Plant Disease Suppressiveness Enhancement via Soil Health Management
Simple Summary
Abstract
1. Introduction
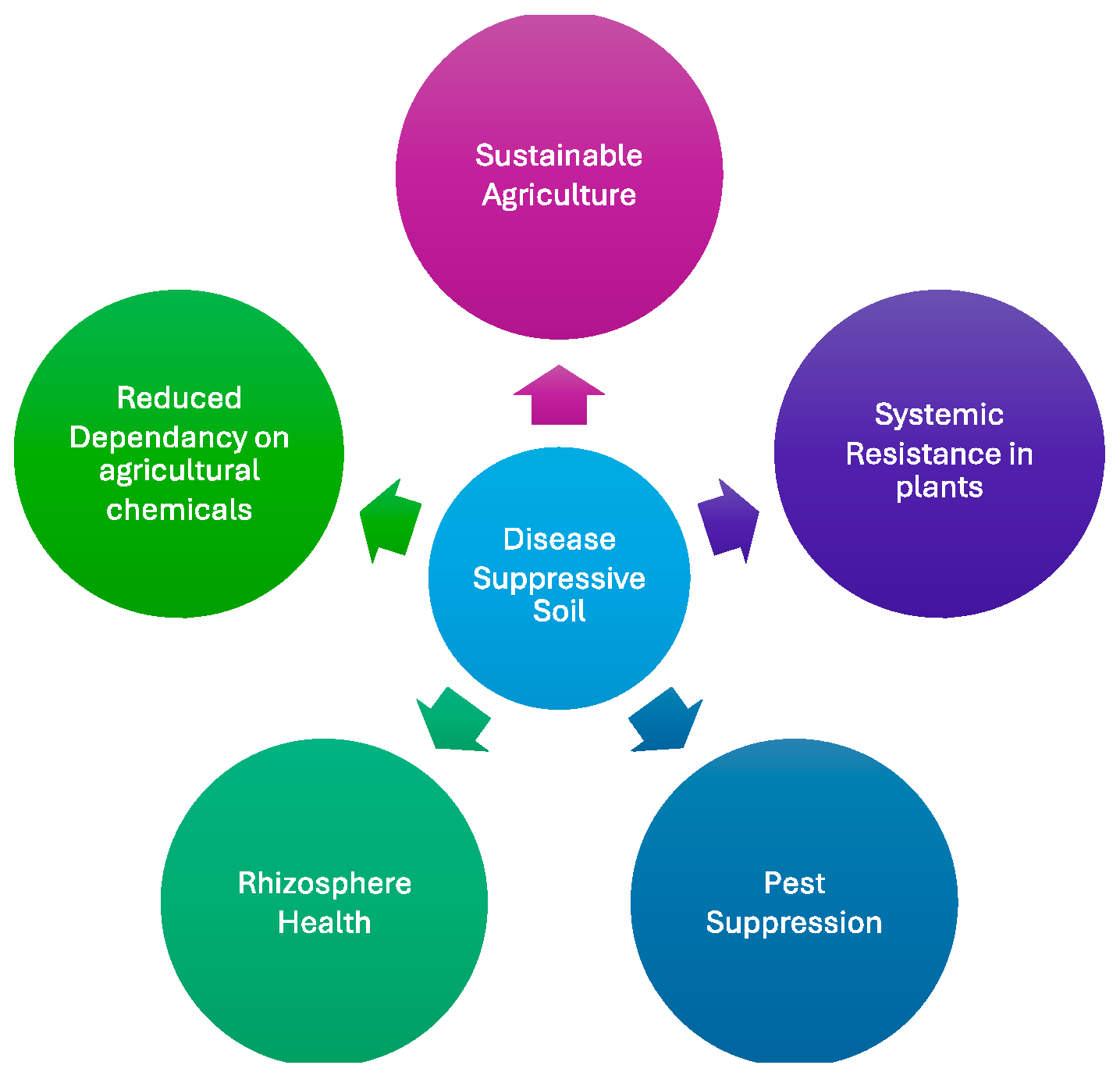
- What is Disease-Suppressive Soil?
1.1. Types of Disease-Suppressive Soils
1.2. History of Disease-Suppressive Soils
- Early Observations and Foundations
- ▪
- Pre–1900s
- Emergence of Modern Disease Suppressiveness Research
- ▪
- Mid–20th Century
- Expansion of the Concept: General vs. Specific Suppressiveness
- ▪
- 1970s–1980s
- Advances in Microbial Ecology and Molecular Techniques
- ▪
- 1990s–2000s
- Disease-Suppressive Soils and Sustainable Agriculture
- ▪
- 2000s–Present
2. Diverse and Dynamic Mechanisms of the Pathogen Inhibition Caused by Disease-Suppressive Soils
2.1. General Suppression Mechanisms (GSMs) and Specific Suppression Mechanisms (SSMs)
2.1.1. General Antibiosis (Production of General Antibiotics)
General Antibiosis
Competition
2.1.2. Specific Suppression Mechanisms (SSMs) Refers to the Targeted Biological Processes in Disease-Suppressive Soils That Inhibit Specific Soil-Borne Pathogens Through Different Mechanisms
Specific Antibiosis (Production of Specific Antimicrobial Substances)
Predation
Parasitism
Induced Systemic Resistance (ISR) of Plants Against Pathogens Activated by Beneficial Microbes in Soils
2.2. Reprogramming of Microbial Community in Soil
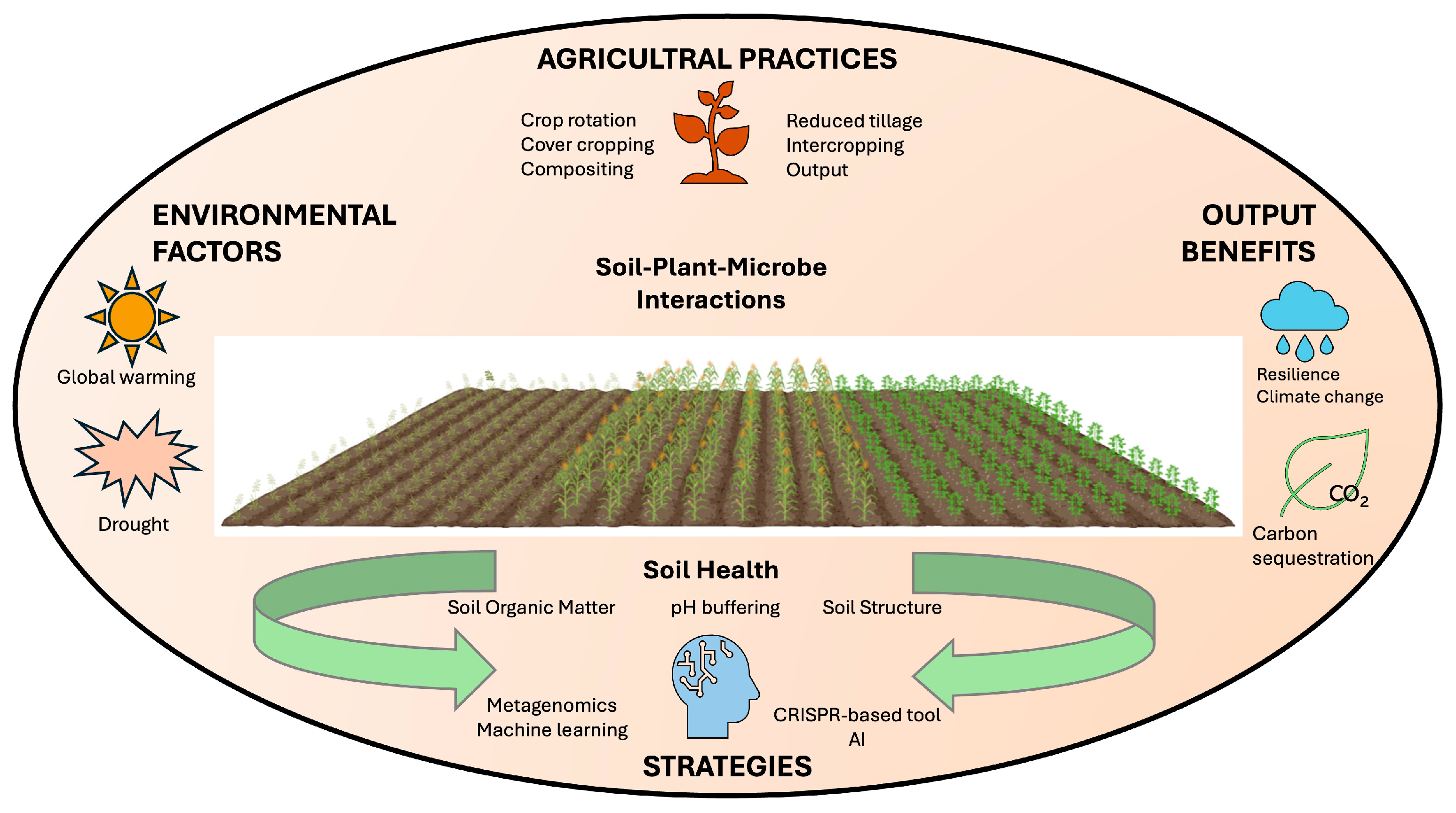
2.3. Soil-Driven Mechanisms of Disease Suppression in Soils
3. Soil Health Parameters Affecting the Activities of Disease Suppression
3.1. Soil pH and Soil Electrical Conductivity (EC)
3.2. Soil Temperature and Moisture
3.3. Soil Clay Content and Soil Aggregation
3.4. Soil Carbon and Nitrogen Pools and Stocks
3.5. Soil Respiration
4. Disease-Suppressive Soils—Management Trends
5. Limitations of Disease-Suppressive Soils
6. Conclusions and Future Insights
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMF | Arbuscular Mycorrhizal Fungi |
| ASD | Anaerobic Soil Disinfestation |
| BNF | Biological Nitrogen Fixation |
| C | Carbon |
| CEC | Cation Exchange Capacity |
| Cmic | Microbial Biomass Carbon |
| DAPG | 2,4-Diacetylphoroglucinol |
| DSS | Disease-Suppressive Soils |
| EC | Electrical Conductivity |
| ET | Ethylene |
| FoC | Fusarium oxysporum sp. cubense |
| GDS | General Disease Suppression |
| GSMs | General Suppression Mechanisms |
| HCN | Hydrogen Cyanide |
| HR | Hypersensitive Response |
| IPM | Integrated Pest Management |
| ISFM | Integrated Soil Fertility Management |
| ISR | Induced Systemic Resistance |
| JA | Jasmonic Acid |
| KSB | Potassium-Solubilizing Bacteria |
| N | Nitrogen |
| OAS | Organic Amendments |
| OM | Organic Matter |
| PGPM | Plant Growth-Promoting Microorganisms |
| PGPR | Plant Growth-Promoting Rhizobacteria |
| POC | Particulate Organic Matter |
| POM-C | Particulate Organic Matter Carbon |
| POX-C | Permanganate Oxidizable Carbon |
| ROS | Reactive Oxygen Species |
| SA | Salicylic Acid |
| SAR | Systemic Acquired Resistance |
| SDS | Specific Disease Suppression |
| SOM | Soil Organic Matter |
| SSMs | Specific Suppression Mechanisms |
| USDA | The United States Department of Agriculture |
References
- Liu, C.; Zhang, M.; Wang, H. Impact of soil-borne pathogens on crop yield and plant health. Plant Pathol. J. 2023, 15, 89–102. [Google Scholar]
- Yang, F.; Zhang, G.; Li, X. Soil-borne diseases and their effects on soil microbial communities. Environ. Soil Sci. 2024, 13, 234–245. [Google Scholar]
- Zhao, J.; Wang, Z.; Zhang, H. Role of disease-suppressive soils in managing soil-borne pathogens. Soil Biol. Biochem. 2024, 75, 56–68. [Google Scholar]
- Vurro, M.; Bonciani, B.; Vannacci, G. Emerging infectious diseases of crop plants in developing countries: Impact on agriculture and socio-economic consequences. Food Secur. 2010, 2, 113–132. [Google Scholar] [CrossRef]
- Karamura, E.; Kayobyo, G.; Tushemereirwe, W.; Benin, S.; Blomme, G.; Eden Green, S.; Markham, R. Assessing the Impacts of Banana Bacterial Wilt Disease on Banana (musa spp.) Productivity and Livelihoods of Ugandan Farm Households. Acta Hortic. 2010, 879, 749–755. [Google Scholar] [CrossRef]
- Kalyebara, M.R.; Ragama, P.E.; Kikulwe, E.; Bagamba, F.; Nankinga, K.C.; Tushemereirwe, W.K. Economic importance of the banana bacterial wilt in Uganda. Afr. Crop Sci. J. 2007, 14, 93–104. [Google Scholar] [CrossRef]
- Xu, Y.; Li, T.; Zhang, L. Microbial communities and their role in disease suppression in agricultural soils. Microbiol. Res. 2024, 12, 121–136. [Google Scholar]
- Wang, L.; Sun, Y.; Li, Q. Soil properties and their influence on disease-suppressive soils. Soil Health Sustain. 2023, 11, 45–58. [Google Scholar]
