Liming-Induced Nitrous Oxide Emissions from Acidic Soils Dominated by Stimulative Nitrification
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Physicochemical Characteristics
2.2. The Alkaline Mineral Amendment
2.3. Robotized Batch Cultivations for Respiratory Phenotype Experiment
2.4. Measurements and Analysis
2.4.1. Chemical Analysis
2.4.2. DNA Extraction and qPCR of Targeted Genes
2.5. Statistical Analysis
3. Results
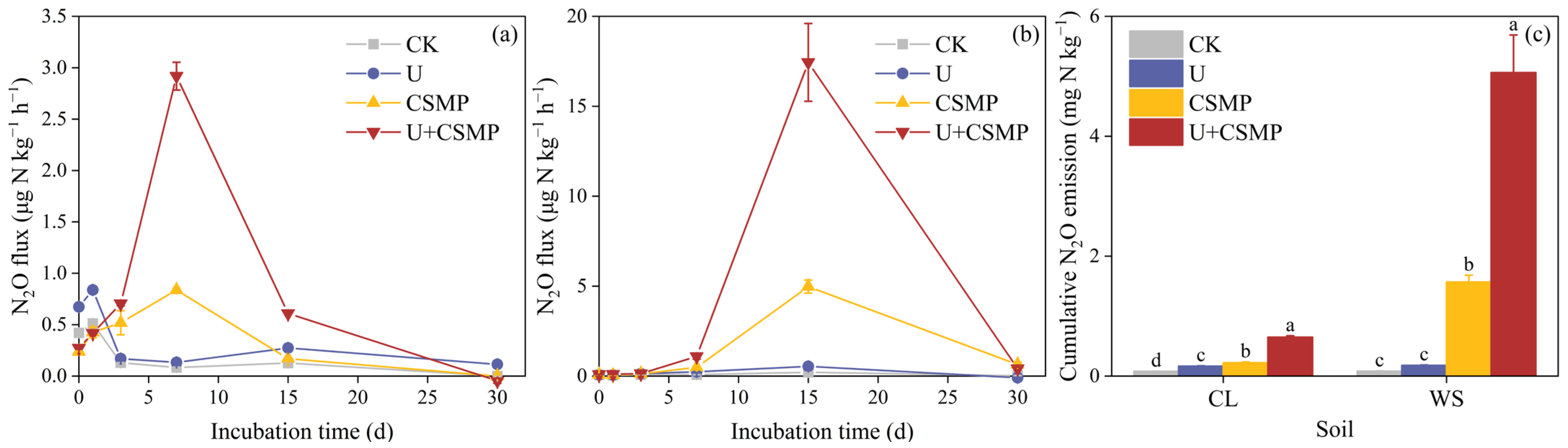
3.1. Soil N2O Emissions in Response to CSMP and Urea Application
3.2. Dynamics of NH4+-N and NO3−-N Concentrations and Net Nitrification Rate in Response to CSMP and Urea Application
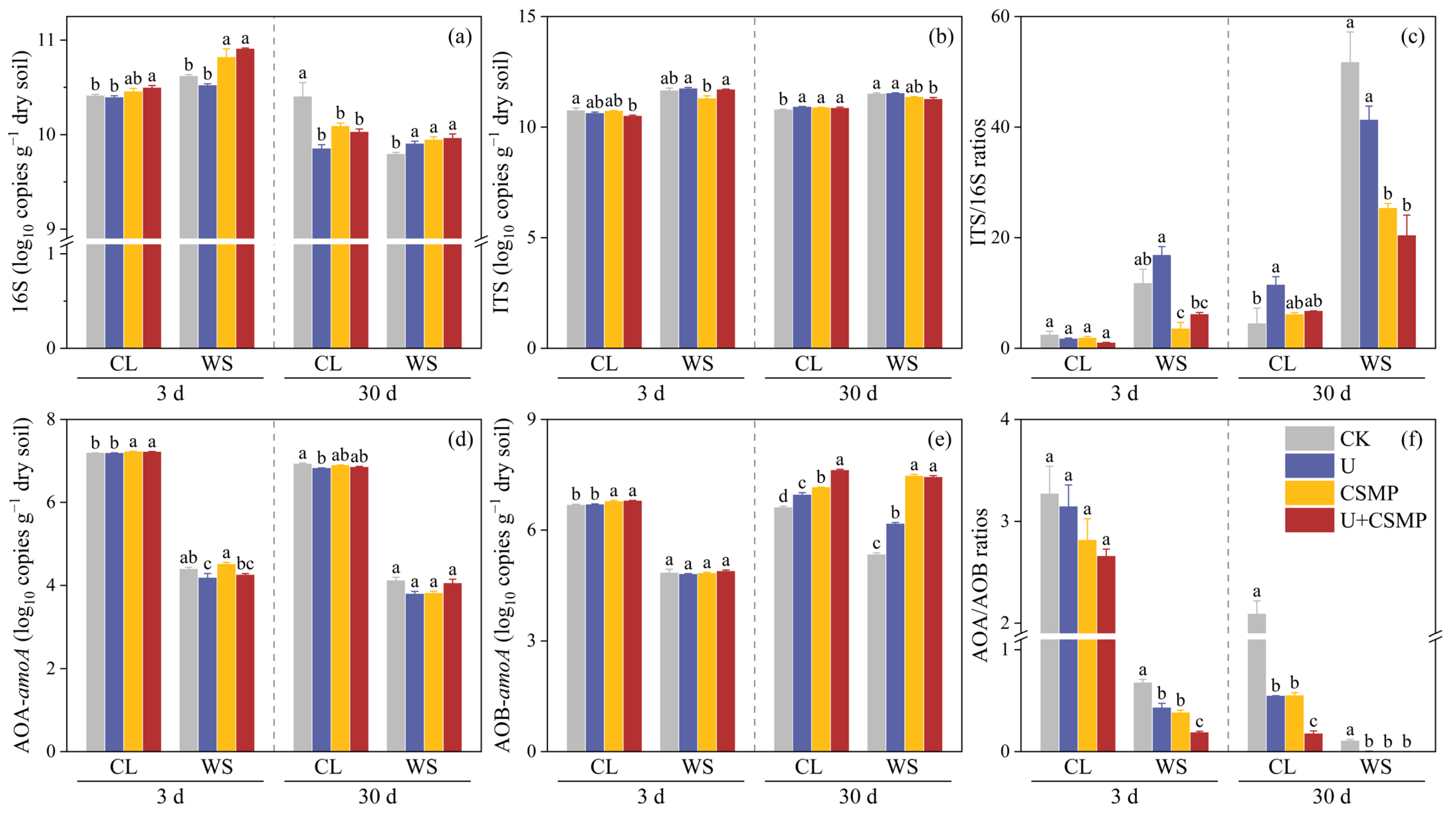
3.3. Abundance of Functional Genes Related to N2O Production and Reduction
3.4. Structural Equation Modeling
4. Discussion
4.1. N2O Emission and N Transformations Following CSMP and Urea Application
4.2. CSMP Reduced the Acidic Soils’ N2O Emissions Dominated by Nitrification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse gases emissions and global climate change: Examining the influence of CO2, CH4, and N2O. Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [CrossRef]
- Kanter, D.; Mauzerall, D.L.; Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W.; Grabiel, P.M.; Moomaw, W.R.; Galloway, J.N. A post-Kyoto partner: Considering the stratospheric ozone regime as a tool to manage nitrous oxide. Proc. Natl. Acad. Sci. USA 2013, 110, 4451–4457. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef]
- Du, Y.; Gu, X.; Wang, J.; Niu, W. Yield and gas exchange of greenhouse tomato at different nitrogen levels under aerated irrigation. Sci. Total Environ. 2019, 668, 1156–1164. [Google Scholar] [CrossRef]
- Li, L.; Hong, M.; Zhang, Y.; Paustian, K. Soil N2O emissions from specialty crop systems: A global estimation and meta-analysis. Glob. Chang. Biol. 2024, 30, e17233. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, P.; Liu, J.; Xiao, H.; Zhang, A.; Chen, S.; Chen, J.; Liu, H.; Zhu, X.; Hussain, Q.; et al. Microbial effects of prolonged nitrogen fertilization and straw mulching on soil N2O emissions using metagenomic sequencing. Agric. Ecosyst. Environ. 2025, 382, 109476. [Google Scholar] [CrossRef]
