Leaf Nitrogen Allocation Trade-Offs Promote Efficient Utilization of Different Nitrogen Forms in Hemarthria altissima
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Growth Conditions
2.2. Gas Exchange Measurements and Chlorophyll Fluorescence
2.3. Sample Collection and Chemical Analyses
2.4. Estimation of Nitrogen Allocation in the Photosynthetic Machinery and Its Efficiency in Photosynthetic Nitrogen Utilization
2.5. Statistical Analysis
3. Results
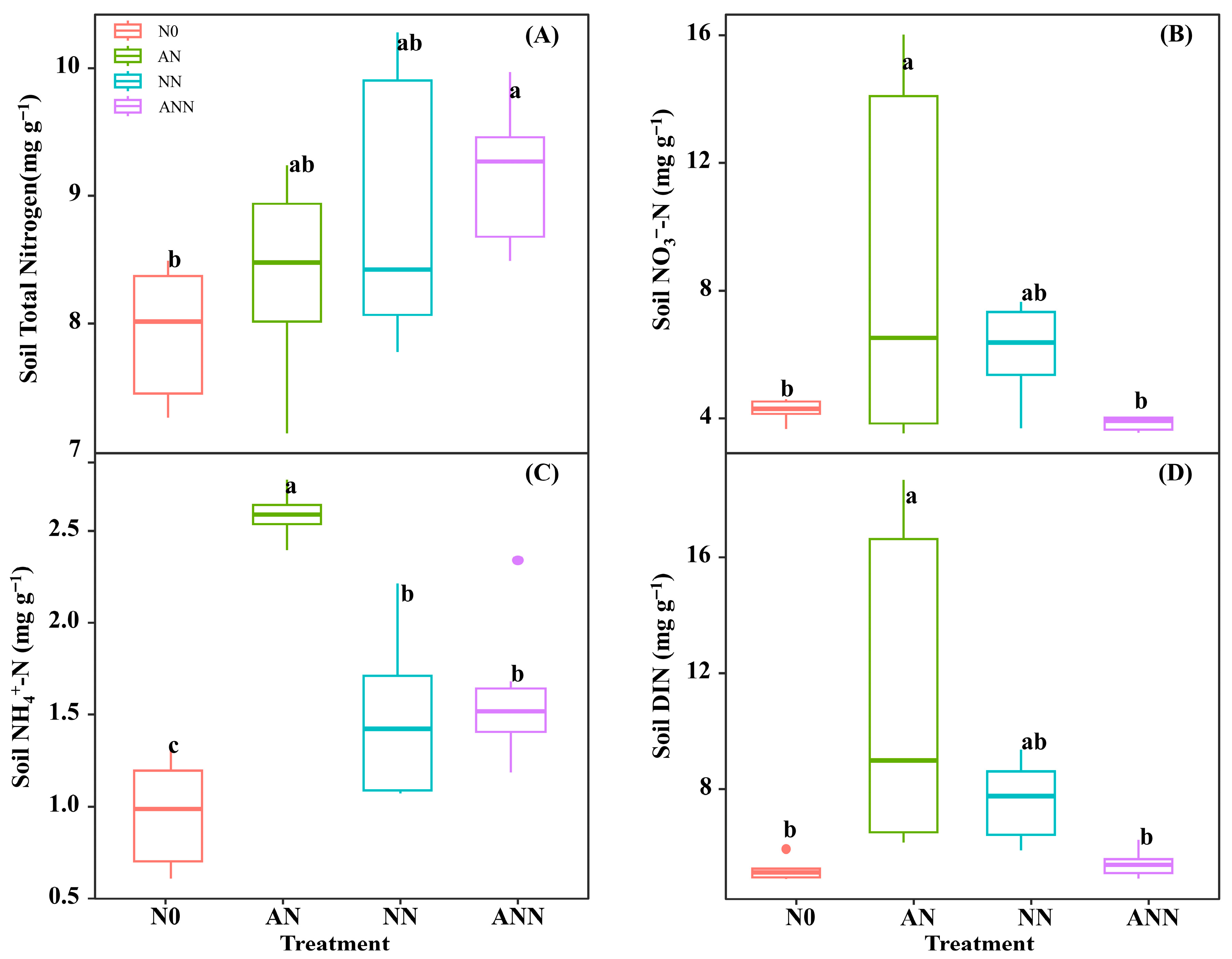
3.1. Soil Nitrogen Characteristics
3.2. Leaf Nitrogen Assimilation Enzyme Activity
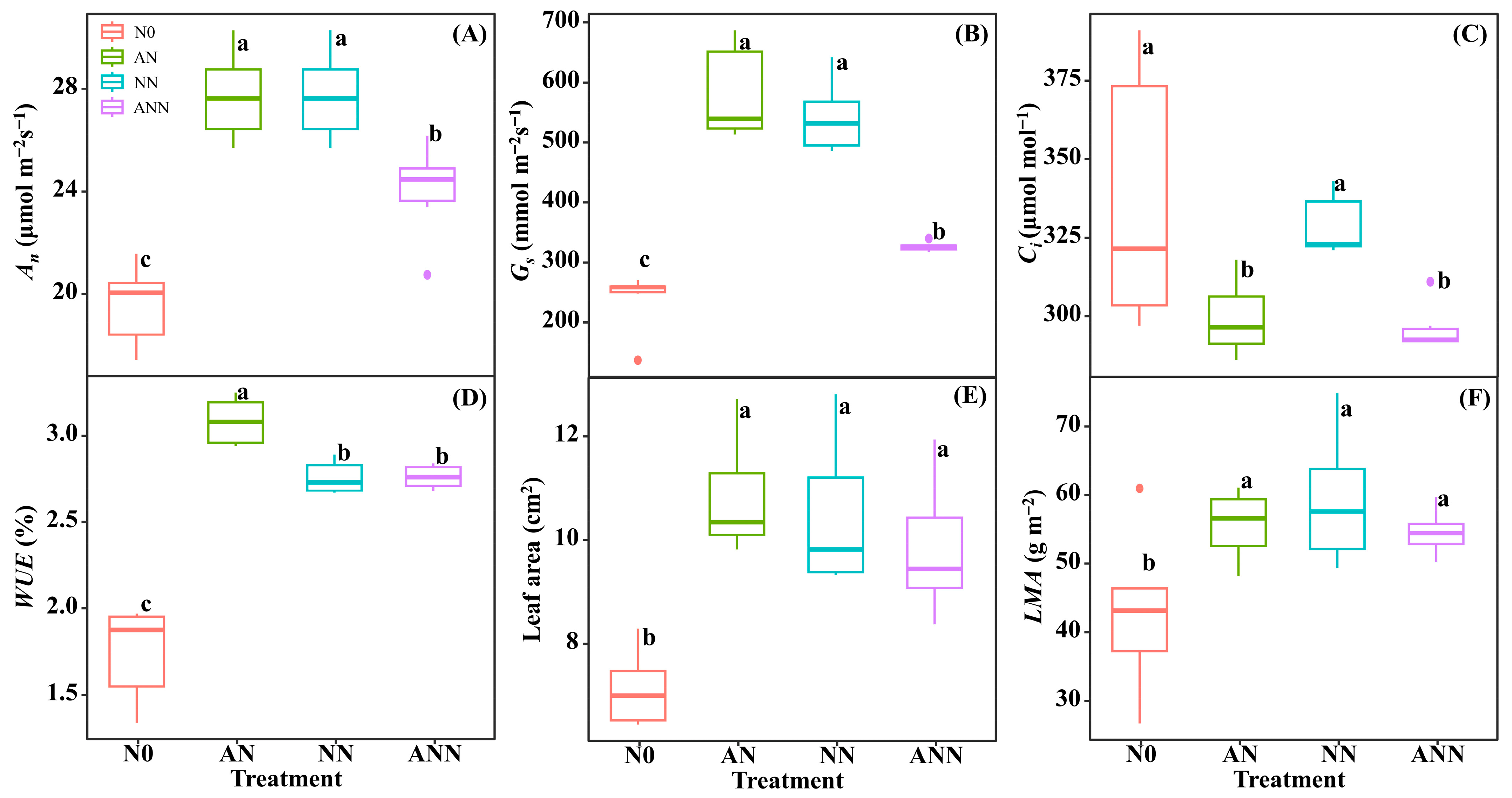
3.3. Leaf Gas Exchange Parameters and Morphological Characteristics
3.4. Leaf Photosynthetic Pigment
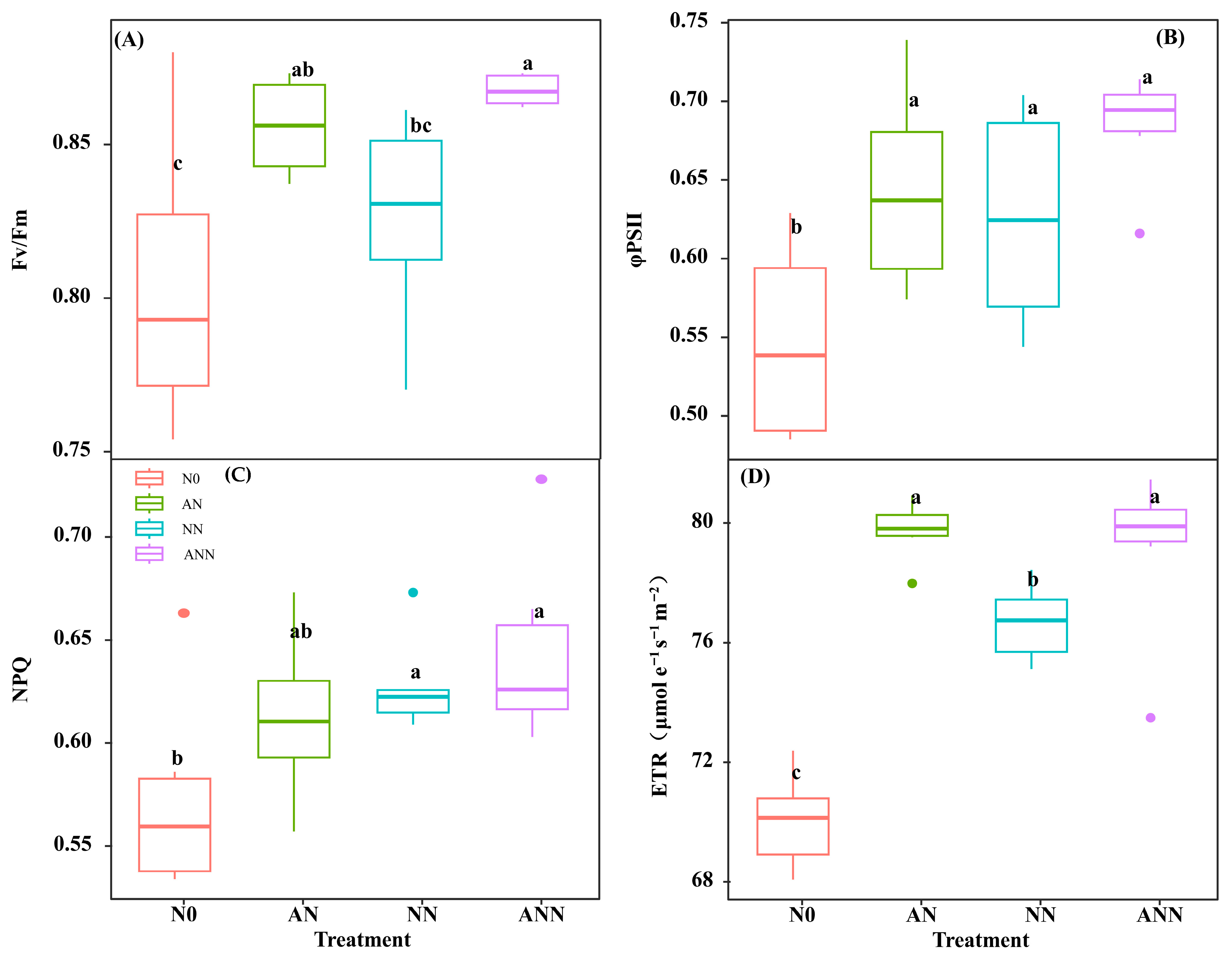
3.5. Leaf Photosynthetic Efficiency and Photosynthetic Nitrogen Utilization Efficiency
3.6. Leaf Photosynthetic Enzyme Activity
3.7. Within-Leaf Nitrogen Allocation Estimate
3.8. PSII Quantum Efficiencies
4. Discussion
4.1. Effects of Different Forms of Nitrogen on the Nitrogen Source and Nitrogen Metabolic Enzyme Activity in H. altissima
4.2. Effects of Different Forms of Nitrogen on Leaf Morphological Traits and Gas Exchange in H. altissima
4.3. Effects of Different Forms of Nitrogen on Leaf Photosynthetic Performance and Photosynthetic Nitrogen Use Efficiency in H. altissima
4.4. Effects of Different Forms of Nitrogen on Leaf Nitrogen Allocation and Trade-Offs in H. altissima
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| An | Assimilation rate |
| Gs | Stomatal conductance |
| Ci | Internal CO2 concentration |
| WUE | Water-use efficiency |
| Vcmax | Maximum rate of Rubisco carboxylation |
| Jmax | Maximum rate of electron transport |
| LMA | A leaf mass per unit leaf area |
| Narea | A leaf nitrogen content per unit leaf area |
| NC | Nitrogen contents in carboxylation |
| NB | Nitrogen contents in bioenergetics |
| NL | Nitrogen contents in light harvesting components |