- Baker, K.F.; Cook, R.J. Biological Control of Plant Pathogens; W.H. Freeman and Company: New York, NY, USA, 1974. [Google Scholar]
- Goh, Y.K.; Zoqratt, M.Z.H.M.; Goh, Y.K.; Ayub, Q.; Ting, A.S.Y. Discovering naturally occurring microbiota in disease suppressive soil: Potential role of biological elements in suppressing Ganoderma boninense. Biol. Control 2022, 165, 104787. [Google Scholar] [CrossRef]
- Almario, J.; Kyselková, M.; Kopecký, J.; Ságová-Marečková, M.; Muller, D.; Grundmann, G.L.; Moënne-Loccoz, Y. Assessment of the relationship between geologic origin of soil, rhizobacterial community composition and soil receptivity to tobacco black root rot in Savoie region (France). Plant Soil 2013, 371, 397–408. [Google Scholar] [CrossRef]
- Almario, J.; Muller, D.; Défago, G.; Moënne-Loccoz, Y. Rhizosphere ecology and phytoprotection in soils naturally suppressive to Thielaviopsis black root rot of tobacco. Environ. Microbiol. 2014, 16, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, N.; Tiedje, J.M.; Quensen, J.F.; Meng, Q.; Hao, J.J. Microbial communities associated with potato common scab-suppressive soil determined by pyrosequencing analyses. Plant Dis. 2012, 96, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Delitte, M.; Caulier, S.; Bragard, C.; Desoignies, N. Plant microbiota beyond farming practices: A review. Front. Sustain. Food Syst. 2021, 5, 62. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Y.; Lin, Z.; Tuo, Y.; Li, H.; Wang, Y. Differences in soil microbial community composition between suppressive and root rotconducive in tobacco fields. Curr. Microbiol. 2021, 78, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Döring, T.F.; Rosslenbroich, D.; Giese, C.; Athmann, M.; Watson, C.; Vágó, I.; Kátai, J.; Tállai, M.; Bruns, C. Disease suppressive soils vary in resilience to stress. Appl. Soil Ecol. 2020, 149, 103482. [Google Scholar] [CrossRef]
- Cao, Y.; Shen, Z.; Zhang, N.; Deng, X.; Thomashow, L.S.; Lidbury, I.; Liu, H.; Li, R.; Shen, Q.; Kowalchuk, G.A. Phosphorus availability influences disease-suppressive soil microbiome through plant-microbe interactions. Microbiome 2024, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, M.; Wang, H. Disease-Suppressive Soils Induce Systemic Resistance in Arabidopsis thaliana Against Pseudomonas syringae pv. Tomato. PhytoFrontiers 2024, 114, 234–245. Available online: https://apsjournals.apsnet.org/doi/10.1094/PHYTOFR-02-24-0012-R (accessed on 13 June 2025).
- Gómez Expósito, R.; de Bruijn, I.; Postma, J.; Raaijmakers, J.M. Current insights into the role of rhizosphere bacteria in disease suppressive soils. Front. Microbiol. 2017, 8, 2529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.; Chen, J. Suppression of leaf-feeding pests by microbiomes in disease-suppressive soils. Microbiome 2024, 13, 45–60. [Google Scholar]
- Kwak, Y.S.; Weller, D.M. Take-all of Wheat and Natural Disease Suppression: A Review. Plant Pathol. J. 2013, 29, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Larkin, R.P.; Fravel, D.R. Efficacy of Various Fungal and Bacterial Biocontrol Organisms for Control of Fusarium Wilt of Tomato. Plant Dis. 1998, 82, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- De Troyer, L.; Audenaert, K.; Ommeslag, S.; Debode, J.; De Gelder, L.; De Zutter, N. The biocontrol agent Streptomyces rimosus subsp. rimosus tempers shifts in the wheat spicosphere microbiome induced by Fusarium Head Blight. Front. Plant Sci. 2025, 16, 154. [Google Scholar] [CrossRef] [PubMed]
- Larkin, R.P.; Fravel, D.R. Development of disease-suppressive soils through crop rotation and organic amendment applications. Phytopathology 2002, 92, 1271–1278. [Google Scholar]
- Cook, R.J.; Baker, K.F. The Nature and Practice of Biological Control of Plant Pathogens. In American Phytopathological Society; American Phytopathological Society: St. Paul, MN, USA, 1983. [Google Scholar]
- Mazzola, M. Soil suppressiveness to soilborne diseases. Soil Biol. Biochem. 2002, 34, 435–444. [Google Scholar]
- Löffler, W. Untersuchungen über die Fusariosen der Tomatenpflanze. Z. Für Pflanzenkrankheiten 1887, 2, 391–422. [Google Scholar]
- Van Wees, S.C.M.; Van der Ent, S.; Pieterse, C.M.J. Plant growth-promoting rhizobacteria (PGPR) and their role in plant stress tolerance. Soil Biol. Biochem. 2008, 40, 2592–2600. [Google Scholar] [CrossRef]
- Glick, B.R. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 1995, 41, 109–117. [Google Scholar] [CrossRef]
