The Mammalian Circadian Timing System and the Suprachiasmatic Nucleus as Its Pacemaker
Abstract
:1. Introduction
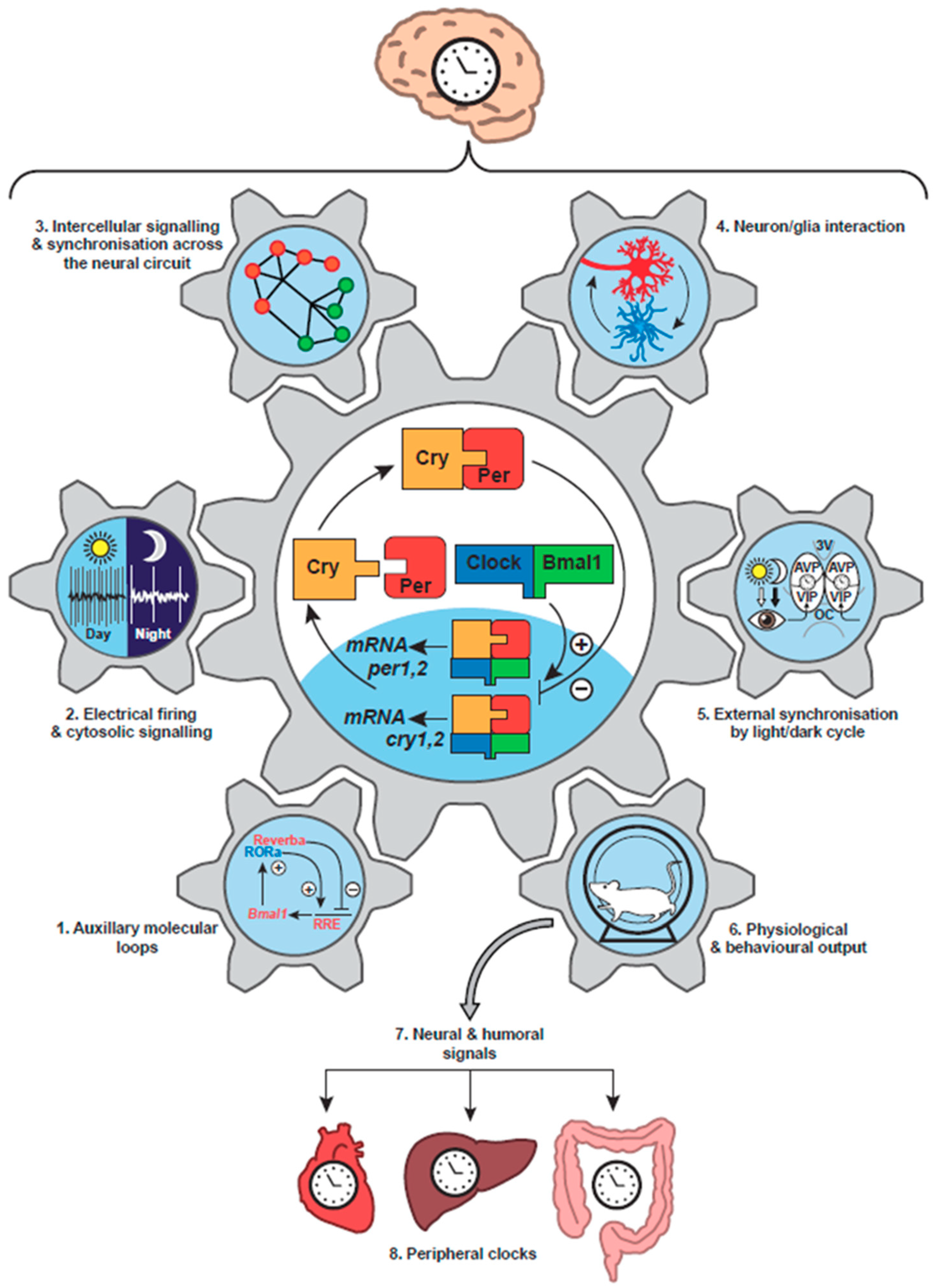
2. Circadian Feedback Loops: A Recurrent Motif
2.1. Comparative Circadian Clocks: Post-Translational Oscillators
2.2. Comparative Circadian Clocks: Transcriptional/Post-Translational Oscillators
2.3. The Intra-Cellular Circadian Clock Mechanism of Mammals
3. Circadian Clock Networks in Mammals
3.1. Tissue Localisation of Clocks
3.2. Internal Synchronisation of the Mammalian Circadian Timing System
3.3. External Synchronisation of the Mammalian Circadian Timing System
4. Neurobiology of the Suprachiasmatic Nucleus
4.1. Cellular Organisation of the SCN
4.2. Cellular Oscillations in the SCN: Electrical, Transcriptional and Metabolic
4.3. Entrainment of the SCN TTFL Cellular Clocks
5. The Suprachiasmatic Nucleus as a Neuronal Network
5.1. Synchronisation across the SCN Circuit
5.2. Are There Pacemaker Cells in the SCN?
5.3. Exploring SCN Cell-Autonomous and Network-Level Timing by Genetic Complementation
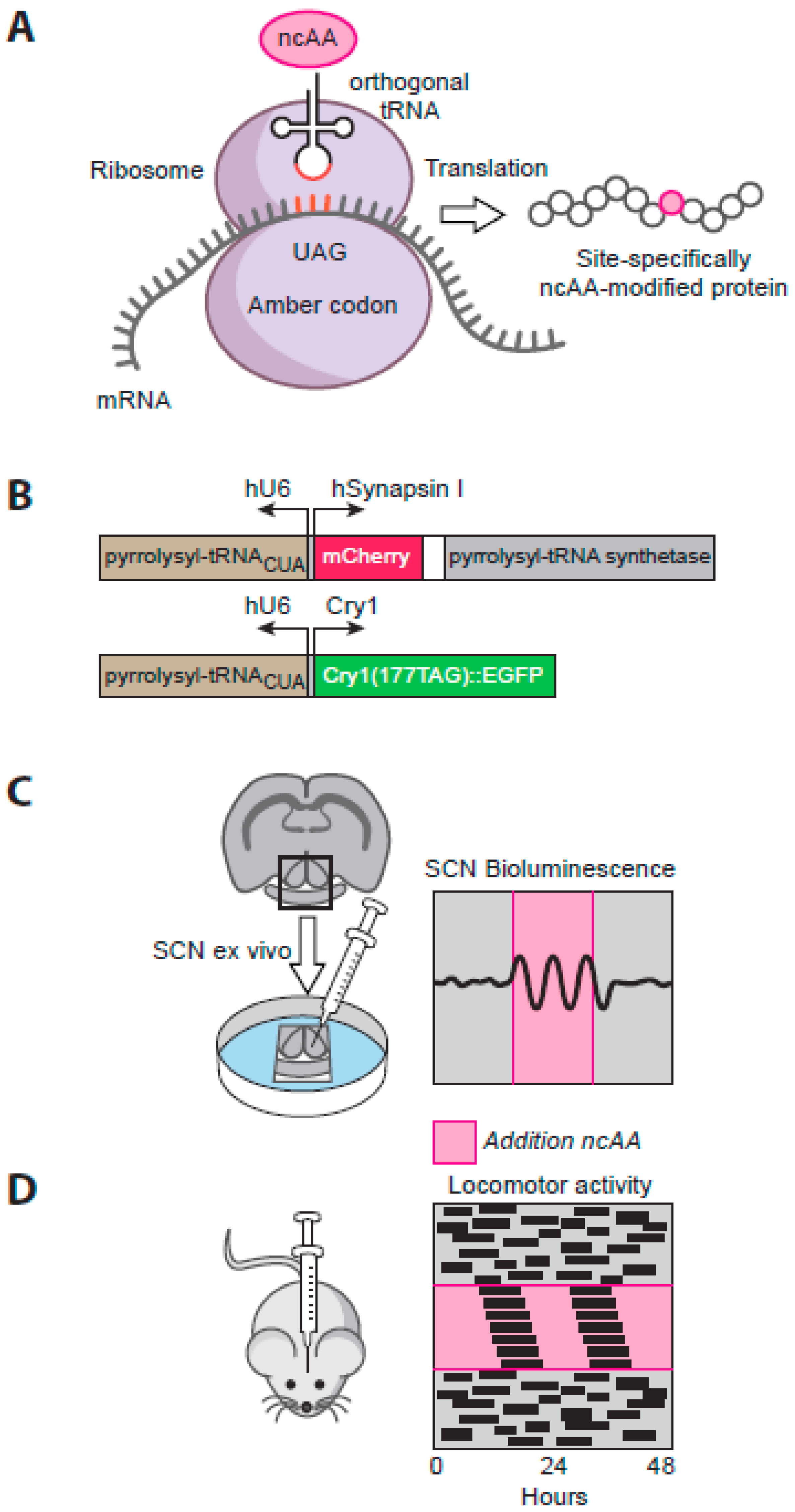
5.4. Translational Switching as a Novel Means to Explore SCN Circadian Timing
6. Astrocytes as Clock Cells: The “Dark” Side of the SCN Timing Network
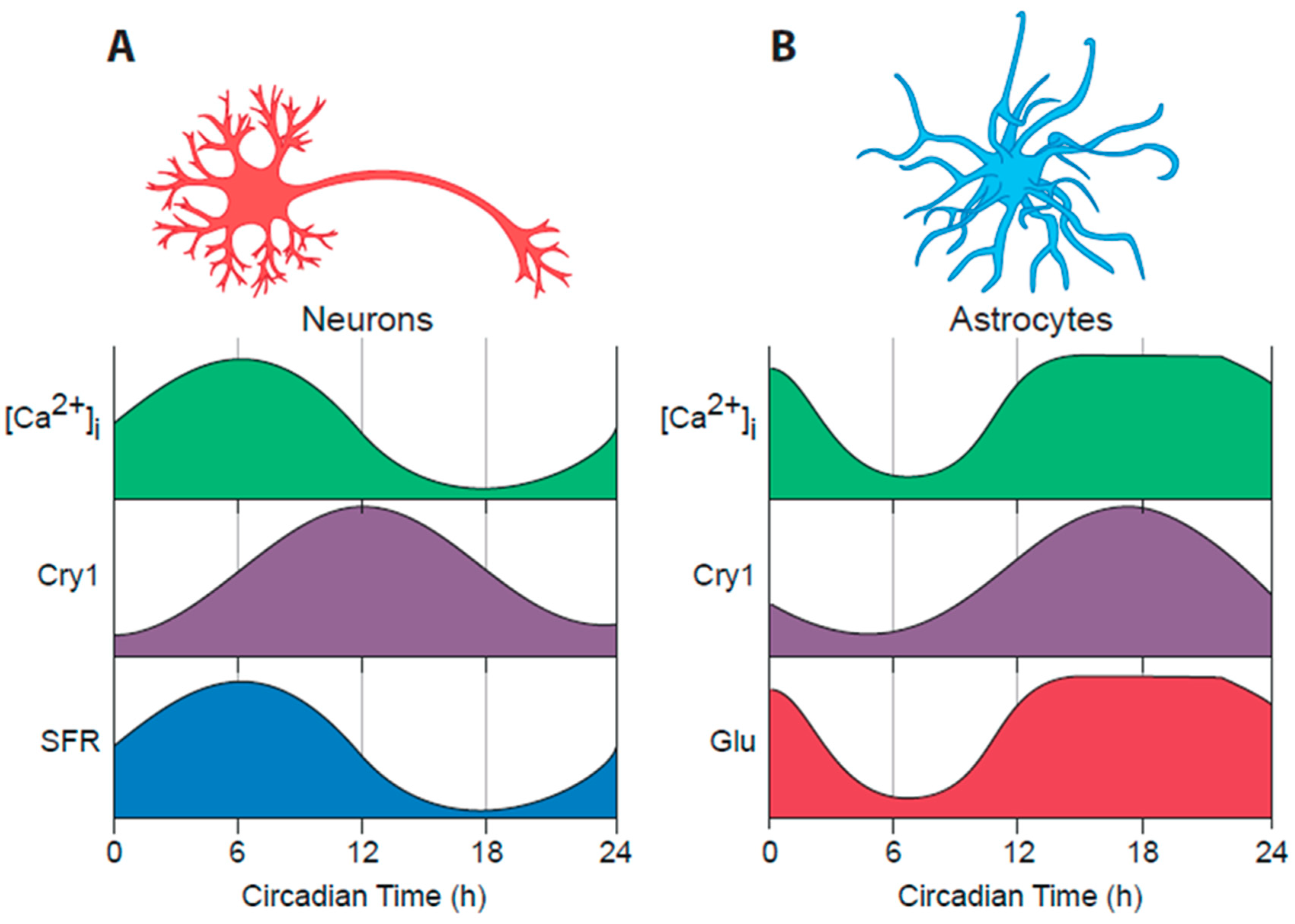
6.1. Circadian Properties of SCN Astrocytes
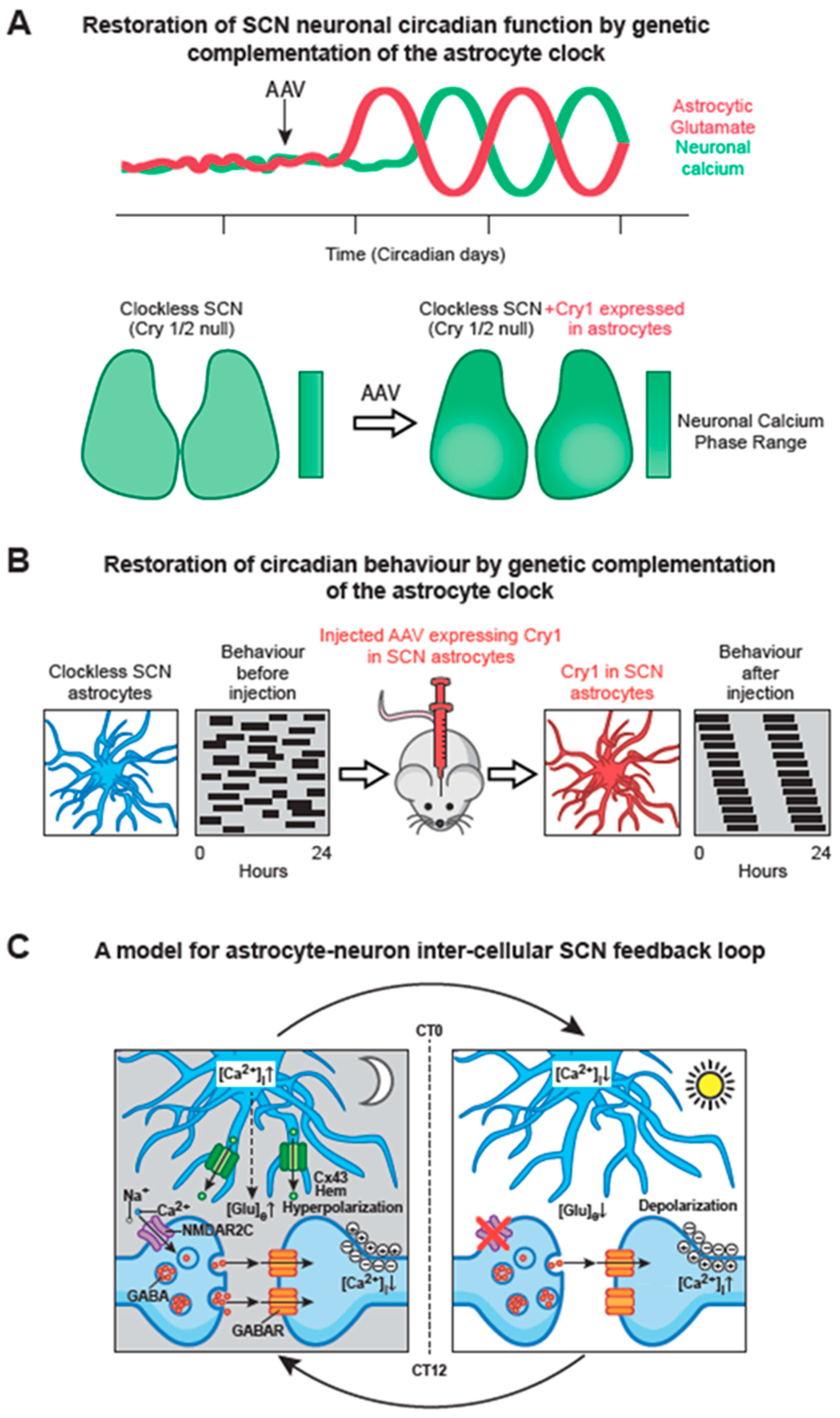
6.2. SCN Astrocytes Control SCN Molecular Rhythms and Behavioural Rhythms
6.3. Circadian Signalling from SCN Astrocytes to SCN Neurons
7. Conclusion: Future Prospects
Funding
Acknowledgments
Conflicts of Interest
References
- Woelfle, M.A.; Ouyang, Y.; Phanvijhitsiri, K.; Johnson, C.H. The adaptive value of circadian clocks; an experimental assessment in cyanobacteria. Curr. Biol. 2004, 14, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Bell-Pedersen, D.; Cassone, V.M.; Earnest, D.J.; Golden, S.S.; Hardin, P.E.; Thomas, T.L.; Zoran, M.J. Circadian rhythms from multiple oscillators: Lessons from diverse organisms. Nat. Rev. Genet. 2005, 6, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, E.R.; Dijk, D.J.; Lightman, S.; Vgontzas, A.; Van Cauter, E. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr. Rev. 2017, 38, 3–45. [Google Scholar] [PubMed]
- Duffy, J.F.; Czeisler, C.A. Effect of light on human circadian physiology. Sleep Med. Clin. 2009, 4, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Kim, J.K.; Eng, G.W.; Forger, D.B.; Virshup, D.M. A period2 phosphoswitch regulates and temperature compensates circadian period. Mol. Cell 2015, 60, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Bodenstein, C.; Heiland, I.; Schuster, S. Temperature compensation and entrainment in circadian rhythms. Phys. Biol. 2012, 9, 036011. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, Y.; Koyama, Y.M.; Ukai-Tadenuma, M.; Hirokawa, T.; Kikuchi, M.; Yamada, R.G.; Ukai, H.; Fujishima, H.; Umehara, T.; Tainaka, K.; et al. Temperature-sensitive substrate and product binding underlie temperature-compensated phosphorylation in the clock. Mol. Cell 2017, 67, 783–798 e720. [Google Scholar] [CrossRef] [PubMed]
- Bartness, T.J.; Powers, J.B.; Hastings, M.H.; Bittman, E.L.; Goldman, B.D. The timed infusion paradigm for melatonin delivery: What has it taught us about the melatonin signal, its reception and the photoperiodic control of seasonal responses. J. Pineal Res. 1993, 15, 161–190. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Inagaki, N.F.; Takagi, S.; Kuroda, S.; Yamasaki, M.; Watanabe, M.; Honma, S.; Honma, K.I. Localization of photoperiod responsive circadian oscillators in the mouse suprachiasmatic nucleus. Sci. Rep. 2017, 7, 8210. [Google Scholar] [CrossRef]
