Responses of Soil Quality and Microbial Community Composition to Vegetation Restoration in Tropical Coastal Forests
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Sampling and Physicochemical Properties
2.3. Soil Microbial Biomass and Community Composition
2.4. Soil Quality Index (SQI) Evaluation
2.5. Data Analysis
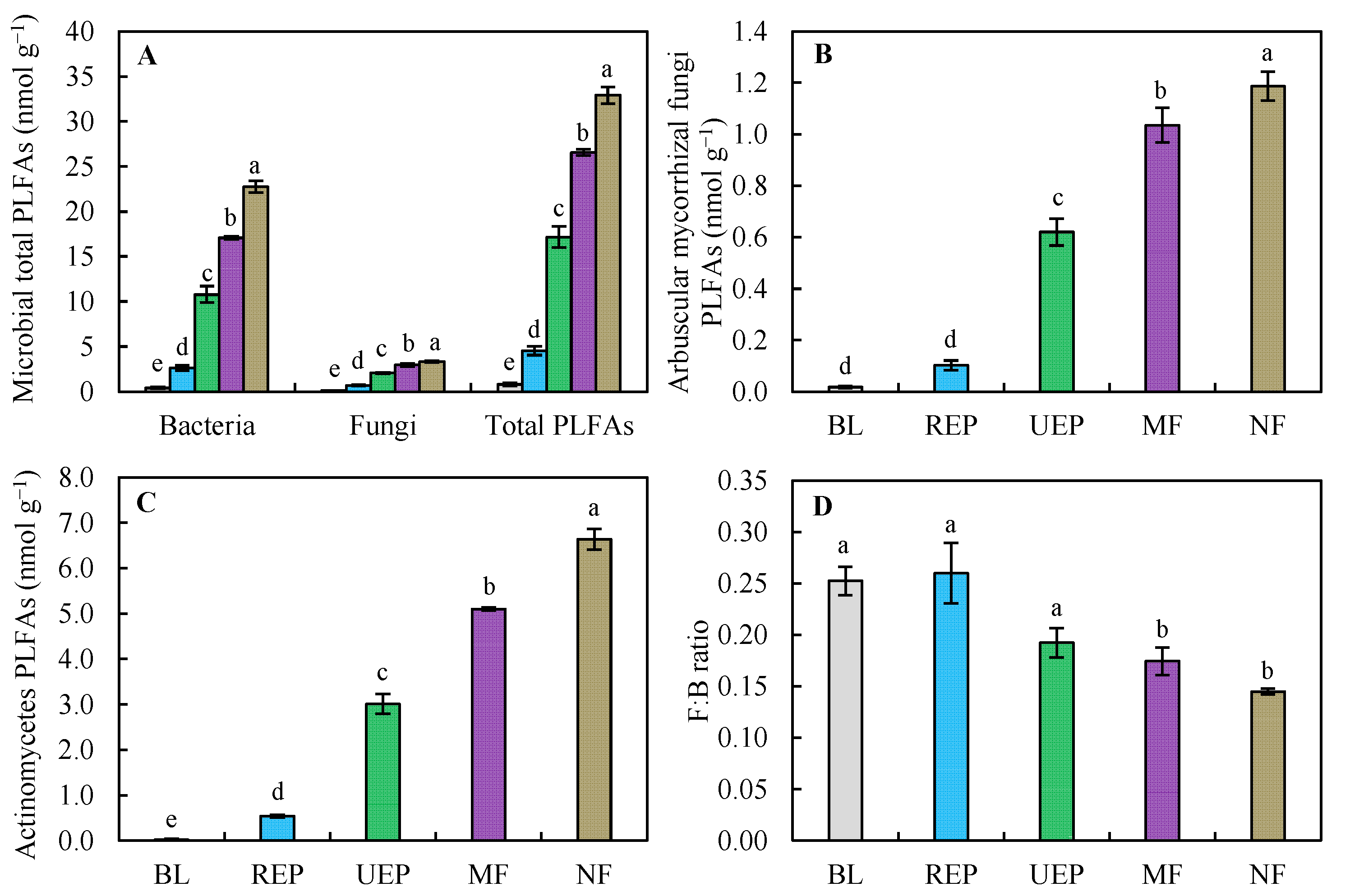
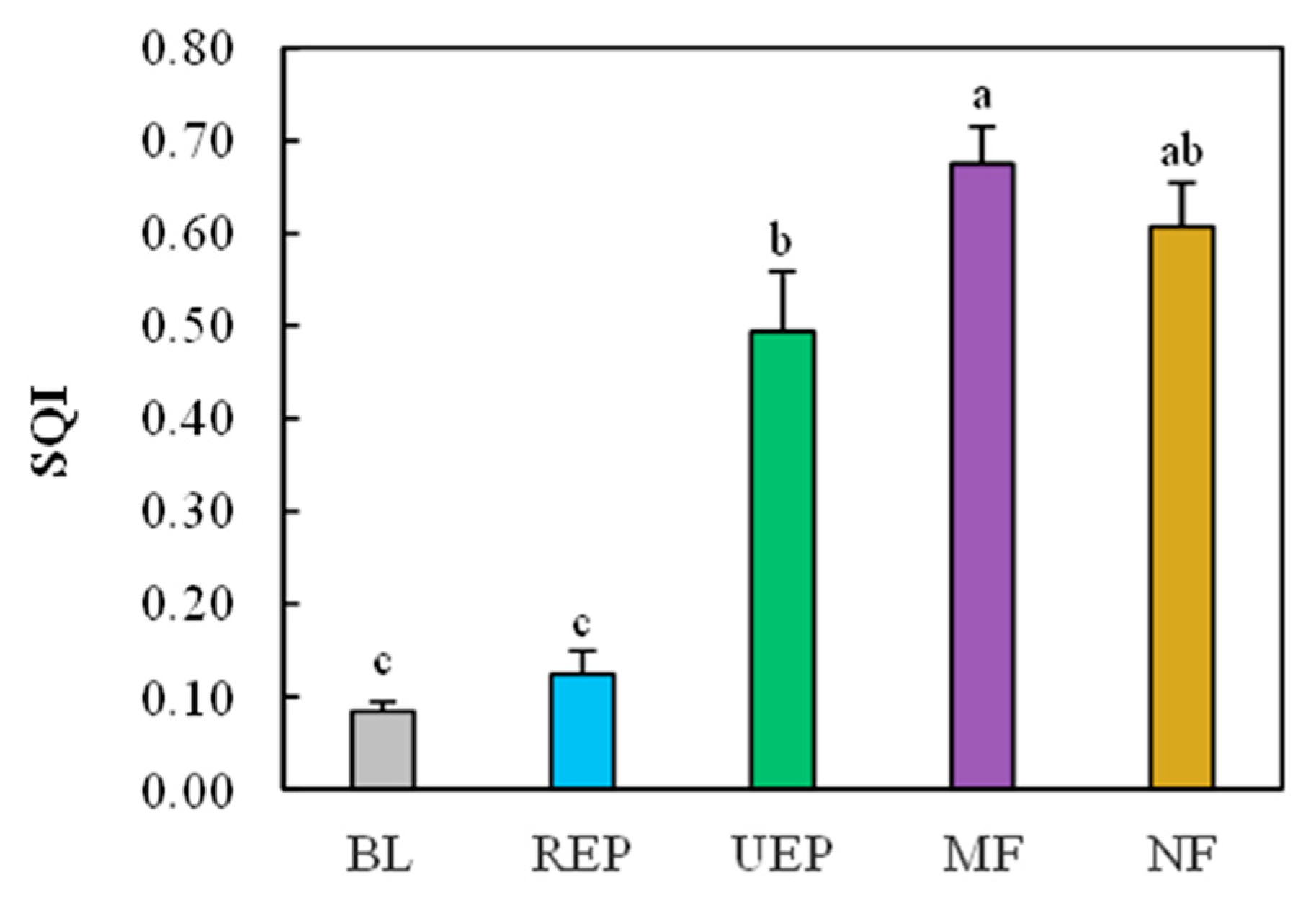
3. Results
3.1. Soil Physicochemical Properties
3.2. Soil Microbial Properties
3.3. Soil Quality Index and Its Relationships with Microbial Community
4. Discussion
4.1. Effects of Vegetation Recovery on Soil Quality
4.2. Effects of Vegetation Recovery on Soil Microbial Community
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SQI | Soil quality index |
| BL | Barren land |
| REP | Disturbed short-rotation Eucalyptus plantation |
| UEP | Undisturbed long-term Eucalyptus plantation |
| MF | Mixed native-species plantation |
| NF | Natural forest |
| AMF | Arbuscular mycorrhizal fungi |
Appendix A
| Parameter | Average | Curve Type | Slope (b) | Normalization Equation | Weighting Value (W) |
|---|---|---|---|---|---|
| (x0) | |||||
| DOC | 0.40 | More is better | −2.5 | S = 1/(1 + (X/0.4)−2.5) | 0.346 |
| TN | 452.81 | More is better | −2.5 | S = 1/(1 + (X/452.81)−2.5) | 0.340 |
| NH4+-N | 10.75 | More is better | −2.5 | S = 1/(1 + (X/10.75)−2.5) | 0.314 |
| SMC | pH | SOC | TN | TP | DOC | DN | NH4+-N | NO3−-N | |
|---|---|---|---|---|---|---|---|---|---|
| B | 0.95 *** | −0.71 ** | 0.94 *** | 0.64 ** | 0.72 *** | 0.97 *** | 0.94 *** | 0.51 | 0.74 *** |
| F | 0.94 *** | −0.72 ** | 0.96 *** | 0.69 ** | 0.74 *** | 0.93 *** | 0.90 *** | 0.30 | 0.64 ** |
| TPLFAs | 0.95 *** | −0.71 ** | 0.95 *** | 0.66 ** | 0.74 *** | 0.96 *** | 0.94 *** | 0.24 | 0.71 ** |
| Act | 0.94 *** | −0.71 ** | 0.94 *** | 0.67 ** | 0.75 *** | 0.96 *** | 0.95 *** | 0.22 | 0.76 *** |
| AMF | 0.94 ** | −0.65 ** | 0.93 *** | 0.71 ** | 0.77 *** | 0.94 *** | 0.91 *** | 0.25 | 0.66 ** |
| F:B | −0.83 *** | 0.56 * | −0.76 | −0.52 * | −0.63 ** | −0.84 *** | −0.85 *** | −0.20 | −0.65 ** |
References
- Bodo, T.; Gimah, B.G.; Seomoni, K.J. Deforestation and habitat loss: Human causes, consequences and possible solutions. J. Geogr. Sci. 2021, 4, 22–30. [Google Scholar] [CrossRef]
- Qu, X.; Li, X.; Bardgett, R.D.; Kuzyakov, Y.; Revillini, D.; Sonne, C.; Xia, C.; Ruan, H.; Cao, F.; Reich, P.B.; et al. Deforestation impacts soil biodiversity and ecosystem services worldwide. Proc. Natl. Acad. Sci. USA 2024, 121, e2318475121. [Google Scholar] [CrossRef]