- Zhang, J.; Mavrodi, D.V.; Yang, M.; Thomashow, L.S.; Mavrodi, O.V.; Kelton, J.; Weller, D.M. Pseudomonas synxantha 2-79 Transformed with Pyrrolnitrin Biosynthesis Genes Has Improved Biocontrol Activity Against Soilborne Pathogens of Wheat and Canola. Phytopathology 2020, 110, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Harwoko, H.; Daletos, G.; Stuhldreier, F.; Lee, J.; Wesselborg, S.; Feldbrügge, M.; Müller, W.E.G.; Kalscheuer, R.; Ancheeva, E.; Proksch, P. Dithiodiketopiperazine derivatives from endophytic fungi Trichoderma harzianum and Epicoccum nigrum. Nat. Prod. Res. 2019, 35, 257–265. [Google Scholar] [CrossRef] [PubMed]
- West, S.A.; Cooper, G.A. Division of labour in microorganisms: An evolutionary perspective. Nat. Rev. Microbiol. 2016, 14, 716–723. [Google Scholar] [CrossRef] [PubMed]
- García-Bayona, L.; Comstock, L.E. Bacterial antagonism in host-associated microbial communities. Science 2018, 361, eaat2456. [Google Scholar] [CrossRef] [PubMed]
- Braga, L.P.P.; Spor, A.; Kot, W.; Breuil, M.-C.; Hansen, L.H.; Setubal, J.C.; Philippot, L. Impact of phages on soil bacterial communities and nitrogen availability under different assembly scenarios. Microbiome 2020, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Van der Waals, J.E.; Mostert, L. Competition between plant pathogens and beneficial microorganisms in Soil. Plant Pathol. J. 2008, 24, 339–354. [Google Scholar]
- Wei, Z.; Yang, T.; Friman, V.-P.; Xu, Y.; Shen, Q.; Jousset, A. Trophic network architecture of root-associated bacterial communities determines pathogen invasion and plant health. Nat. Commun. 2015, 6, 8413. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, L.; Chen, Q.; Liu, L. The role of mycorrhizal fungi in carbon cycling and sequestration. Soil Biol. Biochem. 2016, 94, 1–8. [Google Scholar] [CrossRef]
- Niu, B.; Wang, W.; Yuan, Z.; Sederoff, R.R.; Sederoff, H.; Chiang, V.L.; Borriss, R. Microbial Interactions Within Multiple-Strain Biological Control Agents Impact Soil-Borne Plant Disease. Front. Microbiol. 2020, 11, 585404. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Modi, D.; Picot, A. Soil and Phytomicrobiome for Plant Disease Suppression and Management under Climate Change: A Review. Plants 2023, 12, 2736. [Google Scholar] [CrossRef] [PubMed]
- Schlatter, D.; Kinkel, L.; Thomashow, L.; Weller, D.; Paulitz, T. Disease Suppressive Soils: New Insights from the Soil Microbiome. Phytopathology 2017, 107, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Weller, D.M.; Raaijmakers, J.M.; Gardener, B.B.; Thomashow, L.S. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 2002, 40, 309–348. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Bishnoi, S.K.; Chandra, S. Assessment of antibiosis potential of Bacillus sp. against the soil-borne fungal pathogen Sclerotium rolfsii Sacc. (Athelia rolfsii (Curzi) Tu & Kimbrough). Egypt. J. Biol. Pest Control 2021, 31, 17. [Google Scholar] [CrossRef]
- Ferris, H.; Mullaney, M.J. The role of soil nematodes in plant disease suppression: A review. Soil Biol. Biochem. 2007, 39, 2145–2154. [Google Scholar] [CrossRef]
- Gadd, G.M. Fungal production of volatile organic compounds. Mycol. Res. 2001, 105, 1393–1400. [Google Scholar] [CrossRef]
- Erktan, A.; Rillig, M.C.; Carminati, A.; Jousset, A.; Scheu, S. Protists and collembolans alter microbial community composition, C dynamics and soil aggregation in simplified consumer–prey systems. Biogeosciences 2020, 17, 4961–4980. [Google Scholar] [CrossRef]
- Blumenshine, S.C.; Hambright, K.D. Top-down control in pelagic systems: A role for invertebrate predation. Hydrobiologia 2003, 491, 347–356. [Google Scholar] [CrossRef]
- Larkin, R.P.; Fravel, D.R. Effects of Coniothyrium minitans on Sclerotinia disease and sclerotia in the Soil. Plant Dis. 1999, 83, 668–674. [Google Scholar]
- Menge, J.A.; Katan, J. Biological control of soilborne plant diseases. Annu. Rev. Phytopathol. 1989, 27, 195–214. [Google Scholar]
- Li, X.; Chen, D.; Carrión, V.J.; Revillini, D.; Yin, S.; Dong, Y.; Zhang, T.; Wang, X.; Delgado-Baquerizo, M. Acidification suppresses the natural capacity of soil microbiome to fight pathogenic Fusarium infections. Nat. Commun. 2023, 14, 5090. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zhao, Y.; Yuan, Y.; Li, J. Parasitism Shifts the Effects of Native Soil Microbes on the Growth of the Invasive Plant Alternanthera philoxeroides. Life 2023, 13, 150. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Oduor, A.M.O.; Yu, F.; Dong, M. A native parasitic plant and soil microorganisms facilitate a native plant co-occurrence with an invasive plant. Ecol. Evol. 2019, 9, 8652–8663. [Google Scholar] [CrossRef] [PubMed]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Verma, J.P. Does plant—Microbe interaction confer stress tolerance in plants: A review. Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant growth-promoting rhizobacteria for sustainable agricultural production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef] [PubMed]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of arbuscular mycorrhizal fungi on plant growth and performance: Importance in biotic and abiotic stressed regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Hu, Y.; Pandey, A.K.; Wu, X.; Fang, P.; Xu, P. The role of arbuscular mycorrhiza fungi in drought tolerance in legume crops: A review. Legume Res. Int. J. 2022, 45, 1–9. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Awasthi, A.; Soni, S.K.; Singh, R.; Verma, R.K.; Kalra, A. Complementarity among plant growth promoting traits in rhizospheric bacterial communities promotes plant growth. Sci. Rep. 2015, 5, 15500. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Liu, W.; Yuan, P.; Zhao, Z.; Zhang, C.; Opiyo, S.O.; Adhikari, A.; Zhao, L.; Harsh, G.; Xia, Y. Plant Growth Promotion and Stress Tolerance Enhancement through Inoculation with Bacillus proteolyticus OSUB18. Biology 2023, 12, 1495. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Zahra, N.; Hafeez, M.B.; Ahmad, M.; Iqbal, S.; Shaukat, K.; Ahmad, G. Nitrogen fixation of legumes: Biology and Physiology. In The Plant Family Fabaceae: Biology and Physiological Responses to Environmental Stresses; Springer: Berlin/Heidelberg, Germany, 2020; pp. 43–74. [Google Scholar]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects A review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Gangwar, M.; Saini, P.; Nikhanj, P.; Kaur, S. Plant growth-promoting microbes (PGPM) as potential microbial bio-agents for eco-friendly agriculture. In Advances in Soil Microbiology: Recent Trends and Future Prospects: Volume 2: Soil-Microbe-Plant Interaction; Springer: Berlin/Heidelberg, Germany, 2017; pp. 37–55. [Google Scholar]
- Dixit, R.; Kamat, S.; Srivastava, A.; Kumari, M. Molecular basis of plant-PGPM interactions during amelioration of biotic stress. In Microbial Biocontrol: Food Security and Post Harvest Management: Volume 2; Springer International Publishing: Cham, Switzerland, 2022; pp. 129–165. [Google Scholar]
- Ali, A.; Shah, L.; Rahman, S.; Riaz, M.W.; Yahya, M.; Xu, Y.J.; Liu, F.; Si, W.; Jiang, H.; Cheng, B. Plant defense mechanism and current understanding of salicylic acid and NPRs in activating SAR. Physiol. Mol. Plant Pathol. 2018, 104, 15–22. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced systemic resistance (ISR) in plants: Mechanism of action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Van der Ent, S.; Verhagen, B.W.M.; Van Doorn, R.; Bakker, D.; Verlaan, M.G.; Pel, M.J.C.; Joosten, R.G.; Proveniers, M.C.G.; Van Loon, L.C.; Ton, J. MYB72 is required in early signaling steps of rhizobacteria-induced systemic resistance in Arabidopsis. Plant Physiol. 2008, 146, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, U.; Chakraborty, B.; Basnet, M. Plant growth promotion and induction of resistance in Camellia sinensis by Bacillus megaterium. J. Basic. Microb. 2006, 46, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Van Wees, S.C.; Hoffland, E.; Van Pelt, J.A.; Van Loon, L.C. Systemic resistance in Arabidopsis induced by biocontrol bacteria is independent of salicylic acid accumulation and pathogenesis-related gene expression. Plant Cell 1996, 8, 1225–1237. [Google Scholar] [PubMed]
- Van der Ent, S.; Van Wees, S.C.; Pieterse, C.M. Jasmonate signaling in plant interactions with resistance-inducing beneficial microbes. Phytochemistry 2009, 70, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhao, Z.; Fan, J.; Liang, Y.; Bernier, M.C.; Gao, Y.; Zhao, L.; Opiyo, S.O.; Xia, Y. Bacillus proteolyticus OSUB18 triggers induced systemic resistance against bacterial and fungal pathogens in Arabidopsis. Front. Plant Sci. 2023, 14, 1078100. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, L.; Zeriouh, H.; Romero, D.; Cubero, J.; de Vicente, A.; Pérez-García, A. The antagonistic strain Bacillus acillus subtilis UMAF 6639 also confers protection to melon plants against cucurbit powdery mildew by activation of jasmonate-and salicylic acid-dependent defense responses. Microb. Biotechnol. 2013, 6, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, P.; Singh, A. Systemic Acquired Resistance vs Induced Systemic Resistance: A Review. Agric. Rev. 2024, 45, 410–419. [Google Scholar] [CrossRef]
