrossRef]
- Gerhart-Hines, Z.; Rodgers, J.T.; Bare, O.; Lerin, C.; Kim, S.-H.; Mostoslavsky, R.; Alt, F.W.; Wu, Z.; Puigserver, P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J. 2007, 26, 1913–1923. [Google Scholar] [CrossRef]
- Egan, B.; Carson, B.P.; Garcia-Roves, P.M.; Chibalin, A.V.; Sarsfield, F.M.; Barron, N.; McCaffrey, N.; Moyna, N.M.; Zierath, J.R.; O’Gorman, D.J.; et al. Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor γ coactivator-1α mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J. Physiol. 2010, 588, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Hussien, R.; Oommen, S.; Gohil, K.; Brooks, G. A Lactate sensitive transcription factor network in L6 cells: Activation of MCT1 and mitochondrial biogenesis. FASEB J. 2007, 21, 2602–2612. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Takeda, K.; Tamura, Y.; Hatta, H. Lactate administration increases mRNA expression of PGC-1α and UCP3 in mouse skeletal muscle. Appl. Physiol. Nutr. Metab. 2016, 41, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Cell-cell and intracellular lactate shuttles. J. Physiol. 2009, 587, 5591–5600. [Google Scholar] [CrossRef] [PubMed]
- Talanian, J.L.; Galloway, S.D.R.; Heigenhauser, G.J.F.; Bonen, A.; Spriet, L.L. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J. Appl. Physiol. 2007, 102, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, D.; Kitaoka, Y.; Hatta, H. High-intensity interval training enhances oxidative capacity and substrate availability in skeletal muscle. J. Phys. Fit. Sport Med. 2016, 5, 13–23. [Google Scholar] [CrossRef]
- Trapp, E.G.; Chisholm, D.J.; Freund, J.; Boutcher, S.H. The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int. J. Obes. 2008, 32, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Millet, G.; Bentley, D.J.; Roels, B.; Mc Naughton, L.R.; Mercier, J.; Cameron-Smith, D. Effects of Intermittent Training on Anaerobic Performance and MCT Transporters in Athletes. PLoS ONE 2014, 9, e95092. [Google Scholar] [CrossRef] [PubMed]
- Cochran, A.J.R.; Percival, M.E.; Tricarico, S.; Little, J.P.; Cermak, N.; Gillen, J.B.; Tarnopolsky, M.A.; Gibala, M.J. Intermittent and continuous high-intensity exercise training induce similar acute but different chronic muscle adaptations. Exp. Physiol. 2014, 99, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; McGee, S.L.; Garnham, A.P.; Howlett, K.F.; Snow, R.J.; Hargreaves, M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1 in human skeletal muscle. J. Appl. Physiol. 2009, 106, 929–934. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef]
- Wright, D.C.; Han, D.-H.; Garcia-Roves, P.M.; Geiger, P.C.; Jones, T.E.; Holloszy, J.O. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J. Biol. Chem. 2007, 282, 194–199. [Google Scholar] [CrossRef]
- Taylor, C.W.; Ingham, S.A.; Hunt, J.E.A.; Martin, N.R.W.; Pringle, J.S.M.; Ferguson, R.A. Exercise duration-matched interval and continuous sprint cycling induce similar increases in AMPK phosphorylation, PGC-1α and VEGF mRNA expression in trained individuals. Eur. J. Appl. Physiol. 2016, 116, 1445–1454. [Google Scholar] [CrossRef]