- Chen, S.; Wen, Z.; Zhang, S.; Huang, P.; Ma, M.; Zhou, X.; Liao, T.; Wu, S. Effects of long-term and large-scale ecology projects on forest dynamics in Yangtze River Basin, China. For. Ecol. Manag. 2021, 496, 119463. [Google Scholar] [CrossRef]
- Shao, Q.; Liu, S.; Ning, J.; Liu, G.; Yang, F.; Zhang, X.; Niu, L.; Huang, H.; Fan, J.; Liu, J. Remote sensing assessment of the ecological benefits provided by national key ecological projects in China during 2000–2019. J. Geogr. Sci. 2023, 33, 1587–1613. [Google Scholar] [CrossRef]
- Lu, F.; Hu, H.; Sun, W.; Zhu, J.; Liu, G.; Zhou, W.; Zhang, Q.; Shi, P.; Liu, X.; Wu, X.; et al. Effects of national ecological restoration projects on carbon sequestration in China from 2001 to 2010. Proc. Natl. Acad. Sci. USA 2018, 115, 4039–4044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, J.; You, C.; Cao, R.; Tan, B.; Li, H.; Yang, W. Contributions of National Key Forestry Ecology Projects to the forest vegetation carbon storage in China. For. Ecol. Manag. 2020, 462, 117981. [Google Scholar] [CrossRef]
- Gatica-Saavedra, P.; Echeverría, C.; Nelson, C.R. Ecological indicators for assessing ecological success of forest restoration: A world review. Restor. Ecol. 2017, 25, 850–857. [Google Scholar] [CrossRef]
- Mirghaed, F.A.; Souri, B. Contribution of land use, soil properties and topographic features for providing of ecosystem services. Ecol. Eng. 2023, 189, 106898. [Google Scholar] [CrossRef]
- Paré, D.; Bognounou, F.; Emilson, E.J.; Laganière, J.; Leach, J.; Mansuy, N.; Martineau, C.; Norris, C.; Venier, L.; Webster, K. Connecting forest soil properties with ecosystem services: Toward a better use of digital soil maps-A review. Soil Sci. Soc. Am. J. 2024, 88, 981–999. [Google Scholar] [CrossRef]
- Sandra, P.R.; Shankar, A.; Garkoti, S.C.; Adarsh, C.K. Understanding the effects of forest types, vegetation structural diversity, and soil properties on above-and below-ground carbon stock of moist deciduous forest ecosystems in Western Ghats, India. Catena 2025, 257, 109198. [Google Scholar] [CrossRef]
- Oraon, P.R.; Sagar, V.; Beauty, K. Ecological restoration of degraded land through afforestation activities. In Land and Environmental Management Through Forestry; Raj, A., Jhariya, M.K., Banerjee, A., Nema, S., Bargali, K., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2023; pp. 201–216. [Google Scholar] [CrossRef]
- Ou, H.B.; Liu, X.S.; Wei, S.X.; Jiang, Y.; Gao, F.; Wang, Z.H.; Fu, W.; Du, H. The effects of different vegetation restoration models on soil quality in karst areas of southwest China. Forests 2024, 15, 1061. [Google Scholar] [CrossRef]
- Marron, N.; Epron, D. Are mixed-tree plantations including a nitrogen-fixing species more productive than monocultures? For. Ecol. Manag. 2019, 441, 242–252. [Google Scholar] [CrossRef]