- Thompson, R.L.; Lassaletta, L.; Patra, P.K.; Wilson, C.; Wells, K.C.; Gressent, A.; Koffi, E.N.; Chipperfield, M.P.; Winiwarter, W.; Davidson, E.A.; et al. Acceleration of global N2O emissions seen from two decades of atmospheric inversion. Nat. Clim. Chang. 2019, 9, 993–998. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Vogt, R.D.; Mulder, J.; Wang, J.; Zhang, X. Soil pH as the chief modifier for regional nitrous oxide emissions: New evidence and implications for global estimates and mitigation. Glob. Change Biol. 2018, 24, e617–e626. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, Y.; Zhang, K.; Xu, X.; Zhao, Y.; Bai, T.; Zhao, Y.; Wang, H.; Sheng, X.; Bloszies, S.; et al. Intermediate soil acidification induces highest nitrous oxide emissions. Nat. Commun. 2024, 15, 2695. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, X.; He, X. A global meta-analysis of crop yield and agricultural greenhouse gas emissions under nitrogen fertilizer application. Sci. Total Environ. 2022, 831, 154982. [Google Scholar] [CrossRef]
- Baggs, E.M.; Smales, C.L.; Bateman, E.J. Changing pH shifts the microbial sourceas well as the magnitude of N2O emission from soil. Biol. Fert. Soils 2010, 46, 793–805. [Google Scholar] [CrossRef]
- Holland, J.E.; Bennett, A.E.; Newton, A.C.; White, P.; McKenzie, B.; George, T.; Pakeman, R.; Bailey, J.; Fornara, D.; Hayes, R. Liming impacts on soils, crops and biodiversity in the UK: A review. Sci. Total Environ. 2018, 610, 316–332. [Google Scholar] [CrossRef]
- Zheng, Y.; Abbott, L.K.; Bolan, N.; Hu, H.; Jenkins, S.N.; Mickan, B.S. Biochar interacted with organic compounds from digestate in controlling N2O emissions. J. Environ. Manag. 2025, 385, 125591. [Google Scholar] [CrossRef]
- Russenes, A.L.; Korsaeth, A.; Bakken, L.R.; Dörsch, P. Spatial variation in soil pH controls off-season N2O emission in an agricultural soil. Soil. Biol. Biochem. 2016, 99, 36–46. [Google Scholar] [CrossRef]
- Wu, G.; Liang, F.; Wu, Q.; Feng, X.; Shang, W.; Li, H.; Li, X.; Che, Z.; Dong, Z.; Song, H. Soil pH differently affects N2O emissions from soils amended with chemical fertilizer and manure by modifying nitrification and denitrification in wheat-maize rotation system. Biol. Fert. Soils 2024, 60, 101–113. [Google Scholar] [CrossRef]
- Zhang, C.; Ju, X.; Zhang, J.; Rees, R.M.; Müller, C. Soil pH and long-term fertilization affect gross N transformation and N2O production pathways in Chinese and UK croplands. Biol. Fert. Soils 2023, 59, 527–539. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Z.; Zhan, Y.; Zheng, X.; Zhou, M.; Yan, G.; Wang, L.; Werner, C.; Butterbach-Bahl, K. Potential benefits of liming to acid soils on climate change mitigation and food security. Glob. Chang. Biol. 2021, 27, 2807–2821. [Google Scholar] [CrossRef] [PubMed]