- Welkie, D.G.; Rubin, B.E.; Diamond, S.; Hood, R.D.; Savage, D.F.; Golden, S.S. A hard day’s night: Cyanobacteria in diel cycles. Trends Microbiol. 2018, 27, 231–242. [Google Scholar] [CrossRef]
- Tomita, J.; Nakajima, M.; Kondo, T.; Iwasaki, H. No transcription-translation feedback in circadian rhythm of kaic phosphorylation. Science 2005, 307, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Imai, K.; Ito, H.; Nishiwaki, T.; Murayama, Y.; Iwasaki, H.; Oyama, T.; Kondo, T. Reconstitution of circadian oscillation of cyanobacterial kaic phosphorylation in vitro. Science 2005, 308, 414–415. [Google Scholar] [CrossRef] [PubMed]
- Welkie, D.G.; Rubin, B.E.; Chang, Y.G.; Diamond, S.; Rifkin, S.A.; LiWang, A.; Golden, S.S. Genome-wide fitness assessment during diurnal growth reveals an expanded role of the cyanobacterial circadian clock protein kaia. Proc. Natl. Acad. Sci. USA 2018, 115, E7174–E7183. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.S.; Green, E.W.; Zhao, Y.; van Ooijen, G.; Olmedo, M.; Qin, X.; Xu, Y.; Pan, M.; Valekunja, U.K.; Feeney, K.A.; et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature 2012, 485, 459–464. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.S.; Reddy, A.B. Circadian clocks in human red blood cells. Nature 2011, 469, 498–503. [Google Scholar] [CrossRef]
- O’Neill, J.S.; van Ooijen, G.; Dixon, L.E.; Troein, C.; Corellou, F.; Bouget, F.Y.; Reddy, A.B.; Millar, A.J. Circadian rhythms persist without transcription in a eukaryote. Nature 2011, 469, 554–558. [Google Scholar] [CrossRef]
- Rosbash, M. A 50-year personal journey: Location, gene expression, and circadian rhythms. Cold Spring Harb. Perspect. Biol. 2017, 9, a032516. [Google Scholar] [CrossRef]
- Loros, J.J.; Dunlap, J.C.; Larrondo, L.F.; Shi, M.; Belden, W.J.; Gooch, V.D.; Chen, C.H.; Baker, C.L.; Mehra, A.; Colot, H.V.; et al. Circadian output, input, and intracellular oscillators: Insights into the circadian systems of single cells. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 201–214. [Google Scholar] [CrossRef]
- Shalit-Kaneh, A.; Kumimoto, R.W.; Filkov, V.; Harmer, S.L. Multiple feedback loops of the arabidopsis circadian clock provide rhythmic robustness across environmental conditions. Proc. Natl. Acad. Sci. USA 2018, 115, 7147–7152. [Google Scholar] [CrossRef]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef]
- Sancar, A.; Thompson, C.; Thresher, R.J.; Araujo, F.; Mo, J.; Ozgur, S.; Vagas, E.; Dawut, L.; Selby, C.P. Photolyase/cryptochrome family blue-light photoreceptors use light energy to repair DNA or set the circadian clock. Cold Spring Harb. Symp. Quant. Biol. 2000, 65, 157–171. [Google Scholar] [CrossRef]
- Preitner, N.; Damiola, F.; Lopez-Molina, L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The orphan nuclear receptor rev-erbalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef]
- Cho, H.; Zhao, X.; Hatori, M.; Yu, R.T.; Barish, G.D.; Lam, M.T.; Chong, L.W.; DiTacchio, L.; Atkins, A.R.; Glass, C.K.; et al. Regulation of circadian behaviour and metabolism by rev-erb-alpha and rev-erb-beta. Nature 2012, 485, 123–127. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef]