| Npsn | Nitrogen contents in photosynthetic apparatus |
| PNUE | Photosynthetic nitrogen use efficiency |
| Fv/Fm | The maximum quantum yield of PSII |
| NPQ | The non-photochemical quenching coefficient |
| φPSII | The effective quantum yield of PSII |
| ETR | The electron transport rate |
References
- Liang, X.; Zhang, T.; Lu, X.; Ellsworth, D.S.; BassiriRad, H.; You, C.; Wang, D.; He, P.; Deng, Q.; Liu, H. Global response patterns of plant photosynthesis to nitrogen addition: A meta-analysis. Glob. Change Biol. 2020, 26, 3585–3600. [Google Scholar] [CrossRef]
- Xia, J.; Wan, S. Global response patterns of terrestrial plant species to nitrogen addition. New Phytol. 2008, 179, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Hessini, K.; Hamed, K.B.; Gandour, M.; Mejri, M.; Abdelly, C.; Cruz, C. Ammonium nutrition in the halophyte Spartina alterniflora under salt stress: Evidence for a priming effect of ammonium? Plant Soil 2013, 370, 163–173. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, H.; Liu, H.; Yang, L.; Mi, G.; Wang, P. Enhancing Crop Nitrogen Efficiency: The Role of Mixed Nitrate and Ammonium Supply in Plant Growth and Development. Biology 2025, 14, 546. [Google Scholar] [CrossRef] [PubMed]
- Prinsi, B.; Espen, L. Time-Course of Metabolic and Proteomic Responses to Different Nitrate/Ammonium Availabilities in Roots and Leaves of Maize. Int. J. Mol. Sci. 2018, 19, 2202. [Google Scholar] [CrossRef]
- Li, Z.; Gao, W.; Jiang, C.; Lu, Y.; Kamran, M.; Yang, X. Appropriate nitrogen application rate with decreased basal/topdressing ratio improves yield, quality, water productivity, and N-use efficiency of forage maize in a rainfed region. Agric. Water Manag. 2025, 317, 109629. [Google Scholar] [CrossRef]
- Cao, L.; Yan, C.; He, T.; Zhong, Q.; Yuan, Y.; Cao, L. Coordinated Regulation of Photosynthesis, Stomatal Traits, and Hormonal Dynamics in Camellia oleifera During Drought and Rehydration. Biology 2025, 14, 965. [Google Scholar] [CrossRef]