- Hiis, E.G.; Vick, S.H.W.; Molstad, L.; Røsdal, K.; Jonassen, K.R.; Winiwarter, W.; Bakken, L.R. Unlocking bacterial potential to reduce farmland N2O emissions. Nature 2024, 630, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Sanford, R.A.; Chee-Sanford, J.; Ooi, S.K.; Löffler, F.E.; Konstantinidis, K.T.; Yang, W.H. Beyond denitrification: The role of microbial diversity in controlling nitrous oxide reduction and soil nitrous oxide emissions. Glob. Chang. Biol. 2021, 27, 2669–2683. [Google Scholar] [CrossRef]
- Bakken, L.R.; Bergaust, L.; Liu, B.; Frostegård, Å. Regulation of denitrification at the cellular level: A clue to the understanding of N2O emissions from soils. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1226–1234. [Google Scholar] [CrossRef]
- Shaaban, M.; Peng, Q.; Hu, R.; Wu, Y.; Lin, S.; Zhao, J. Dolomite application to acidic soils: A promising option for mitigating N2O emissions. Environ. Sci. Pollut. Res. 2015, 22, 19961–19970. [Google Scholar] [CrossRef]
- Wan, S.; Lin, Y.; Fan, J.; Hu, H.; Zhang, J.; Jin, S.; Deng, M.; Müller, C.; He, J. Different responses of nitrous oxide emissions to liming and manure amendment of an acidic ultisol are controlled by autotrophic and heterotrophic nitrification. Soil Biol. Biochem. 2023, 178, 108960. [Google Scholar] [CrossRef]
- Xie, L.; Li, L.; Xie, J.; Wang, J.; Mumtaz, M.Z.; Effah, Z.; Fudjoe, S.K.; Khaskheli, M.A.; Luo, Z.; Li, L. Optimal substitution of inorganic fertilizer with organic amendment sustains rainfed maize production and decreases soil N2O emissions by modifying denitrifying bacterial communities in Northern China. Eur. J. Agron. 2024, 160, 127287. [Google Scholar] [CrossRef]
- Lourenço, K.S.; Costa, O.Y.D.A.; Cantarella, H.; Kuramae, E.E. Ammonia-oxidizing bacteria and fungal denitrifier diversity are associated with N2O production in tropical soils. Soil Biol. Biochem. 2022, 166, 108563. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil. Soil Biol. Biochem. 2010, 42, 926–934. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Y.; Ma, J.; Jiang, J.; You, X.; Lv, R.; Zhou, S.; Pan, C.; Liu, B.; Xu, Q.; et al. How does biochar influence soil nitrification and nitrification-induced N2O emissions? Sci. Total Environ. 2024, 908, 168530. [Google Scholar] [CrossRef]
- Clough, T.J.; Bertram, J.E.; Ray, J.L.; Condron, L.; O’CAllaghan, M.; Sherlock, R.; Wells, N. Unweathered Wood Biochar Impact on Nitrous Oxide Emissions from a Bovine-Urine-Amended Pasture Soil. Soil Sci. Soc. Am. J. 2010, 74, 852–860. [Google Scholar] [CrossRef]