- Guo, J.; Feng, H.; McNie, P.; Liu, Q.; Xu, X.; Pan, C.; Yan, K.; Feng, L.; Goitom, E.A.; Yu, Y. Species mixing improves soil properties and enzymatic activities in Chinese fir plantations: A meta-analysis. Catena 2023, 220, 106723. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, S.; Liu, Y.; Yao, B.; Yu, M.; Ma, J.; Yang, X.; Xue, J.; Xiang, Y.; Li, Y.; et al. Impact of mixed plantations on soil physicochemical properties: Variations and controlling factors in China. For. Ecol. Manag. 2024, 568, 122107. [Google Scholar] [CrossRef]
- Wang, X.; Zhuo, Z.; Zhou, M.; Li, S.; Lin, G.; Zhang, Y.; Jiang, F.; Huang, Y.; Lin, J. Response of the soil bacterial community to soil fertility during vegetation restoration in soil and water loss areas in south China. J. Soil Sci. Plant Nutr. 2024, 24, 3687–3698. [Google Scholar] [CrossRef]
- Brown, H.C.A.; Appiah, M.; Quansah, G.W.; Adjei, E.O.; Berninger, F. Soil carbon and bio-physicochemical properties dynamics under forest restoration sites in southern Ghana. Geoderma Reg. 2024, 38, e00838. [Google Scholar] [CrossRef]
- Dong, R.; Wang, X.; Wang, Y.; Ma, Y.; Yang, S.; Zhang, L.; Zhang, M.; Qin, J.; Quzha, R. Differences in soil microbial communities with successional stage depend on vegetation coverage and soil substrates in alpine desert shrublands. Plant Soil 2023, 485, 549–568. [Google Scholar] [CrossRef]
- Peng, S.; Chen, A.; Fang, H.; Wu, J.; Liu, G. Effects of vegetation restoration types on soil quality in Yuanmou dry-hot valley, China. Soil Sci. Plant Nutr. 2013, 59, 347–360. [Google Scholar] [CrossRef]
- Riestra, D.; Noellemeyer, E.; Quiroga, A. Soil texture and forest species condition the effect of afforestation on soil quality parameters. Soil Sci. 2012, 177, 279–287. [Google Scholar] [CrossRef]
- Augusto, L.; Boča, A. Tree functional traits, forest biomass, and tree species diversity interact with site properties to drive forest soil carbon. Nat. Commun. 2022, 13, 1097. [Google Scholar] [CrossRef]
- Xie, H.; Tang, Y.; Yu, M.; Wang, G.G. The effects of afforestation tree species mixing on soil organic carbon stock, nutrients accumulation, and understory vegetation diversity on reclaimed coastal lands in Eastern China. Glob. Ecol. Conserv. 2021, 26, e01478. [Google Scholar] [CrossRef]
- Luo, X.; Hou, E.; Zhang, L.; Kuang, Y.; Wen, D. Altered soil microbial properties and functions after afforestation increase soil carbon and nitrogen but not phosphorus accumulation. Biol. Fertil. Soils 2023, 59, 645–658. [Google Scholar] [CrossRef]