- Onoda, Y.; Wright, I.J.; Evans, J.R.; Hikosaka, K.; Kitajima, K.; Niinemets, Ü.; Poorter, H.; Tosens, T.; Westoby, M. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 2017, 214, 1447–1463. [Google Scholar] [CrossRef]
- Sugiura, D.; Terashima, I.; Evans, J.R. A decrease in mesophyll conductance by cell-wall thickening contributes to photosynthetic downregulation. Plant Physiol. 2020, 183, 1600–1611. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiao, J.; Liu, S.; He, T.; Rong, J.; Zheng, Y. Effect of Nitrogen Application Rate on Growth Physiology, Yield Quality, and Nitrogen Fertilizer Utilization Rate of Liriope muscari in Pots. Biology 2025, 14, 1104. [Google Scholar] [CrossRef]
- Wei, X.; Han, L.; Xu, N.; Sun, M.; Yang, X. Nitrate nitrogen enhances the efficiency of photoprotection in Leymus chinensis under drought stress. Front. Plant Sci. 2024, 15, 1348925. [Google Scholar] [CrossRef]
- Li, T.; Yang, L.; Zhao, D.; Li, T.; Liu, Y.; Wang, X. Induced photosynthesis significantly influences biomass in Betula platyphylla seedlings compared to steady-state photosynthesis under different nitrogen forms. Plant Physiol. Biochem. 2025, 228, 110225. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, J.; Ao, Y.; Han, J.; Guo, Z.; Liu, X.; Zhang, J.; Mu, C.; Le Roux, X. Responses of soil N2O emissions and their abiotic and biotic drivers to altered rainfall regimes and co-occurring wet N deposition in a semi-arid grassland. Glob. Change Biol. 2021, 27, 4894–4908. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Xu, Y.; Meng, B.; Loik, M.E.; Ma, J.-Y.; Sun, W. Nitrogen addition increases the sensitivity of photosynthesis to drought and re-watering differentially in C3 versus C4 grass species. Front. Plant Sci. 2019, 10, 815. [Google Scholar] [CrossRef]
- Wei, X.; Yang, Y.; Yao, J.; Han, J.; Yan, M.; Zhang, J.; Shi, Y.; Wang, J.; Mu, C. Improved utilization of nitrate nitrogen through within-leaf nitrogen allocation trade-offs in Leymus chinensis. Front. Plant Sci. 2022, 13, 870681. [Google Scholar] [CrossRef]