- Akhtar, R.A.; Reddy, A.B.; Maywood, E.S.; Clayton, J.D.; King, V.M.; Smith, A.G.; Gant, T.W.; Hastings, M.H.; Kyriacou, C.P. Circadian cycling of the mouse liver transcriptome, as revealed by cdna microarray, is driven by the suprachiasmatic nucleus. Curr. Biol. 2002, 12, 540–550. [Google Scholar] [CrossRef]
- Li, J.; Grant, G.R.; Hogenesch, J.B.; Hughes, M.E. Considerations for rna-seq analysis of circadian rhythms. Methods Enzymol. 2015, 551, 349–367. [Google Scholar]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef]
- Reddy, A.B.; Karp, N.A.; Maywood, E.S.; Sage, E.A.; Deery, M.; O’Neill, J.S.; Wong, G.K.; Chesham, J.; Odell, M.; Lilley, K.S.; et al. Circadian orchestration of the hepatic proteome. Curr. Biol. 2006, 16, 1107–1115. [Google Scholar] [CrossRef]
- Takahashi, J.S. Enriching the Circadian Proteome. Cell Metab. 2017, 25, 1–2. [Google Scholar] [CrossRef]
- Mauvoisin, D.; Wang, J.; Jouffe, C.; Martin, E.; Atger, F.; Waridel, P.; Quadroni, M.; Gachon, F.; Naef, F. Circadian clock-dependent and -independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proc. Natl. Acad. Sci. USA 2014, 111, 167–172. [Google Scholar] [CrossRef]
- Green, C.B.; Takahashi, J.S.; Bass, J. The meter of metabolism. Cell 2008, 134, 728–742. [Google Scholar] [CrossRef]
- Young, M.E.; Reddy, A.B.; Pollock, D.M. Introduction to special issue: Circadian regulation of metabolism, redox signaling and function in health and disease. Free Radic. Biol. Med. 2018, 119, 1–2. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef]
- Bloch, K.E.; Brack, T.; Wirz-Justice, A. Transient short free running circadian rhythm in a case of aneurysm near the suprachiasmatic nuclei. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1178–1180. [Google Scholar] [CrossRef]
- Borgers, A.J.; Romeijn, N.; van Someren, E.; Fliers, E.; Alkemade, A.; Bisschop, P.H. Compression of the optic chiasm is associated with permanent shorter sleep duration in patients with pituitary insufficiency. Clin. Endocrinol. (Oxf.) 2011, 75, 347–353. [Google Scholar] [CrossRef]
- Ralph, M.R.; Foster, R.G.; Davis, F.C.; Menaker, M. Transplanted suprachiasmatic nucleus determines circadian period. Science 1990, 247, 975–978. [Google Scholar] [CrossRef]
- King, V.M.; Chahad-Ehlers, S.; Shen, S.; Harmar, A.J.; Maywood, E.S.; Hastings, M.H. A hvipr transgene as a novel tool for the analysis of circadian function in the mouse suprachiasmatic nucleus. Eur. J. Neurosci. 2003, 17, 822–832. [Google Scholar] [CrossRef]
- Patton, A.P.; Hastings, M.H. The suprachiasmatic nucleus. Curr. Biol. 2018, 28, R816–R822. [Google Scholar] [CrossRef]
- Balsalobre, A.; Damiola, F.; Schibler, U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 1998, 93, 929–937. [Google Scholar] [CrossRef]
- Nagoshi, E.; Saini, C.; Bauer, C.; Laroche, T.; Naef, F.; Schibler, U. Circadian gene expression in individual fibroblasts: Cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell 2004, 119, 693–705. [Google Scholar] [CrossRef]
- Welsh, D.K.; Yoo, S.H.; Liu, A.C.; Takahashi, J.S.; Kay, S.A. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr. Biol. 2004, 14, 2289–2295. [Google Scholar] [CrossRef]
- Yoo, S.H.; Yamazaki, S.; Lowrey, P.L.; Shimomura, K.; Ko, C.H.; Buhr, E.D.; Siepka, S.M.; Hong, H.K.; Oh, W.J.; Yoo, O.J.; et al. Period2: Luciferase real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. USA 2004, 101, 5339–5346. [Google Scholar] [CrossRef]