- Guo, Y.; Abdalla, M.; Espenberg, M.; Hastings, A.; Hallett, P.; Smith, P. A systematic analysis and review of the impacts of afforestation on soil quality indicators as modified by climate zone, forest type and age. Sci. Total Environ. 2021, 757, 143824. [Google Scholar] [CrossRef]
- Xu, Y.; Du, A.; Wang, Z.; Zhu, W.; Li, C.; Wu, L. Effects of different rotation periods of Eucalyptus plantations on soil physiochemical properties, enzyme activities, microbial biomass and microbial community structure and diversity. For. Ecol. Manag. 2020, 456, 117683. [Google Scholar] [CrossRef]
- Bose, T.; Hammerbacher, A.; Slippers, B.; Roux, J.; Wingfield, M.J. Continuous replanting could degrade soil health in short-rotation plantation forestry. Curr. For. Rep. 2023, 9, 230–250. [Google Scholar] [CrossRef]
- Zhou, X.; Wen, Y.; Goodale, U.M.; Zuo, H.; Zhu, H.; Li, X.; You, Y.; Yan, L.; Su, Y.; Huang, X. Optimal rotation length for carbon sequestration in Eucalyptus plantations in subtropical China. New For. 2017, 48, 609–627. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, X.A.; Zhang, Y.; Rao, X.; Fu, S. Dynamics of understory shrub biomass in six young plantations of southern subtropical China. Forests 2017, 8, 419. [Google Scholar] [CrossRef]
- Başkent, E.Z.; Kašpar, J. Exploring the effects of various rotation lengths on the ecosystem services within a multiple-use management framework. For. Ecol. Manag. 2023, 538, 120974. [Google Scholar] [CrossRef]
- Furey, G.N.; Tilman, D. Plant biodiversity and the regeneration of soil fertility. Proc. Natl. Acad. Sci. USA 2021, 118, e2111321118. [Google Scholar] [CrossRef]
- Cagnoni, L.B.; Weidlich, E.W.; Guillemot, J.; Morselo, C.; Weih, M.; Adler, A.; Brancalion, P.H. Stakeholders’ perspectives of species diversity in tree plantations: A global review. Curr. For. Rep. 2023, 9, 251–262. [Google Scholar] [CrossRef]
- Yao, X.; Hui, D.; Xing, S.; Zhang, Q.; Chen, J.; Li, Z.; Yang, X.; Deng, Q. Mixed plantations with N-fixing tree species maintain ecosystem C: N: P stoichiometry: Implication for sustainable production. Soil Biol. Biochem. 2024, 191, 109356. [Google Scholar] [CrossRef]
- Shao, G.; Ai, J.; Sun, Q.; Hou, L.; Dong, Y. Soil quality assessment under different forest types in the Mount Tai, central Eastern China. Ecol. Indic. 2020, 115, 106439. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wu, G.; Lie, Z.; Sheng, H.; Aguila, L.C.R.; Khan, M.S.; Liu, X.; Zhou, S.; Wu, T.; et al. Mixed plantations do not necessarily provide higher ecosystem multifunctionality than monoculture plantations. Sci. Total Environ. 2024, 914, 170156. [Google Scholar] [CrossRef]