- Shen, J.; Tang, H.; Liu, J.; Wang, C.; Li, Y.; Ge, T.; Jones, D.L.; Wu, J. Contrasting effects of straw and straw-derived biochar amendments on greenhouse gas emissions within double rice cropping systems. Agric. Ecosyst. Environ. 2014, 188, 264–274. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, M.; Zhu, B.; Kemmann, B.; Pfülb, L.; Burkart, S.; Liu, H.; Butterbach-Bahl, K.; Well, R. Threshold-like effect of soil NO3− concentrations on denitrification product N2O/(N2O+N2) ratio is mediated by soil pH. Soil Biol. Biochem. 2023, 187, 109213. [Google Scholar] [CrossRef]
- Clough, T.J.; Kelliher, F.M.; Sherlock, R.R.; Ford, C.D. Lime and Soil Moisture Effects on Nitrous Oxide Emissions from a Urine Patch. Soil Sci. Soc. Am. J. 2004, 68, 1600–1609. [Google Scholar] [CrossRef]
- Shaaban, M.; Hu, R.; Wu, Y.; Song, L.; Xu, P. Soil pH management for mitigating N2O emissions through nosZ (Clade I and II) gene abundance in rice paddy system. Environ. Res. 2023, 225, 115542. [Google Scholar] [CrossRef]
- Liang, Z.; Elsgaard, L. Nitrous oxide fluxes from long-term limed soils following P and glucose addition: Nonlinear response to liming rates and interaction from added P. Sci. Total Environ. 2021, 797, 148933. [Google Scholar] [CrossRef]
- Hink, L.; Nicol, G.W.; Prosser, J.I. Archaea produce lower yields of N2O than bacteria during aerobic ammonia oxidation in soil. Environ. Microbiol. 2017, 19, 4829–4837. [Google Scholar] [CrossRef]
- Abalos, D.; Liang, Z.; Dörsch, P.; Elsgaard, L. Trade-offs in greenhouse gas emissions across a liming-induced gradient of soil pH: Role of microbial structure and functioning. Soil Biol. Biochem. 2020, 150, 108006. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, H.; Chen, Z.; Wang, J.; Cai, Z.; Sun, N.; Wang, S.; Zhang, J.; Chang, S.X.; Xu, M.; et al. Contrasting effects of different pH-raising materials on N2O emissions in acidic upland soils. Eur. J. Soil Sci. 2021, 72, 432–445. [Google Scholar] [CrossRef]
- Yin, J.; Cui, W.; Xu, Y.; Ma, Y.; Chen, H.; Guo, J.; Liu, R.; Chen, Q. Understanding the relative contributions of fungi and bacteria led nitrous oxide emissions in an acidic soil amended with industrial waste. Ecotoxicol. Environ. Saf. 2023, 255, 114727. [Google Scholar] [CrossRef]
- GB/T 36207-2018; Fertilizer of Calcium Silicon Magnesium Potassium. China National Standards: Shanghai, China, 2018.