- Yamazaki, S.; Numano, R.; Abe, M.; Hida, A.; Takahashi, R.; Ueda, M.; Block, G.D.; Sakaki, Y.; Menaker, M.; Tei, H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 2000, 288, 682–685. [Google Scholar] [CrossRef]
- Brown, S.A.; Fleury-Olela, F.; Nagoshi, E.; Hauser, C.; Juge, C.; Meier, C.A.; Chicheportiche, R.; Dayer, J.M.; Albrecht, U.; Schibler, U. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biol. 2005, 3, e338. [Google Scholar] [CrossRef]
- Brandstaetter, R. Circadian lessons from peripheral clocks: Is the time of the mammalian pacemaker up? Proc. Natl. Acad. Sci. USA 2004, 101, 5699–5700. [Google Scholar] [CrossRef]
- Schibler, U. The 2008 pittendrigh/aschoff lecture: Peripheral phase coordination in the mammalian circadian timing system. J. Biol. Rhythms 2009, 24, 3–15. [Google Scholar] [CrossRef]
- Stokkan, K.A.; Yamazaki, S.; Tei, H.; Sakaki, Y.; Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science 2001, 291, 490–493. [Google Scholar] [CrossRef]
- Vollmers, C.; Gill, S.; DiTacchio, L.; Pulivarthy, S.R.; Le, H.D.; Panda, S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc. Natl. Acad. Sci. USA 2009, 106, 21453–21458. [Google Scholar] [CrossRef]
- Strohmaier, S.; Devore, E.E.; Zhang, Y.; Schernhammer, E.S. A review of data of findings on night shift work and the development of dm and cvd events: A synthesis of the proposed molecular mechanisms. Curr. Diab Rep. 2018, 18, 132. [Google Scholar] [CrossRef]
- Shan, Z.; Li, Y.; Zong, G.; Guo, Y.; Li, J.; Manson, J.E.; Hu, F.B.; Willett, W.C.; Schernhammer, E.S.; Bhupathiraju, S.N. Rotating night shift work and adherence to unhealthy lifestyle in predicting risk of type 2 diabetes: results from two large US cohorts of female nurses. BMJ 2018, 363, k4641. [Google Scholar] [CrossRef]
- Lieu, S.J.; Curhan, G.C.; Schernhammer, E.S.; Forman, J.P. Rotating night shift work and disparate hypertension risk in African-Americans. J. Hypertens. 2012, 30, 61–66. [Google Scholar] [CrossRef]
- Wegrzyn, L.R.; Tamimi, R.M.; Rosner, B.A.; Brown, S.B.; Stevens, R.G.; Eliassen, A.H.; Laden, F.; Willett, W.C.; Hankinson, S.E.; Schernhammer, E.S. Rotating night-shift work and the risk of breast cancer in the nurses’ health studies. Am. J. Epidemiol. 2017, 186, 532–540. [Google Scholar] [CrossRef]
- Abrahamson, E.E.; Moore, R.Y. Suprachiasmatic nucleus in the mouse: Retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001, 916, 172–191. [Google Scholar] [CrossRef]
- LeGates, T.A.; Fernandez, D.C.; Hattar, S. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci. 2014, 15, 443–454. [Google Scholar] [CrossRef]
- Schmidt, T.M.; Do, M.T.; Dacey, D.; Lucas, R.; Hattar, S.; Matynia, A. Melanopsin-positive intrinsically photosensitive retinal ganglion cells: From form to function. J. Neurosci. 2011, 31, 16094–16101. [Google Scholar] [CrossRef]
- Freedman, M.S.; Lucas, R.J.; Soni, B.; von Schantz, M.; Munoz, M.; David-Gray, Z.; Foster, R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science 1999, 284, 502–504. [Google Scholar] [CrossRef]
- Sekaran, S.; Foster, R.G.; Lucas, R.J.; Hankins, M.W. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr. Biol. 2003, 13, 1290–1298. [Google Scholar] [CrossRef]
- Provencio, I.; Rodriguez, I.R.; Jiang, G.; Hayes, W.P.; Moreira, E.F.; Rollag, M.D. A novel human opsin in the inner retina. J. Neurosci. 2000, 20, 600–605. [Google Scholar] [CrossRef]