- Bastida, F.; Zsolnay, A.; Hernández, T.; García, C. Past, present and future of soil quality indices: A biological perspective. Geoderma 2008, 147, 159–171. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.; Zhang, W.; Tang, Z.; Zhang, Y. How forest types shape soil quality: The evidence from eastern China’s north sub-tropical ecosystems. Ecol. Indic. 2025, 175, 113583. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Ma, L.; He, X.; Liu, Z.; Wang, F.; Chu, G.; Tang, X. Accumulation of glomalin-related soil protein benefits soil carbon sequestration: Tropical coastal forest restoration experiences. Land Degrad. Dev. 2022, 33, 1541–1551. [Google Scholar] [CrossRef]
- Ren, H.; Li, Z.; Shen, W.; Yu, Z.; Peng, S.; Liao, C.; Ding, M.; Wu, J. Changes in biodiversity and ecosystem function during the restoration of a tropical forest in South China. Sci China Ser. C 2007, 50, 277–284. [Google Scholar] [CrossRef]
- Wu, W.; Kuang, L.; Li, Y.; He, L.; Mou, Z.; Wang, F.; Zhang, J.; Wang, J.; Li, Z.; Lambers, H.; et al. Faster recovery of soil biodiversity in native species mixture than in Eucalyptus monoculture after 60 years afforestation in tropical degraded coastal terraces. Glob. Change Biol. 2021, 27, 5329–5340. [Google Scholar] [CrossRef]
- Lu, R.K. Method of Analysis in Soil and Agrochemistry; Agricultural Press: Beijing, China, 1999; pp. 31–33. (In Chinese) [Google Scholar]
- Bao, S. Analysis in Soil and Agrochemistry, 3rd ed.; Agricultural Press: Beijing, China, 2000; pp. 25–76. (In Chinese) [Google Scholar]
- Witt, C.; Gaunt, J.L.; Galicia, C.C.; Ottow, J.C.; Neue, H.U. A rapid chloroform-fumigation extraction method for measuring soil microbial biomass carbon and nitrogen in flooded rice soils. Biol. Fertil. Soils 2000, 30, 510–519. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Frostegård, A.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Joergensen, R.G. Phospholipid fatty acids in soil—Drawbacks and future prospects. Biol. Fertil. Soils 2022, 58, 1–6. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Mitchell, J.P. A comparison of soil quality indexing methods for vegetable production systems in northern California. Agr. Ecosyst. Environ. 2022, 90, 25–45. [Google Scholar] [CrossRef]
- Bastida, F.; Moreno, J.L.; Hernández, T.; García, C. Microbiological degradation index of soils in a semiarid climate. Soil Biol. Biochem. 2006, 38, 3463–3473. [Google Scholar] [CrossRef]
- Chen, L.; Xiang, W.; Ouyang, S.; Wu, H.; Xia, Q.; Ma, J.; Zeng, Y.; Lei, P.; Xiao, W.; Li, S.; et al. Tight coupling of fungal community composition with soil quality in a Chinese fir plantation chronosequence. Land Degrad. Dev. 2021, 32, 1164–1178. [Google Scholar] [CrossRef]