- Zhou, K.; Zhou, Q.; Gong, K.; Zhang, J. Waste-to-resource strategy to fabricate environmentally benign flame retardants from waste phosphorus tailings. Compos. Commun. 2020, 19, 173–176. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, Y.; Liu, Z.; Lin, H.; Zhang, Y.; Song, X.; Liu, P.; Li, Y.; Chen, J.; Chen, H.; et al. Effects of byproduct amendment on enzyme activities and physicochemical properties of acidic orchard soil from Jiaodong Peninsula of China. Commun. Soil Sci. Plant Anal. 2018, 49, 913–922. [Google Scholar] [CrossRef]
- Bakken, L. Spreadsheet for Gas Kinetics in Batch Cultures: KINCALC; Researchgate: Berlin, Germany, 2024. [Google Scholar]
- Li, S.; Cai, D.; Shaaban, M.; Ma, J.; Liu, S. Liming promotes soil nitrite accumulation but reduces subsequent abiotic nitrous oxide emissions. Res. Sq. 2023, preprint. [Google Scholar] [CrossRef]
- Wu, L.; Xiao, Q.; Wang, J.; Huang, Y.; Wu, D.; Liu, J.; Wang, B.; Zhang, H.; Xu, M.; Zhang, W. Liming decreases the emission and temperature sensitivity of N2O following labile carbon addition. Geoderma 2022, 425, 116032. [Google Scholar] [CrossRef]
- Li, Y.; Chapman, S.J.; Nicol, G.W.; Yao, H. Nitrification and nitrifiers in acidic soils. Soil Biol. Biochem. 2018, 116, 290–301. [Google Scholar] [CrossRef]
- Inatomi, M.; Hajima, T.; Ito, A. Fraction of nitrous oxide production in nitrification and its effect on total soil emission: A meta-analysis and global-scale sensitivity analysis using a process-based model. PLoS ONE 2019, 14, e219159. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, H.; Ye, G.; Fan, J.; Ding, W.; He, Z.; Zheng, Y.; He, J. Ammonia-oxidizing bacteria play an important role in nitrification of acidic soils: A meta-analysis. Geoderma 2021, 404, 115395. [Google Scholar] [CrossRef]
- Pan, B.; Lam, S.K.; Wang, E.; Mosier, A.R.; Chen, D. New approach for predicting nitrification and its fraction of N2O emissions in global terrestrial ecosystems. Environ. Res. Lett. 2021, 16, 34053. [Google Scholar] [CrossRef]
- Yu, C.; Wang, G.; Zhang, H.; Chen, H.; Ma, Q. Biochar and nitrification inhibitor (dicyandiamide) combination had a double-win effect on saline-alkali soil improvement and soybean production in the Yellow River Delta, China. Agronomy 2022, 12, 3154. [Google Scholar] [CrossRef]
- Farquharson, R. Nitrification rates and associated nitrous oxide emissions from agricultural soils—A synopsis. Soil Res. 2016, 54, 469–480. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, J.; Wang, J.; Wang, S.; Chang, S.X.; Cai, Z.; Zhang, J.; Niu, S.; Hu, S. Nitrogen deposition differentially affects soil gross nitrogen transformations in organic and mineral horizons. Earth-Sci. Rev. 2020, 201, 103033. [Google Scholar] [CrossRef]
- Samad, M.S.; Ganasamurthy, S.; Highton, M.P.; Bakken, L.R.; Clough, T.J.; de Klein, C.A.; Richards, K.G.; Lanigan, G.J.; Morales, S.E. Urea treatment decouples intrinsic pH control over N2O emissions in soils. Soil Biol. Biochem. 2021, 163, 108461. [Google Scholar] [CrossRef]
- Norton, J.; Ouyang, Y. Controls and Adaptive Management of Nitrification in Agricultural Soils. Front. Microbiol. 2019, 10, 1931. [Google Scholar] [CrossRef]