- Vincent, M.N.; Harrison, L.A.; Brackin, J.M.; Kovacevich, P.A.; Mukerji, P.; Weller, D.M.; Pierson, E.A. Genetic Analysis of the Antifungal Activity of a Soilborne Pseudomonas Aureofaciens Strain. Appl. Environ. Microbiol. 1991, 57, 2928–2934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Qiao, X.; Fan, X.; Huo, X.; Zhang, D. Isolation and Yield Optimization of Lipopeptides from Bacillus subtilis Z-14 Active against Wheat Take-All Caused by Gaeumannomyces graminis Var. Tritici. J. Sep. Sci. 2021, 44, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Salgado, E.A.; Sánchez-Morán, A.G.; Rodríguez-Preciado, S.Y.; Sifuentes-Franco, S.; Rodríguez-Rodríguez, R.; Macías-Barragán, J.; Díaz-Zaragoza, M. Multifaceted Applications of Synthetic Microbial Communities: Advances in Biomedicine, Bioremediation, and Industry. Microbiol. Res. 2024, 15, 1709–1727. [Google Scholar] [CrossRef]
- Han, L.Z. Optimizing synthetic microbial communities for sustainable agriculture: Design, functionality, and field performance. Mol. Microbiol. Res. 2024, 14, 31–38. [Google Scholar] [CrossRef]
- Chaudhary, P.; Xu, M.; Ahamad, L.; Chaudhary, A.; Kumar, G.; Adeleke, B.S.; Verma, K.K.; Hu, D.-M.; Širić, I.; Kumar, P.; et al. Application of Synthetic Consortia for Improvement of Soil Fertility, Pollution Remediation, and Agricultural Productivity: A Review. Agronomy 2023, 13, 643. [Google Scholar] [CrossRef]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic Amendments, Beneficial Microbes, and Soil Microbiota: Toward a Unified Framework for Disease Suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Zotti, M.; Idbella, M.; Cesarano, G.; Al-Rowaily, S.L.; Abd-ElGawad, A.M. Mixtures of organic amendments and biochar promote beneficial soil microbiota and affect Fusarium oxysporum f. sp. lactucae, Rhizoctonia solani and Sclerotinia minor disease suppression. Plant Pathol. 2022, 71, 818–829. [Google Scholar] [CrossRef]
- Bonanomi, G.; Antignani, V.; Pane, C.; Scala, F. Suppression of Soilborne Fungal Diseases with Organic Amendments. J. Plant Pathol. 2007, 89, 311–324. [Google Scholar]
- Fukui, R. Suppression of soilborne plant pathogens through community evolution of soil microorganisms. Microbes Environ. 2003, 18, 1–9. [Google Scholar] [CrossRef]
- Wakelin, S.A. Managing soil microbiology: Realising opportunities for the productive land-based sectors. N. Z. J. Agric. Res. 2018, 61, 358–376. [Google Scholar] [CrossRef]
- Garg, D.; Patel, N.; Rawat, A.; Rosado, A.S. Cutting edge tools in the field of soil microbiology. Curr. Res. Microb. Sci. 2024, 6, 100226. [Google Scholar] [CrossRef] [PubMed]
- Djemiel, C.; Dequiedt, S.; Karimi, B.; Cottin, A.; Horrigue, W.; Bailly, A.; Boutaleb, A.; Sadet-Bourgeteau, S.; Maron, P.A.; Chemidlin Prévost-Bouré, N.; et al. Potential of Meta-Omics to Provide Modern Microbial Indicators for Monitoring Soil Quality and Securing Food Production. Front. Microbiol. 2022, 13, 889788. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Hofmockel, K.S. The soil microbiome-from metagenomics to metaphenomics. Curr. Opin. Microbiol. 2018, 43, 162–168. [Google Scholar] [CrossRef] [PubMed]
- De Corato, U. Disease-suppressive compost enhances natural soil suppressiveness against soil-borne plant pathogens: A critical review. Rhizosphere 2020, 13, 100192. [Google Scholar] [CrossRef]
- Yadav, R.; Beniwal, R.; Ramakrishna, W. Soil Diseases Suppressiveness Conferred by Organic Farming, Practices and Microbial Metabolites. Arch. Agron. Soil Sci. 2023, 69, 3201–3221. [Google Scholar] [CrossRef]
- Mazzola, M.; Freilich, S. Prospects for Biological Soilborne Disease Control: Application of Indigenous Versus Synthetic Microbiomes. Phytopathology 2017, 107, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Wang, J.; Bai, W.; Qi, J.; Chen, W. Soil bacterial diversity correlates with precipitation and soil pH in long-term maize cropping systems. Sci. Rep. 2020, 10, 6012. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tahvanainen, T.; Malard, L.; Chen, L.; Pérez-Pérez, J.; Berninger, F. Global analysis of soil bacterial genera and diversity in response to pH. Soil Biol. Biochem. 2024, 198, 109552. [Google Scholar] [CrossRef]
- Larkin, R.P. Soil Health Paradigms and Implications for Disease Management. Annu. Rev. Phytopathol. 2015, 53, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Sansinenea, E. The Role of Beneficial Microorganisms in Soil Quality and Plant Health. Sustainability 2022, 14, 5358. [Google Scholar] [CrossRef]