- Masto, R.E.; Chhonkar, P.K.; Singh, D.; Patra, A.K. Alternative soil quality indices for evaluating the effect of intensive cropping, fertilization and manuring for 31 years in the semi-arid soils of India. Environ. Monit. Assess. 2008, 136, 419–435. [Google Scholar] [CrossRef]
- Guan, H.; Fan, J. Effects of vegetation restoration on soil quality in fragile karst ecosystems of southwest China. PeerJ 2020, 8, e9456. [Google Scholar] [CrossRef]
- Panico, S.C.; Memoli, V.; Esposito, F.; Maisto, G.; De Marco, A.D. Plant cover and management practices as drivers of soil quality. Appl. Soil Ecol. 2018, 129, 34–42. [Google Scholar] [CrossRef]
- Ren, Q.; Qiang, F.; Liu, G.; Liu, C.; Ai, N. Response of soil quality to ecosystems after revegetation in a coal mine reclamation area. Catena 2025, 257, 109038. [Google Scholar] [CrossRef]
- Wang, F.; Ding, Y.; Sayer, E.J.; Li, Q.; Zou, B.; Mo, Q.; Li, Y.; Lu, X.; Tang, J.; Zhu, W.; et al. Tropical forest restoration: Fast resilience of plant biomass contrasts with slow recovery of stable soil C stocks. Funct. Ecol. 2017, 31, 2344–2355. [Google Scholar] [CrossRef]
- Shen, Y.; Li, J.; Chen, F.; Cheng, R.; Xiao, W.; Wu, L.; Zeng, L. Correlations between forest soil quality and aboveground vegetation characteristics in Hunan Province, China. Front. Plant Sci. 2022, 13, 1009109. [Google Scholar] [CrossRef] [PubMed]
- Schoenholtz, S.H.; Van Miegroet, H.; Burger, J.A. A review of chemical and physical properties as indicators of forest soil quality: Challenges and opportunities. For. Ecol. Manag. 2000, 138, 335–356. [Google Scholar] [CrossRef]
- Lv, X.; Tang, Q.; Han, C.; Song, M.; Yuan, C.; Yang, Q.; Wei, J.; He, X.; Collins, A.L. Farmland abandonment and vegetation succession mediate soil properties but are determined by the duration of conversion. Catena 2024, 238, 107877. [Google Scholar] [CrossRef]
- Xiang, W.; Xu, L.; Lei, P.; Ouyang, S.; Deng, X.; Chen, L.; Zeng, Y.; Hu, Y.; Zhao, Z.; Wu, H.; et al. Rotation age extension synergistically increases ecosystem carbon storage and timber production of Chinese fir plantations in southern China. J. Environ. Manag. 2022, 317, 115426. [Google Scholar] [CrossRef]
- Sasanifar, S.; Alijanpour, A.; Shafiei, A.B.; Rad, J.E.; Molaei, M. Forest conservation mediating soil quality relationship with diversity of various plant layers in the biosphere of Arasabran, Iran. Sci. Total Environ. 2024, 928, 172475. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, X.; Wu, J.; Zhao, J.; Zhao, M.; Chu, G.; Hui, D.; Zhou, G.; Deng, Q.; Zhang, D. Changes in soil microbial biomass, community composition, and enzyme activities after half-century forest restoration in degraded tropical lands. Forests 2019, 10, 1124. [Google Scholar] [CrossRef]