- Nalbandian, H.M.; Radak, Z.; Takeda, M. Active Recovery between Interval Bouts Reduces Blood Lactate While Improving Subsequent Exercise Performance in Trained Men. Sports 2017, 5, 40. [Google Scholar] [CrossRef]
- De Filippis, E.; Alvarez, G.; Berria, R.; Cusi, K.; Everman, S.; Meyer, C.; Mandarino, L.J. Insulin-resistant muscle is exercise resistant: Evidence for reduced response of nuclear-encoded mitochondrial genes to exercise. Am. J. Physiol. Metab. 2008, 294, E607–E614. [Google Scholar] [CrossRef] [PubMed]
- Nordsborg, N.B.; Lundby, C.; Leick, L.; Pilegaard, H. Relative workload determines exercise-induced increases in PGC-1alpha mRNA. Med. Sci. Sports Exerc. 2010, 42, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Brandt, N.; Dethlefsen, M.M.; Bangsbo, J.; Pilegaard, H. PGC-1α and exercise intensity dependent adaptations in mouse skeletal muscle. PLoS ONE 2017, 12, e0185993. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl. Physiol. Nutr. Metab. 2009, 34, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 2011, 1813, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Marton, O.; Koltai, E.; Takeda, M.; Koch, L.G.; Britton, S.L.; Davies, K.J.A.; Boldogh, I.; Radak, Z. Mitochondrial biogenesis-associated factors underlie the magnitude of response to aerobic endurance training in rats. Pflügers Arch. Eur. J. Physiol. 2015, 467, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Messonnier, L.; Kristensen, M.; Juel, C.; Denis, C. Importance of pH regulation and lactate/H+ transport capacity for work production during supramaximal exercise in humans. J. Appl. Physiol. 2007, 102, 1936–1944. [Google Scholar] [CrossRef]
- Cupeiro, R.; Pérez-Prieto, R.; Amigo, T.; Gortázar, P.; Redondo, C.; González-Lamuño, D. Role of the monocarboxylate transporter MCT1 in the uptake of lactate during active recovery. Eur. J. Appl. Physiol. 2016, 116, 1005–1010. [Google Scholar] [CrossRef][Green Version]
- Kitaoka, Y.; Machida, M.; Takemasa, T. Expression of monocarboxylate transporter (MCT) 1 and MCT4 in overloaded mice plantaris muscle. J. Physiol. Sci. 2011, 61, 467–472. [Google Scholar] [CrossRef]
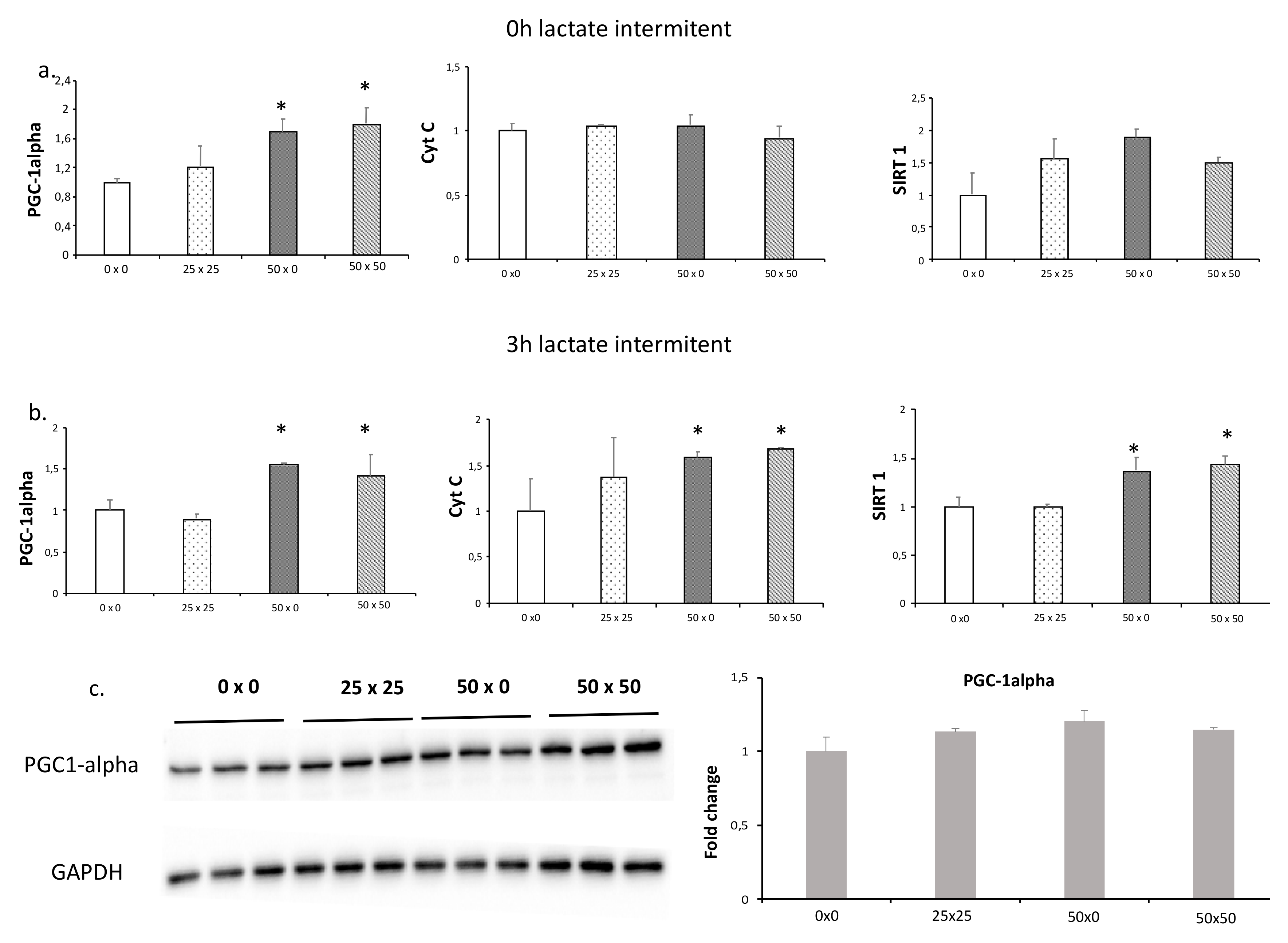
| Pattern Name | Lactate Concentration in Interval 1 (mM) | Lactate Concentration in Interval 2 (mM) |
|---|---|---|
| 0 × 0 | 0 | 0 |
| 25 × 25 | 25 | 25 |
| 50 × 0 | 50 | 0 |
| 50 × 50 | 50 | 50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nalbandian, M.; Radak, Z.; Takeda, M. N-acetyl-L-cysteine Prevents Lactate-Mediated PGC1-alpha Expression in C2C12 Myotubes. Biology 2019, 8, 44. https://doi.org/10.3390/biology8020044
Nalbandian M, Radak Z, Takeda M. N-acetyl-L-cysteine Prevents Lactate-Mediated PGC1-alpha Expression in C2C12 Myotubes. Biology. 2019; 8(2):44. https://doi.org/10.3390/biology8020044
Chicago/Turabian StyleNalbandian, Minas, Zsolt Radak, and Masaki Takeda. 2019. "N-acetyl-L-cysteine Prevents Lactate-Mediated PGC1-alpha Expression in C2C12 Myotubes" Biology 8, no. 2: 44. https://doi.org/10.3390/biology8020044
APA StyleNalbandian, M., Radak, Z., & Takeda, M. (2019). N-acetyl-L-cysteine Prevents Lactate-Mediated PGC1-alpha Expression in C2C12 Myotubes. Biology, 8(2), 44. https://doi.org/10.3390/biology8020044