- Cardoso, E.J.B.N.; Vasconcellos, R.L.F.; Bini, D.; Miyauchi, M.Y.H.; Santos, C.A.D.; Alves, P.R.L.; Paula, A.M.D.; Nakatani, A.S.; Pereira, J.D.M.; Nogueira, M.A. Soil health: Looking for suitable indicators. What should be considered to assess the effects of use and management on soil health? Sci. Agric. 2013, 70, 274–289. [Google Scholar] [CrossRef]
- Jayaraman, S.; Naorem, A.K.; Lal, R.; Dalal, R.C.; Sinha, N.K.; Patra, A.K.; Chaudhari, S.K. Disease-Suppressive Soils—Beyond Food Production: A Critical Review. J. Soil Sci. Plant Nutr. 2021, 21, 1437–1465. [Google Scholar] [CrossRef] [PubMed]
- Janvier, C.; Villeneuve, F.; Alabouvette, C.; Edel-Hermann, V.; Mateille, T.; Steinberg, C. Soil health through soil disease suppression: Which strategy from descriptors to indicators? Soil Biol. Biochem. 2007, 39, 274–289. [Google Scholar] [CrossRef]
- Sagova-Mareckova, M.; Omelka, M.; Kopecky, J. The Golden Goal of Soil Management: Disease-Suppressive Soils. Phytopathology 2023, 113, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Mitsuboshi, M.; Kioka, Y.; Noguchi, K.; Asakawa, S. Evaluation of Disease Suppressiveness of Soils in Croplands by Co-Cultivation of Pathogenic Fusarium oxysporum and Indigenous Soil Microorganisms. Microbes Environ. 2022, 37, ME21063. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Wu, M.; Liu, M.; Li, G.; Liu, K.; Qiu, C.; Bao, Y.; Li, Z. Comparative analysis on root exudate and rhizosphere soil bacterial assembly between tomatoes and peppers infected by Ralstonia. Chem. Biol. Technol. Agric. 2024, 11, 36. [Google Scholar] [CrossRef]
- Abawi, G.S.; Widmer, T.L. Impact of soil health management practices on soilborne pathogens, nematodes and root diseases of vegetable crops. Appl. Soil Ecol. 2000, 15, 37–47. [Google Scholar] [CrossRef]
- Bonilla, N.; Gutiérrez-Barranquero, J.; Vicente, A.; Cazorla, F. Enhancing Soil Quality and Plant Health Through Suppressive Organic Amendments. Diversity 2012, 4, 475–491. [Google Scholar] [CrossRef]
- Stotzky, G.; Martin, R.T. Soil mineralogy in relation to the spread of Fusarium wilt of banana in Central America. Plant Soil 1963, 18, 317–337. [Google Scholar] [CrossRef]
- Persson, L.; Olsson, Å. Abiotic characteristics of soils suppressive to Aphanomyces root rot. Soil Biol. Biochem. 2000, 32, 1141–1150. [Google Scholar] [CrossRef]
- Palojarvi, A.; Kellock, M.; Parikka, P.; Jauhiainen, L.; Alakukku, L. Tillage System and Crop Sequence Affect Soil Disease Suppressiveness and Carbon Status in Boreal Climate. Front. Microbiol. 2020, 11, 534786. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2018, 23, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.; Raaijmakers, J.M. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, M. Microbial communities in Soil. Plant Soil 2004, 274, 1–10. [Google Scholar]
- Mazzola, M. Assessment and management of soil microbial community structure for disease suppression. Annu. Rev. Phytopathol. 2004, 42, 35–59. [Google Scholar] [CrossRef] [PubMed]
- Larkin, R.P.; Honeycutt, C.W.; Griffin, T.S. Effect of rotation and cover crops on soilborne potato diseases, tuber yield, and soil microbial communities. Plant Dis. 2011, 95, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Van Elsas, J.D.; Chiurazzi, M.; Mallon, C.A.; Elhottová, D.; Krištůfek, V.; Salles, J.F. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. USA 2002, 109, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Jousset, A.; Rochat, L.; Pechy-Tarr, M.; Keel, C.; Scheu, S.; Bonkowski, M. Predators shape the evolution of bacterial niche specialization. Proc. Natl. Acad. Sci. USA 2017, 114, 394–399. [Google Scholar]
- Cotxarrera, L.; Trillas-Gay, M.I.; Steinberg, C.; Alabouvette, C. Use of compost as a suppressive substrate for the control of Fusarium wilt of tomato. Soil Biol. Biochem. 2002, 34, 735–742. [Google Scholar] [CrossRef]
- Lehman, R.M.; Cambardella, C.A.; Stott, D.E.; Acosta-Martinez, V.; Manter, D.K.; Buyer, J.S.; Halvorson, J.J. Understanding and enhancing soil biological health: The solution for reversing soil degradation. Sustainability 2015, 7, 988–1027. [Google Scholar] [CrossRef]
- Fierer, N.; Grandy, A.S.; Six, J.; Paul, E.A. Searching for unifying principles in soil ecology. Soil Biol. Biochem. 2009, 41, 2249–2256. [Google Scholar] [CrossRef]
- Penton, C.R.; Gupta, V.V.S.R.; Tiedje, J.M.; Neate, S.M.; Ophel-Keller, K.; Gillings, M.; Harvey, P.; Pham, A.; Roget, D.K. Fungal community structure in disease suppressive soils assessed by 28S LSU gene sequencing. PLoS ONE 2014, 9, e93893. [Google Scholar] [CrossRef] [PubMed]
- Shafique, H.; Sultana, V.; Ehteshamul-Haque, S.; Athar, M. Management of soil-borne diseases of organic vegetables. J. Plant Prot. Res. 2016, 56, 221–229. [Google Scholar] [CrossRef]