- Zhao, C.; Long, J.; Liao, H.; Zheng, C.; Li, J.; Liu, L.; Zhang, M. Dynamics of soil microbial communities following vegetation succession in a karst mountain ecosystem, Southwest China. Sci. Rep. 2019, 9, 2160. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, G.; Hai, X.; Li, J.; Shangguan, Z.; Peng, C.; Deng, L. Long-term forest succession improves plant diversity and soil quality but not significantly increase soil microbial diversity: Evidence from the Loess Plateau. Ecol. Eng. 2020, 142, 105631. [Google Scholar] [CrossRef]
- Qiang, W.; He, L.; Zhang, Y.; Liu, B.; Liu, Y.; Liu, Q.; Pang, X. Aboveground vegetation and soil physicochemical properties jointly drive the shift of soil microbial community during subalpine secondary succession in southwest China. Catena 2021, 202, 105251. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.Y.; Chen, X.; Huang, Z. Meta-analysis shows positive effects of plant diversity on microbial biomass and respiration. Nat. Commun. 2019, 10, 1332. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, W.; Jia, S.; Chen, S.; Xiong, D.; Xu, C.; Yang, Z.; Liu, X.; Yang, Y. Effects of litter and root inputs on soil microbial community structure in subtropical natural and plantation forests. Plant Soil 2025, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yuan, Y.; Mou, Z.; Li, Y.; Kuang, L.; Zhang, J.; Wu, W.; Wang, F.; Wang, J.; Lambers, H.; et al. Faster accumulation and greater contribution of glomalin to the soil organic carbon pool than amino sugars do under tropical coastal forest restoration. Glob. Change Biol. 2023, 29, 533–546. [Google Scholar] [CrossRef]
- Li, Q.; Feng, J.; Wu, J.; Jia, W.; Zhang, Q.; Chen, Q.; Zhang, D.; Cheng, X. Spatial variation in soil microbial community structure and its relation to plant distribution and local environments following afforestation in central China. Soil Till. Res. 2019, 193, 8–16. [Google Scholar] [CrossRef]
- Lemessa, D.; Mewded, B.; Legesse, A.; Atinfau, H.; Alemu, S.; Maryo, M.; Tilahun, H. Do Eucalyptus plantation forests support biodiversity conservation? For. Ecol. Manag. 2022, 523, 120492. [Google Scholar] [CrossRef]
- Melo, R.S.; Alexandrino, E.R.; de Paula, F.R.; Boscolo, D.; de Barros Ferraz, S.F. Promoting bird functional diversity on landscapes with a matrix of planted Eucalyptus spp. in the Atlantic Forest. Environ. Manag. 2024, 73, 395–407. [Google Scholar] [CrossRef] [PubMed]
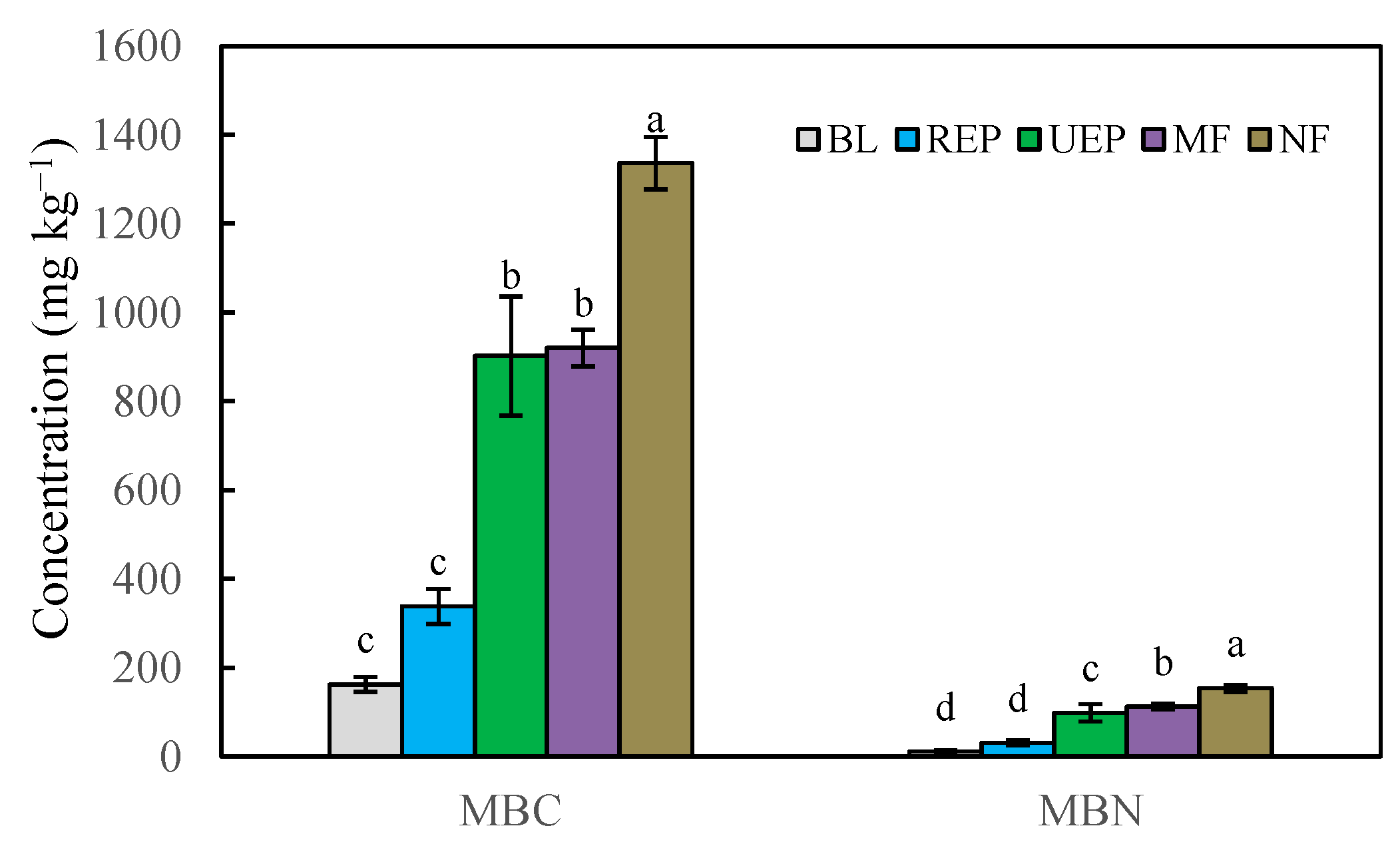
| Parameters | BL | REP | UEP | MF | NF |
|---|---|---|---|---|---|