- Deng, N.; Gubry-Rangin, C.; Song, X.; Ju, X.; Liu, S.; Shen, J.; Di, H.; Han, L.; Zhang, L. AOB Nitrosospira cluster 3a.2 (D11) dominates N2O emissions in fertilised agricultural soils. J. Environ. Manag. 2024, 355, 120504. [Google Scholar] [CrossRef]
- Hui, D.; Ray, A.; Kasrija, L.; Christian, J. Impacts of Climate Change and Agricultural Practices on Nitrogen Processes, Genes, and Soil Nitrous Oxide Emissions: A Quantitative Review of Meta-Analyses. Agriculture 2024, 14, 240. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, Y.; Li, Y.; Xiang, T.; Wang, F.; Peng, J.; Yang, S.; Cao, W. Nitrification regulates the responses of soil nitrous oxide emissions to nitrogen addition in China: A meta-analysis from a gene perspective. Environ. Sci. Process. Impacts 2025, 27, 2367–2378. [Google Scholar] [CrossRef]
- Han, B.; Yao, Y.; Liu, B.; Wang, Y.; Su, X.; Ma, L.; Liu, D.; Niu, S.; Chen, X.; Li, Z. Relative importance between nitrification and denitrification to N2O from a global perspective. Glob. Change Biol. 2024, 30, e17082. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Song, Y.; Li, S.; Xiong, Z. Responses of N2O production pathways and related functional microbes to temperature across greenhouse vegetable field soils. Geoderma 2019, 355, 113904. [Google Scholar] [CrossRef]
- Prosser, J.I.; Hink, L.; Gubry Rangin, C.; Nicol, G.W. Nitrous oxide production by ammonia oxidizers: Physiological diversity, niche differentiation and potential mitigation strategies. Glob. Chang. Biol. 2020, 26, 103–118. [Google Scholar] [CrossRef]
- Nadeem, S.; Bakken, L.R.; Frostegård, Å.; Gaby, J.C.; Dörsch, P. Contingent effects of liming on N2O-emissions driven by autotrophic nitrification. Front. Environ. Sci. 2020, 8, 598513. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Z.; Li, Y.; Chen, Z.; Zhang, J.; Cai, Z.; Elsgaard, L.; Cheng, Y.; van Groenigen, K.J.; Abalos, D. Liming modifies greenhouse gas fluxes from soils: A meta-analysis of biological drivers. Agric. Ecosyst. Environ. 2022, 340, 108182. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, L.; Li, Y.; Brookes, P.C.; Xu, J.; Luo, Y. The effects of combinations of biochar, lime, and organic fertilizer on nitrification and nitrifiers. Biol. Fert. Soils 2017, 53, 77–87. [Google Scholar] [CrossRef]
- Ji, C.; Li, S.; Geng, Y.; Miao, Y.; Ding, Y.; Liu, S.; Zou, J. Differential responses of soil N2O to biochar depend on the predominant microbial pathway. Appl. Soil Ecol. 2020, 145, 103348. [Google Scholar] [CrossRef]
- Smith, C.J.; Osborn, A.M. Advantages and limitations of quantitative PCR (Q-PCR)-based approaches in microbial ecology. FEMS Microbiol. Ecol. 2009, 67, 6–20. [Google Scholar] [CrossRef]
- Yu, L.; Harris, E.; Lewicka-Szczebak, D.; Barthel, M.; Blomberg, M.R.; Harris, S.J.; Johnson, M.S.; Lehmann, M.F.; Liisberg, J.; Müller, C.; et al. What can we learn from N2O isotope data?—Analytics, processes and modelling. Rapid Commun. Mass Spectrom. 2020, 34, e8858. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Fan, Q.; Yu, J.; Liu, R. A meta-analysis to examine whether nitrification inhibitors work through selectively inhibiting ammonia-oxidizing bacteria. Front. Microbiol. 2022, 13, 962146. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hayden, H.L.; Hu, H.; He, J.; Suter, H.; Chen, D. Effects of the nitrification inhibitor acetylene on nitrous oxide emissions and ammonia-oxidizing microorganisms of different agricultural soils under laboratory incubation conditions. Appl. Soil Ecol. 2017, 119, 80–90. [Google Scholar] [CrossRef]