- Stinziano, J.R.; Morgan, P.B.; Lynch, D.J.; Saathoff, A.J.; McDermitt, D.K.; Hanson, D.T. The rapid A–Ci response: Photosynthesis in the phenomic era. Plant Cell Environ. 2017, 40, 1256–1262. [Google Scholar] [CrossRef]
- Von Caemmerer, S. Biochemical Models of Leaf Photosynthesis; Csiro Publishing: Canberra, Australia, 2000. [Google Scholar]
- Long, S.P.; Bernacchi, C.J. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 2003, 54, 2393–2401. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Niu, D.; Liu, X. Effects of abiotic stress on chlorophyll metabolism. Plant Sci. 2024, 342, 112030. [Google Scholar] [CrossRef] [PubMed]
- Garmay, A.V.; Oskolok, K.V.; Monogarova, O.V.; Demidov, M.I. Determination of ammonium and nitrate in soils by digital colorimetry. Environ. Monit. Assess. 2024, 196, 948. [Google Scholar] [CrossRef]
- de Castro, T.A.V.T.; Berbara, R.L.L.; Tavares, O.C.H.; da Graca Mello, D.F.; Pereira, E.G.; de Souza, C.d.C.B.; Espinosa, L.M.; García, A.C. Humic acids induce a eustress state via photosynthesis and nitrogen metabolism leading to a root growth improvement in rice plants. Plant Physiol. Biochem. 2021, 162, 171–184. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Chen, Y.; Jiang, S.; Ye, J.; Ding, P.; Shao, X. Alginate oligosaccharide alleviates chilling injury in postharvest peach fruit via regulating antioxidant and membrane lipid metabolisms. Plant Physiol. Biochem. 2025, 228, 110292. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Hu, W.; Zhou, S.; Robakowski, P.; Kang, H.; An, T.; Wang, F.; Xiao, Y.; Yang, X. Limitations of the Farquhar–von Caemmerer–Berry Model in Estimating the Maximum Electron Transport Rate: Evidence from Four C3 Species. Biology 2025, 14, 630. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Fujiki, H.; Ozaki, K.; Yanahara, S.; Takeuchi, N.; Suzuki, Y.; Sugiyama, T.; Makino, A.; Ookawa, T.; Hirasawa, T.; et al. Decrease of cytokinin flux from roots enhances degradation of ribulose-1, 5-bisphosphate carboxylase/oxygenase: A mechanism of the accelerated decrease of leaf photosynthesis with senescence under soil moisture stress in rice (Oryza sativa L.). Plant Soil 2024, 496, 391–411. [Google Scholar] [CrossRef]
- Rudenko, N.N. Photosynthesis and Carbon Metabolism in Higher Plants and Algae. Plants 2025, 14, 2161. [Google Scholar] [CrossRef]
- Hikosaka, K.; Terashima, I. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ. 1995, 18, 605–618. [Google Scholar] [CrossRef]
- Poorter, H.; Evans, J.R. Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 1998, 116, 26–37. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen journey in plants: From uptake to metabolism, stress response, and microbe interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Hu, C.-C.; Liu, X.-Y.; Driscoll, A.W.; Kuang, Y.-W.; Brookshire, E.J.; Lü, X.-T.; Chen, C.-J.; Song, W.; Mao, R.; Liu, C.-Q. Global distribution and drivers of relative contributions among soil nitrogen sources to terrestrial plants. Nat. Commun. 2024, 15, 6407. [Google Scholar] [CrossRef]
- Ding, S.; Shao, X.; Li, J.; Ahammed, G.J.; Yao, Y.; Ding, J.; Hu, Z.; Yu, J.; Shi, K. Nitrogen forms and metabolism affect plant defence to foliar and root pathogens in tomato. Plant Cell Environ. 2021, 44, 1596–1610. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Wang, J.; Zhang, M.; Liu, W.; Li, X.; Lin, X. Improving Leaf GOGAT Activity After the Post-Silking Period Contributes to High Grain Yield with Reduced Nitrogen in N-Efficient Maize. Agronomy 2025, 15, 1379. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, Y.; Liu, Z.; Cai, M.; Pan, H.; Zhang, Q. Different responses of Lagerstroemia indica to varied supplies of ammonium and nitrate. Sci. Hortic. 2024, 329, 113001. [Google Scholar] [CrossRef]