| SMC (%) | 10.1 ± 0.5 c | 9.5 ± 0.3 c | 19.6 ± 0.8 b | 19.9 ± 1.1 b | 23.3 ± 0.4 a |
| pH value | 4.7 ± 0.05 a | 4.4 ± 0.02 b | 4.4 ± 0.04 bc | 4.3 ± 0.1 bc | 4.2 ± 0.04 c |
| SOC (mg g−1) | 3.0 ± 0.2 d | 9.8 ± 0.5 c | 19.0 ± 1.6 b | 20.5 ± 0.8 b | 26.7 ± 0.7 a |
| TN (mg g−1) | 0.01 ± 0.01 c | 0.09 ± 0.04 c | 0.35 ± 0.24 bc | 1.16 ± 0.11 a | 0.58 ± 0.22 b |
| TP (mg g−1) | 0.02 ± 0.01 c | 0.01 ± 0.01 c | 0.08 ± 0.03 bc | 0.16 ± 0.03 a | 0.11 ± 0.03 ab |
| DOC (mg kg−1) | 159 ± 26 d | 181 ± 13 d | 407 ± 62 c | 694 ± 35 b | 884 ± 50 a |
| NH4+-N (mg kg−1) | 4.1 ± 0.6 b | 7.0 ± 2.1 b | 28.1 ± 7 a | 9.0 ± 2.3 b | 6.1 ± 1.9 b |
| NO3−-N (mg kg−1) | 3.5 ± 0.3 bc | 1.8 ± 0.5 c | 4.6 ± 0.5 b | 3.7 ± 0.7 bc | 11.2 ± 1.4 a |
| Principal Components | PC-1 | PC-2 | PC-3 |
|---|---|---|---|
| Eigenvalues | 8.20 | 1.38 | 1.13 |
| Variance (%) | 68.35 | 11.46 | 9.38 |
| Cumulative (%) | 68.35 | 79.82 | 89.20 |
| Weighting value | 0.346 | 0.34 | 0.314 |
| Factor loading | |||
| SMC | 0.962 | −0.093 | −0.088 |
| pH | −0.771 | 0.206 | −0.021 |
| SOC | 0.968 | −0.051 | −0.028 |
| TN | 0.645 | 0.734 | 0.053 |
| TP | 0.743 | 0.630 | 0.118 |
| MBC | 0.967 | −0.149 | 0.017 |
| DOC | 0.978 | −0.059 | 0.117 |
| MBN | 0.976 | −0.039 | 0.035 |
| DN | 0.955 | −0.067 | 0.082 |
| MBC/MBN | −0.682 | −0.132 | 0.410 |
| NH4+-N | 0.260 | −0.162 | −0.889 |
| NO3−-N | 0.698 | −0.556 | 0.345 |
| Parameter | SMC (B) | pH (F) | SOC (TMB) | TN (Act) | TP (AMF) | -- (F:B) |
|---|---|---|---|---|---|---|
| SQI | 0.94 *** | −0.68 ** | 0.89 *** | 0.75 *** | 0.79 *** | -- |
| (0.89 ***) | (0.92 ***) | (0.90 ***) | (0.90 ***) | (0.91 ***) | (−0.73 ***) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhang, F.; Cao, J.; Liu, T.; Zhang, Y. Responses of Soil Quality and Microbial Community Composition to Vegetation Restoration in Tropical Coastal Forests. Biology 2025, 14, 1120. https://doi.org/10.3390/biology14091120
Chen Y, Zhang F, Cao J, Liu T, Zhang Y. Responses of Soil Quality and Microbial Community Composition to Vegetation Restoration in Tropical Coastal Forests. Biology. 2025; 14(9):1120. https://doi.org/10.3390/biology14091120
Chicago/Turabian StyleChen, Yuanqi, Feifeng Zhang, Jianbo Cao, Tong Liu, and Yu Zhang. 2025. "Responses of Soil Quality and Microbial Community Composition to Vegetation Restoration in Tropical Coastal Forests" Biology 14, no. 9: 1120. https://doi.org/10.3390/biology14091120
APA StyleChen, Y., Zhang, F., Cao, J., Liu, T., & Zhang, Y. (2025). Responses of Soil Quality and Microbial Community Composition to Vegetation Restoration in Tropical Coastal Forests. Biology, 14(9), 1120. https://doi.org/10.3390/biology14091120