- Rollag, M.D.; Berson, D.M.; Provencio, I. Melanopsin, ganglion-cell photoreceptors, and mammalian photoentrainment. J. Biol. Rhythms 2003, 18, 227–234. [Google Scholar] [CrossRef]
- Qiu, X.; Kumbalasiri, T.; Carlson, S.M.; Wong, K.Y.; Krishna, V.; Provencio, I.; Berson, D.M. Induction of photosensitivity by heterologous expression of melanopsin. Nature 2005, 433, 745–749. [Google Scholar] [CrossRef]
- Chen, S.K.; Badea, T.C.; Hattar, S. Photoentrainment and pupillary light reflex are mediated by distinct populations of iprgcs. Nature 2011, 476, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Leise, T.L.; Kingsbury, N.J.; Diemer, T.; Wang, L.L.; Henson, M.A.; Welsh, D.K. Calcium circadian rhythmicity in the suprachiasmatic nucleus: Cell autonomy and network modulation. eNeuro 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Zhu, H.; O’Sullivan, S.; Ogunnaike, B.A.; Weaver, D.R.; Schwaber, J.S.; Vadigepalli, R. Single-cell transcriptional analysis reveals novel neuronal phenotypes and interaction networks involved in the central circadian clock. Front. Neurosci. 2016, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, A.; Butler, R.; Godinho, S.I.; Couch, Y.; Brown, L.A.; Vasudevan, S.R.; Flanagan, K.C.; Anthony, D.; Churchill, G.C.; Wood, M.J.; et al. The crtc1-sik1 pathway regulates entrainment of the circadian clock. Cell 2013, 154, 1100–1111. [Google Scholar] [CrossRef]
- Meijer, J.H.; Michel, S. Neurophysiological analysis of the suprachiasmatic nucleus: A challenge at multiple levels. Methods Enzymol. 2015, 552, 75–102. [Google Scholar] [PubMed]
- Yamaguchi, Y.; Suzuki, T.; Mizoro, Y.; Kori, H.; Okada, K.; Chen, Y.; Fustin, J.M.; Yamazaki, F.; Mizuguchi, N.; Zhang, J.; et al. Mice genetically deficient in vasopressin v1a and v1b receptors are resistant to jet lag. Science 2013, 342, 85–90. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Kreier, F.; Fliers, E.; Sauerwein, H.P.; Romijn, J.A.; Buijs, R.M. Minireview: Circadian control of metabolism by the suprachiasmatic nuclei. Endocrinology 2007, 148, 5635–5639. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Cheng, M.Y. Prokineticin 2 and circadian clock output. FEBS J. 2005, 272, 5703–5709. [Google Scholar] [CrossRef]
- Prosser, H.M.; Bradley, A.; Chesham, J.E.; Ebling, F.J.; Hastings, M.H.; Maywood, E.S. Prokineticin receptor 2 (prokr2) is essential for the regulation of circadian behavior by the suprachiasmatic nuclei. Proc. Natl. Acad. Sci. USA 2007, 104, 648–653. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Isejima, H.; Matsuo, T.; Okura, R.; Yagita, K.; Kobayashi, M.; Okamura, H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science 2003, 302, 1408–1412. [Google Scholar] [CrossRef]
- Mei, L.; Fan, Y.; Lv, X.; Welsh, D.K.; Zhan, C.; Zhang, E.E. Long-term in vivo recording of circadian rhythms in brains of freely moving mice. Proc. Natl. Acad. Sci. USA 2018, 115, 4276–4281. [Google Scholar] [CrossRef]
- Brancaccio, M.; Maywood, E.S.; Chesham, J.E.; Loudon, A.S.; Hastings, M.H. A gq-ca(2+) axis controls circuit-level encoding of circadian time in the suprachiasmatic nucleus. Neuron 2013, 78, 714–728. [Google Scholar] [CrossRef]
- Brancaccio, M.; Patton, A.P.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. Astrocytes control circadian timekeeping in the suprachiasmatic nucleus via glutamatergic signaling. Neuron 2017, 93, 1420–1435. [Google Scholar] [CrossRef]