- Salesse-Smith, C.E.; Wang, Y.; Long, S.P. Increasing Rubisco as a simple means to enhance photosynthesis and productivity now without lowering nitrogen use efficiency. New Phytol. 2025, 245, 951–965. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Ni, W.; Liu, C.; Qin, H.; Guan, Y.; Liu, J.; Feng, Z.; Xing, Y.; Tian, G.; et al. Nitrogen-potassium balance improves leaf photosynthetic capacity by regulating leaf nitrogen allocation in apple. Hortic. Res. 2024, 11, uhad253. [Google Scholar] [CrossRef]
- Peng, W.; Zhang, Y.; Yi, S.H. Increasing nitrogen absorption and assimilation ability under mixed NO3− and NH4+ supply is a driver to promote growth of maize seedlings. J. Integr. Agric. 2023, 22, 1896–1908. [Google Scholar]
- Mu, X.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Within-leaf nitrogen allocation in adaptation to low nitrogen supply in maize during grain-filling stage. Front. Plant Sci. 2016, 7, 699. [Google Scholar] [CrossRef]
- Feng, H.; Guo, J.; Peng, C.; Kneeshaw, D.; Roberge, G.; Pan, C.; Ma, X.; Zhou, D.; Wang, W. Nitrogen addition promotes terrestrial plants to allocate more biomass to aboveground organs: A global meta-analysis. Glob. Change Biol. 2023, 29, 3970–3989. [Google Scholar] [CrossRef]
- Feng, Y.L. Nitrogen allocation and partitioning in invasive and native Eupatorium species. Physiol. Plant. 2008, 132, 350–358. [Google Scholar] [CrossRef]
- Feng, Y.L.; Lei, Y.B.; Wang, R.F.; Callaway, R.M.; Valiente-Banuet, A.; Inderjit; Li, Y.P.; Zheng, Y.L. Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proc. Natl. Acad. Sci. USA 2009, 106, 1853–1856. [Google Scholar] [CrossRef]
- Liu, T.; Ren, T.; White, P.J.; Cong, R.; Lu, J. Storage nitrogen co-ordinates leaf expansion and photosynthetic capacity in winter oilseed rape. J. Exp. Bot. 2018, 69, 2995–3007. [Google Scholar] [CrossRef] [PubMed]






| N Form Treatments | Chla (mg g−1) | Chlb (mg g−1) | Chlm (mg g−1) | Chlarea (g m−2) |
|---|---|---|---|---|
| N0 | 0.80 ± 0.06 a | 0.52 ± 0.01 c | 1.32 ± 0.06 c | 0.55 ± 0.04 b |