- Liu, C.; Mo, T.; Zhong, J.; Chen, H.; Xu, H.; Yang, X.; Li, Y. Synergistic benefits of lime and 3,4-dimethylpyrazole phosphate application to mitigate the nitrous oxide emissions from acidic soils. Ecotoxicol. Environ. Saf. 2023, 263, 115387. [Google Scholar] [CrossRef]
- Mori, H.; Maruyama, F.; Kato, H.; Toyoda, A.; Dozono, A.; Ohtsubo, Y.; Nagata, Y.; Fujiyama, A.; Tsuda, M.; Kurokawa, K. Design and experimental application of a novel non-degenerate universal primer set that amplifies prokaryotic 16S rRNA genes with a low possibility to amplify eukaryotic rRNA genes. DNA Res. 2014, 21, 217–227. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol. Biochem. 2017, 110, 56–67. [Google Scholar] [CrossRef]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.C.; Schleper, C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef]
- Schauss, K.; Focks, A.; Leininger, S.; Kotzerke, A.; Heuer, H.; Thiele Bruhn, S.; Sharma, S.; Wilke, B.M.; Matthies, M.; Smalla, K. Dynamics and functional relevance of ammonia–oxidizing archaea in two agricultural soils. Environ. Microbiol. 2009, 11, 446–456. [Google Scholar] [CrossRef]
- Rotthauwe, J.; Witzel, K.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microb. 1997, 63, 4704–4712. [Google Scholar] [CrossRef]
- Harter, J.; Krause, H.-M.; Schuettler, S.; Ruser, R.; Fromme, M.; Scholten, T.; Kappler, A.; Behrens, S. Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J. 2014, 8, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; Bru, D.; Stres, B.; Hallet, S.; Philippot, L. Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl. Environ. Microb. 2006, 72, 5181–5189. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Graf, D.R.H.; Bru, D.; Philippot, L.; Hallin, S. The unaccounted yet abundant nitrous oxide-reducing microbial community: A potential nitrous oxide sink. ISME J. 2013, 7, 417–426. [Google Scholar] [CrossRef]
- Long, A.; Song, B.K.; Fridey, K.; Silva, A. Detection and diversity of copper containing nitrite reductase genes (nirK) in prokaryotic and fungal communities of agricultural soils. FEMS Microbiol. Ecol. 2015, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, X.; Gong, H.; Ahmed, W.; Thompson, R.B.; Shi, W.; Yin, J.; Chen, Q. Liming-Induced Nitrous Oxide Emissions from Acidic Soils Dominated by Stimulative Nitrification. Biology 2025, 14, 1110. https://doi.org/10.3390/biology14091110
Xiang X, Gong H, Ahmed W, Thompson RB, Shi W, Yin J, Chen Q. Liming-Induced Nitrous Oxide Emissions from Acidic Soils Dominated by Stimulative Nitrification. Biology. 2025; 14(9):1110. https://doi.org/10.3390/biology14091110
Chicago/Turabian StyleXiang, Xiaoxiao, Hongyang Gong, Waqar Ahmed, Rodney B. Thompson, Wenxuan Shi, Junhui Yin, and Qing Chen. 2025. "Liming-Induced Nitrous Oxide Emissions from Acidic Soils Dominated by Stimulative Nitrification" Biology 14, no. 9: 1110. https://doi.org/10.3390/biology14091110
APA StyleXiang, X., Gong, H., Ahmed, W., Thompson, R. B., Shi, W., Yin, J., & Chen, Q. (2025). Liming-Induced Nitrous Oxide Emissions from Acidic Soils Dominated by Stimulative Nitrification. Biology, 14(9), 1110. https://doi.org/10.3390/biology14091110