- O’Neill, J.S.; Maywood, E.S.; Chesham, J.E.; Takahashi, J.S.; Hastings, M.H. Camp-dependent signaling as a core component of the mammalian circadian pacemaker. Science 2008, 320, 949–953. [Google Scholar] [CrossRef]
- Colwell, C.S. Linking neural activity and molecular oscillations in the scn. Nat. Rev. Neurosci. 2011, 12, 553–569. [Google Scholar] [CrossRef]
- Deery, M.J.; Maywood, E.S.; Chesham, J.E.; Sládek, M.; Karp, N.A.; Green, E.W.; Charles, P.D.; Reddy, A.B.; Kyriacou, C.P.; Lilley, K.S. Proteomic analysis reveals the role of synaptic vesicle cycling in sustaining the suprachiasmatic circadian clock. Curr. Biol. 2009, 19, 2031–2036. [Google Scholar] [CrossRef]
- Chiang, C.K. The proteomic landscape of the suprachiasmatic nucleus clock reveals large-scale coordination of key biological processes. PLoS Genet. 2014, 10, e1004695. [Google Scholar] [CrossRef]
- Flourakis, M.; Kula-Eversole, E.; Hutchison, A.L.; Han, T.H.; Aranda, K.; Moose, D.L.; White, K.P.; Dinner, A.R.; Lear, B.C.; Ren, D.; et al. A conserved bicycle model for circadian clock control of membrane excitability. Cell 2015, 162, 836–848. [Google Scholar] [CrossRef]
- Wang, T.A.; Yu, Y.V.; Govindaiah, G.; Ye, X.; Artinian, L.; Coleman, T.P.; Sweedler, J.V.; Cox, C.L.; Gillette, M.U. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science 2012, 337, 839–842. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; O’Neill, J.S. Cellular circadian pacemaking and the role of cytosolic rhythms. Curr. Biol. 2008, 18, R805–R815. [Google Scholar] [CrossRef]
- Shigeyoshi, Y.; Taguchi, K.; Yamamoto, S.; Takekida, S.; Yan, L.; Tei, H.; Moriya, T.; Shibata, S.; Loros, J.J.; Dunlap, J.; et al. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mper1 transcript. Cell 1997, 91, 1043–1053. [Google Scholar] [CrossRef]
- Ginty, D.D.; Kornhauser, J.M.; Thompson, M.A.; Bading, H.; Mayo, K.E.; Takahashi, J.S.; Greenberg, M.E. Regulation of creb phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science 1993, 260, 238–241. [Google Scholar] [CrossRef]
- Ebling, F.J.P.; Staley, K.; Maywood, E.S.; Humby, T.; Hancock, D.C.; Waters, C.M.; Evan, G.I.; Hastings, M.H. The role of nmda-type glutamatergic neurotransmission in the photic induction of immediate-early gene expression in the suprachiasmatic nuclei of the syrian hamster. J. Neuroendocrinol. 1991, 3, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Hamnett, R.; Crosby, P.; Chesham, J.E.; Hastings, M.H. Vasoactive intestinal peptide controls the suprachiasmatic circadian clock network via erk1/2 and dusp4 signalling. Nat. Commun. 2019, 10, 542. [Google Scholar] [CrossRef] [PubMed]
- Maywood, E.S.; O’Neill, J.S.; Chesham, J.E.; Hastings, M.H. Minireview: The circadian clockwork of the suprachiasmatic nuclei--analysis of a cellular oscillator that drives endocrine rhythms. Endocrinology 2007, 148, 5624–5634. [Google Scholar] [CrossRef] [PubMed]
- Maywood, E.S.; Chesham, J.E.; O’Brien, J.A.; Hastings, M.H. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc. Natl. Acad. Sci. USA 2011, 108, 14306–14311. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.A.; Leise, T.L.; Castanon-Cervantes, O.; Davidson, A.J. Dynamic interactions mediated by nonredundant signaling mechanisms couple circadian clock neurons. Neuron 2013, 80, 973–983. [Google Scholar] [CrossRef]
- Sheward, W.J.; Maywood, E.S.; French, K.L.; Horn, J.M.; Hastings, M.H.; Seckl, J.R.; Holmes, M.C.; Harmar, A.J. Entrainment to feeding but not to light: Circadian phenotype of vpac2 receptor-null mice. J. Neurosci 2007, 27, 4351–4358. [Google Scholar] [CrossRef]