| AN | 0.73 ± 0.07 ab | 0.97 ± 0.06 a | 1.71 ± 0.05 a | 0.95± 0.05 a |
| NN | 0.79 ± 0.05 ab | 0.69± 0.05 bc | 1.48 ± 0.05 b | 0.88± 0.07 a |
| ANN | 0.59 ± 0.09 b | 0.88 ± 0.11 ab | 1.42 ± 0.05 bc | 0.81 ± 0.04 a |
| Parameters (mg m−2) | Nitrogen Forms Treatments | |||
|---|---|---|---|---|
| N0 | AN | NN | ANN | |
| Rubisco | 83.38 ± 0.38 c | 105.53 ± 0.36 a | 97.63 ± 0.34 b | 107.72 ± 0.29 a |
| Other soluble protein | 473.66 ± 1.86 b | 502.66 ± 2.45 a | 427.35 ± 4.26 c | 401.33 ± 4.28 d |
| Carboxylation | 249.86 ± 6.41 c | 418.06 ± 20.32 a | 227.81 ± 18.91 d | 384.15 ± 23.19 b |
| Bioenergetics | 72.75 ± 1.82 c | 86.37 ± 1.29 b | 108.23 ± 2.86 a | 99.22 ± 4.16 ab |
| Light-harvesting protein | 318.74 ± 5.18 d | 412.23 ± 7.82 c | 482.08 ± 12.94 a | 444.80 ± 11.37 b |
| Other nitrogen | 338.6 ± 9.16 d | 427.35 ± 21.38 c | 466.18 ± 22.35 b | 502.34 ± 29.34 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, N.; Wei, X.; Zhang, J.; Sun, M.; Zhang, J.; Zhao, Z.; Yang, X. Leaf Nitrogen Allocation Trade-Offs Promote Efficient Utilization of Different Nitrogen Forms in Hemarthria altissima. Biology 2025, 14, 1260. https://doi.org/10.3390/biology14091260
Xu N, Wei X, Zhang J, Sun M, Zhang J, Zhao Z, Yang X. Leaf Nitrogen Allocation Trade-Offs Promote Efficient Utilization of Different Nitrogen Forms in Hemarthria altissima. Biology. 2025; 14(9):1260. https://doi.org/10.3390/biology14091260
Chicago/Turabian StyleXu, Nan, Xiaowei Wei, Ju Zhang, Mingyue Sun, Jinwei Zhang, Zihao Zhao, and Xuechen Yang. 2025. "Leaf Nitrogen Allocation Trade-Offs Promote Efficient Utilization of Different Nitrogen Forms in Hemarthria altissima" Biology 14, no. 9: 1260. https://doi.org/10.3390/biology14091260
APA StyleXu, N., Wei, X., Zhang, J., Sun, M., Zhang, J., Zhao, Z., & Yang, X. (2025). Leaf Nitrogen Allocation Trade-Offs Promote Efficient Utilization of Different Nitrogen Forms in Hemarthria altissima. Biology, 14(9), 1260. https://doi.org/10.3390/biology14091260