- Bever, J.D.; Platt, T.G.; Morton, E.R. Microbial community interactions and soil health. Annu. Rev. Phytopathol. 2012, 50, 71–89. [Google Scholar]
- Fravel, D.; Olivain, C.; Alabouvette, C. Fusarium oxysporum and its biocontrol. New Phytol. 2003, 157, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Larkin, R.P. Green manures and plant disease management. CAB Rev. 2013, 8, 037. [Google Scholar] [CrossRef]
- Zhang, J. Brassica cover crops and soilborne pathogens: Effects on the microbial community and disease suppression. Phytopathology 2015, 105, 1129–1139. [Google Scholar]
- De Medeiros, E.V.; Lima, N.T.; De Sousa Lima, J.R.; Pinto, K.M.S.; Da Costa, D.P.; Franco Junior, C.L.; Souza, R.M.S.; Hammecker, C. Biochar as a strategy to manage plant diseases caused by pathogens inhabiting the soil: A critical review. Phytoparasitica 2021, 49, 713–726. [Google Scholar] [CrossRef]
- Termorshuizen, A.J.; van Rijn, E.; van der Gaag, D.J.; Alabouvette, C.; Chen, Y.; Lagerlof, J.; Malandrakis, A.A.; Paplomatas, E.J.; Ramert, B.; Ryckeboer, J. Suppressiveness composts against pathosystems: Variability in pathogen response. Soil Biol. Biochem. 2006, 38, 2461–2477. [Google Scholar] [CrossRef]
- Mahapatra, B.; Ghosh, D.; Mukhopadhyay, R. Biocontrol potential of Trichoderma and Pseudomonas: A review. Int. J. Adv. Biochem. Res. 2024, 8, 129–132. [Google Scholar] [CrossRef]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codón, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2004, 7, 249–260. [Google Scholar]
- Delavaux, C.S.; Smith-Ramesh, L.M.; Kuebbing, S.E. Beyond nutrients: A meta-analysis of the diverse effects of arbuscular mycorrhizal fungi on plants and soils. Ecology 2017, 98, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Liu, Y.; Mu, R.; Wang, X.; Zhou, Q.; Islam, R.; Su, X.; Tian, Y. Biological Control Effect of Antagonistic Bacteria on Potato Black Scurf Disease Caused by Rhizoctonia solani. Agronomy 2024, 14, 351. [Google Scholar] [CrossRef]
- Yağmur, A.; Demir, S.; Canpolat, S.; Rezaee Danesh, Y.; Farda, B.; Djebaili, R.; Pace, L.; Pellegrini, M. Onion Fusarium basal rot disease control by arbuscular mycorrhizal fungi and Trichoderma harzianum. Plants 2024, 13, 386. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, C.; Edel-Hermann, V.; Alabouvette, C.; Lemanceau, P. Soil Suppressiveness to Plant Diseases. Modern Soil Microbiology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Du, J.X.; Li, Y.; Ur-Rehman, S.; Mukhtar, I.; Yin, Z.; Dong, H.; Wang, H.; Zhang, X.; Gao, Z.; Zhao, X.; et al. Synergistically promoting plant health by harnessing synthetic microbial communities and prebiotics. iScience 2021, 24, 102918. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Ashoka, P.; Ahlawat, U.; Changdeo, W.B.; Rehsawla, R.; Naruka, A.; Sharma, D. Mechanisms and Applications of Microbial Biotechnology in Soil Health and Agricultural Productivity: A Review. J. Adv. Biol. Biotechnol. 2024, 27, 1420–1438. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Cui, H.; Li, Y.; Xu, N.; Lu, T.; Chen, J.; Penuelas, J.; Hu, B.; Qian, H. Composition identification and functional verification of bacterial community in disease-suppressive soils by machine learning. Environ. Microbiol. 2022, 24, 3405–3419. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.; Ahmad, A.A.; Abdo, E.-S.; Bakr, M.A.; Khalil, M.A.; Abdallah, Y.; Ogunyemi, S.O.; Mohany, M.; Al-Rejaie, S.S.; Shou, L.; et al. Suppression of Root Rot Fungal Diseases in Common Beans (Phaseolus vulgaris L.) through the Application of Biologically Synthesized Silver Nanoparticles. Nanomaterials 2024, 14, 710. [Google Scholar] [CrossRef] [PubMed]
- Vincent, I.R.; Rosskopf, E.N.; Brecht, J.K.; Dufault, N.S.; Sandoya-Miranda, G.; Zhao, X. Effects of anaerobic soil disinfestation for soilborne disease and weed management on baby-leaf lettuce performance in a high-tunnel organic production system. Agronomy 2024, 14, 764. [Google Scholar] [CrossRef]
- Nonthapa, A.; Boonthai Iwai, C.; Chankaew, S.; Falab, S. Dual-purpose vermicompost for the growth promotion and suppression of damping-off disease on potted vegetable soybean. Plants 2024, 13, 1607. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Feng, H.; Mo, X.; Sun, J.; Qiu, P.; Liu, Y.; Zhang, R.; Kuramae, E.E.; Shen, B.; Shen, Q. Potassium phosphite enhanced the suppressive capacity of the soil microbiome against the tomato pathogen Ralstonia solanacearum. Biol. Fertil. Soils 2022, 58, 553–563. [Google Scholar] [CrossRef]