- Mazuski, C.; Abel, J.H.; Chen, S.P.; Hermanstyne, T.O.; Jones, J.R.; Simon, T.; Doyle, F.J., 3rd; Herzog, E.D. Entrainment of circadian rhythms depends on firing rates and neuropeptide release of vip scn neurons. Neuron 2018, 99, 555–563. [Google Scholar] [CrossRef]
- Patton, A.P.; Chesham, J.E.; Hastings, M.H. Combined pharmacological and genetic manipulations unlock unprecedented temporal elasticity and reveal phase-specific modulation of the molecular circadian clock of the mouse suprachiasmatic nucleus. J. Neurosci. 2016, 36, 9326–9341. [Google Scholar] [CrossRef]
- Mieda, M.; Ono, D.; Hasegawa, E.; Okamoto, H.; Honma, K.; Honma, S.; Sakurai, T. Cellular clocks in avp neurons of the scn are critical for interneuronal coupling regulating circadian behavior rhythm. Neuron 2015, 85, 1103–1116. [Google Scholar] [CrossRef]
- Mieda, M.; Okamoto, H.; Sakurai, T. Manipulating the cellular circadian period of arginine vasopressin neurons alters the behavioral circadian period. Curr. Biol. 2016, 26, 2535–2542. [Google Scholar] [CrossRef]
- Lee, I.T.; Chang, A.S.; Manandhar, M.; Shan, Y.; Fan, J.; Izumo, M.; Ikeda, Y.; Motoike, T.; Dixon, S.; Seinfeld, J.E.; et al. Neuromedin s-producing neurons act as essential pacemakers in the suprachiasmatic nucleus to couple clock neurons and dictate circadian rhythms. Neuron 2015, 85, 1086–1102. [Google Scholar] [CrossRef]
- Smyllie, N.J.; Chesham, J.E.; Hamnett, R.; Maywood, E.S.; Hastings, M.H. Temporally chimeric mice reveal flexibility of circadian period-setting in the suprachiasmatic nucleus. Proc. Natl. Acad. Sci. USA 2016, 113, 3657–3662. [Google Scholar] [CrossRef]
- Jones, J.R.; Tackenberg, M.C.; McMahon, D.G. Manipulating circadian clock neuron firing rate resets molecular circadian rhythms and behavior. Nat. Neurosci. 2015, 18, 373–375. [Google Scholar] [CrossRef]
- Rosensweig, C.; Reynolds, K.A.; Gao, P.; Laothamatas, I.; Shan, Y.; Ranganathan, R.; Takahashi, J.S.; Green, C.B. An evolutionary hotspot defines functional differences between cryptochromes. Nat. Commun. 2018, 9, 1138. [Google Scholar] [CrossRef]
- Ono, D.; Honma, S.; Honma, K. Cryptochromes are critical for the development of coherent circadian rhythms in the mouse suprachiasmatic nucleus. Nat. Commun. 2013, 4, 1666. [Google Scholar] [CrossRef]
- Putker, M.; O’Neill, J.S. Reciprocal control of the circadian clock and cellular redox state—A critical appraisal. Mol. Cells 2016, 39, 6–19. [Google Scholar]
- Henslee, E.A.; Crosby, P.; Kitcatt, S.J.; Parry, J.S.W.; Bernardini, A.; Abdallat, R.G.; Braun, G.; Fatoyinbo, H.O.; Harrison, E.J.; Edgar, R.S.; et al. Rhythmic potassium transport regulates the circadian clock in human red blood cells. Nat. Commun. 2017, 8, 1978. [Google Scholar] [CrossRef]
- Edwards, M.D.; Brancaccio, M.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. Rhythmic expression of cryptochrome induces the circadian clock of arrhythmic suprachiasmatic nuclei through arginine vasopressin signaling. Proc. Natl. Acad. Sci. USA 2016, 113, 2732–2737. [Google Scholar] [CrossRef]
- Koike, N.; Yoo, S.H.; Huang, H.C.; Kumar, V.; Lee, C.; Kim, T.K.; Takahashi, J.S. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 2012, 338, 349–354. [Google Scholar] [CrossRef]
- Maywood, E.S.; Elliott, T.S.; Patton, A.P.; Krogager, T.P.; Chesham, J.E.; Ernst, R.J.; Beranek, V.; Brancaccio, M.; Chin, J.W.; Hastings, M.H. Translational switching of cry1 protein expression confers reversible control of circadian behavior in arrhythmic cry-deficient mice. Proc. Natl. Acad. Sci. USA 2018, 115, E12388–E12397. [Google Scholar] [CrossRef]
- Chin, J.W. Expanding and reprogramming the genetic code. Nature 2017, 550, 53–60. [Google Scholar] [CrossRef]
- Chai, H.; Diaz-Castro, B.; Shigetomi, E.; Monte, E.; Octeau, J.C.; Yu, X.; Cohn, W.; Rajendran, P.S.; Vondriska, T.M.; Whitelegge, J.P.; et al. Neural circuit-specialized astrocytes: Transcriptomic, proteomic, morphological, and functional evidence. Neuron 2017, 95, 531–549. [Google Scholar] [CrossRef]
- Santos, J.W.Q.; Araujo, J.F.; Cunha, M.J.B.; Costa, S.O.; Barbosa, A.L.C.; Mesquita, J.B.; Costa, M.S.M.O. Circadian variation in gfap immunoreactivity in the mouse suprachiasmatic nucleus. Biol. Rhythm Res. 2005, 36, 141–150. [Google Scholar] [CrossRef]
- Prolo, L.M.; Takahashi, J.S.; Herzog, E.D. Circadian rhythm generation and entrainment in astrocytes. J. Neurosci. 2005, 25, 404–408. [Google Scholar] [CrossRef]
- Tso, C.F.; Simon, T.; Greenlaw, A.C.; Puri, T.; Mieda, M.; Herzog, E.D. Astrocytes regulate daily rhythms in the suprachiasmatic nucleus and behavior. Curr. Biol. 2017, 27, 1055–1061. [Google Scholar] [CrossRef]
- Brancaccio, M.; Edwards, M.D.; Patton, A.P.; Smyllie, N.J.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science 2019, 363, 187–192. [Google Scholar] [CrossRef]
- Meng, Q.J.; Logunova, L.; Maywood, E.S.; Gallego, M.; Lebiecki, J.; Brown, T.M.; Sladek, M.; Semikhodskii, A.S.; Glossop, N.R.; Piggins, H.D.; et al. Setting clock speed in mammals: The ck1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing period proteins. Neuron 2008, 58, 78–88. [Google Scholar] [CrossRef]
- Callaway, E.; Ledford, H. Medicine nobel awarded for work on circadian clocks. Nature 2017, 550, 18. [Google Scholar] [CrossRef]
- Chinoy, E.D.; Duffy, J.F.; Czeisler, C.A. Unrestricted evening use of light-emitting tablet computers delays self-selected bedtime and disrupts circadian timing and alertness. Physiol. Rep. 2018, 6, e13692. [Google Scholar] [CrossRef]
- Guo, F.; Holla, M.; Diaz, M.M.; Rosbash, M. A circadian output circuit controls sleep-wake arousal in drosophila. Neuron 2018, 100, 624–635. [Google Scholar] [CrossRef]
- Toda, H.; Williams, J.A.; Gulledge, M.; Sehgal, A. A sleep-inducing gene, nemuri, links sleep and immune function in drosophila. Science 2019, 363, 509–515. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hastings, M.H.; Maywood, E.S.; Brancaccio, M. The Mammalian Circadian Timing System and the Suprachiasmatic Nucleus as Its Pacemaker. Biology 2019, 8, 13. https://doi.org/10.3390/biology8010013
Hastings MH, Maywood ES, Brancaccio M. The Mammalian Circadian Timing System and the Suprachiasmatic Nucleus as Its Pacemaker. Biology. 2019; 8(1):13. https://doi.org/10.3390/biology8010013
Chicago/Turabian StyleHastings, Michael H., Elizabeth S. Maywood, and Marco Brancaccio. 2019. "The Mammalian Circadian Timing System and the Suprachiasmatic Nucleus as Its Pacemaker" Biology 8, no. 1: 13. https://doi.org/10.3390/biology8010013
APA StyleHastings, M. H., Maywood, E. S., & Brancaccio, M. (2019). The Mammalian Circadian Timing System and the Suprachiasmatic Nucleus as Its Pacemaker. Biology, 8(1), 13. https://doi.org/10.3390/biology8010013