- Tracanna, V.; Ossowicki, A.; Petrus, M.L.C.; Overduin, S.; Terlouw, B.R.; Lund, G.; Robinson, S.L.; Warris, S.; Schijlen, E.G.W.M.; van Wezel, G.P.; et al. Dissecting disease suppressive rhizosphere microbiomes by functional amplicon sequencing and 10× metagenomics. mSystems 2021, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Ossowicki, A.; Tracanna, V.; Petrus, M.L.C.; van Wezel, G.; Raaijmakers, J.M.; Medema, M.H.; Garbeva, P. Microbial and volatile profiling of soils suppressive to Fusarium culmorum of wheat. Proc. R. Soc. B Biol. Sci. 2020, 287, 20192527. [Google Scholar] [CrossRef] [PubMed]
- Durán, P.; Jorquera, M.; Viscardi, S.; Carrion, V.J.; de la Luz Mora, M.; Pozo, M.J. Screening and characterization of potentially suppressive soils against Gaeumannomyces graminis under extensive wheat cropping by Chilean indigenous communities. Front. Microbiol. 2017, 8, 1552. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.M.; Mazzola, M. Soil immune responses. Science 2016, 352, 1392–1393. [Google Scholar] [CrossRef] [PubMed]
- Kyselková, M.; Moënne-Loccoz, Y. Pseudomonas and other microbes in disease-suppressive soils. In Organic Fertilisation, Soil Quality and Human Health, Volume 9; Lichtfouse, E., Ed.; Springer Science+Business Media: Dordrecht, The Netherlands, 2012; pp. 93–140. [Google Scholar]
- Löbmann, M.T.; Vetukuri, R.R.; de Zinger, L.; Alsanius, B.W.; GrenvilleBriggs, L.J.; Walter, A.J. The occurrence of pathogen suppressive soils in Sweden in relation to soil biota, soil properties, and farming practices. Appl. Soil Ecol. 2016, 107, 57–65. [Google Scholar] [CrossRef]
- Martinez, J.; Weyman, S. Climate change and plant pathogens: Impacts and management strategies. Front. Microbiol. 2021, 12, 626867. [Google Scholar]
- Leach, J.E.; White, J.D. Impacts of global warming on plant disease: What we know and what we need to learn. Plant Dis. 2022, 106, 1714–1721. [Google Scholar]
- Prieur, D.; van Veen, J.A.; Leveau, J.H. The microbial ecology of soil suppressiveness to pathogens. Soil Biol. Biochem. 2020, 149, 107926. [Google Scholar] [CrossRef]
- Stone, L.; Lee, K.H. Interactions between climate change and soil disease suppressiveness: A focus on microbial diversity. Environ. Microbiol. Rep. 2023, 15, 375–385. [Google Scholar] [CrossRef]

| History of Disease-Suppressive Soil | |||
|---|---|---|---|
| Sl. No. | Year | Advancements | Outcomes |
| 1. | Pre–1900s | Early observations regarding the suppression of Fusarium wilt in tomato in Germany | Foundational work that led to the emergence of disease-suppressive soils |
| 2. | Mid–20th Century | Disease-suppressive soils coined by Kenneth F. Baker and Robert J. Cook | The mechanism behind disease-suppressive soils was explored and paved the way for the study of microbial ecology |
| 3. | 1970s–1980s | General and specific disease-suppressive soil | The complexity in understanding the suppressive mechanisms led to advanced molecular research. |
| 4. | 1990s–2000s | Evolution of DNA sequencing and metagenomic approaches in disease-suppressive soil research. The ISR mechanism was further discovered and studied. The role of beneficial microbes was acknowledged. | The evolution of advanced DNA sequencing and the acknowledgement of beneficial microbes led to the incorporation of these concepts into sustainable agriculture. |
| 5 | 2000s–Present | Using the concept of disease-suppressive soils to promote sustainable agriculture by decreasing the dependence on agricultural chemicals. Identification of genetic markers associated with disease resistance in plants and beneficial microbial strains. | This approach paves the way for resilient agro-ecosystems and region-specific soil health strategies. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priyadarshini, C.; Lal, R.; Yuan, P.; Liu, W.; Adhikari, A.; Bhandari, S.; Xia, Y. Plant Disease Suppressiveness Enhancement via Soil Health Management. Biology 2025, 14, 924. https://doi.org/10.3390/biology14080924
Priyadarshini C, Lal R, Yuan P, Liu W, Adhikari A, Bhandari S, Xia Y. Plant Disease Suppressiveness Enhancement via Soil Health Management. Biology. 2025; 14(8):924. https://doi.org/10.3390/biology14080924
Chicago/Turabian StylePriyadarshini, Chinmayee, Rattan Lal, Pu Yuan, Wenshan Liu, Ashna Adhikari, Santosh Bhandari, and Ye Xia. 2025. "Plant Disease Suppressiveness Enhancement via Soil Health Management" Biology 14, no. 8: 924. https://doi.org/10.3390/biology14080924
APA StylePriyadarshini, C., Lal, R., Yuan, P., Liu, W., Adhikari, A., Bhandari, S., & Xia, Y. (2025). Plant Disease Suppressiveness Enhancement via Soil Health Management. Biology, 14(8), 924. https://doi.org/10.3390/biology14080924








