Role of StAR Gene in Sex Steroid Hormone Regulation and Gonadal Development in Ark Shell Scapharca broughtonii
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. RNA Extraction and cDNA Synthesis
2.3. cDNA Fragment Validation
2.4. Gene Sequence and Evolutionary Analysis
2.5. In Situ Hybridization Analysis
2.6. Analysis of StAR Expression in the Gonads of Ark Shell Across Different Developmental Stages
2.7. Extraction of Sex Steroid Hormone
2.8. Determination of Sex Steroid Hormone
2.9. Statistical Analysis
3. Results
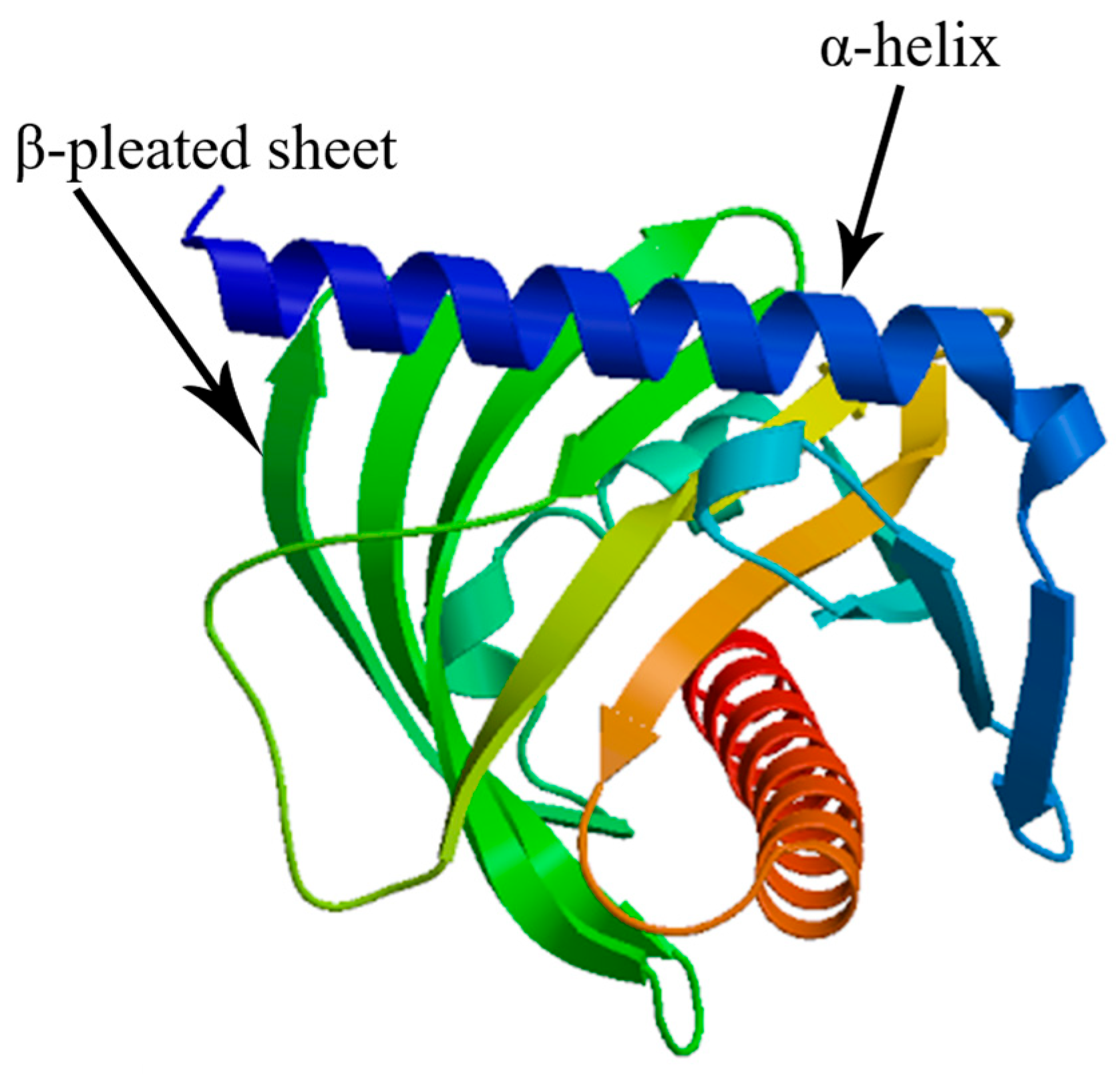
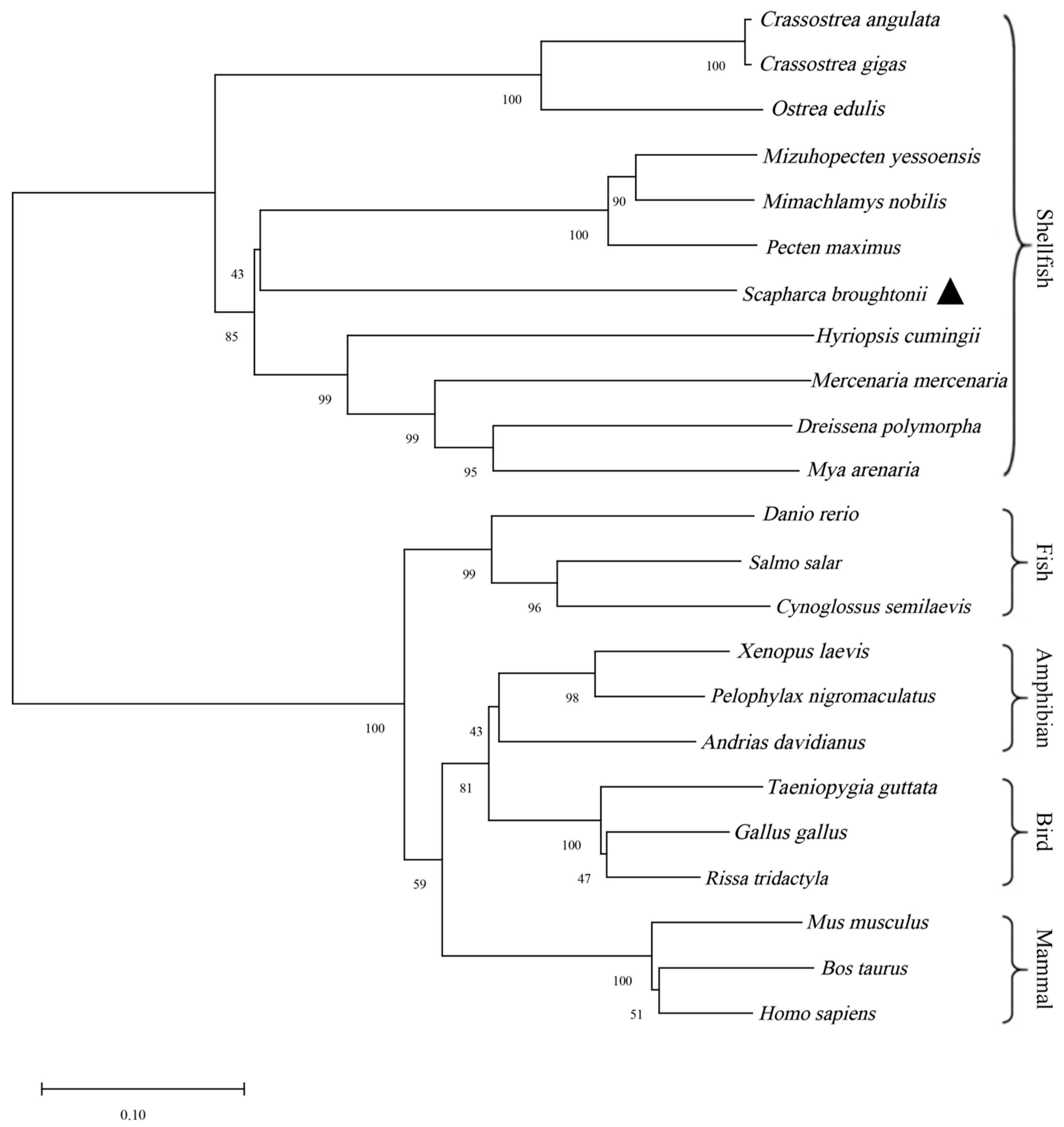
3.1. StAR Gene Sequence and Bioinformatics Analysis
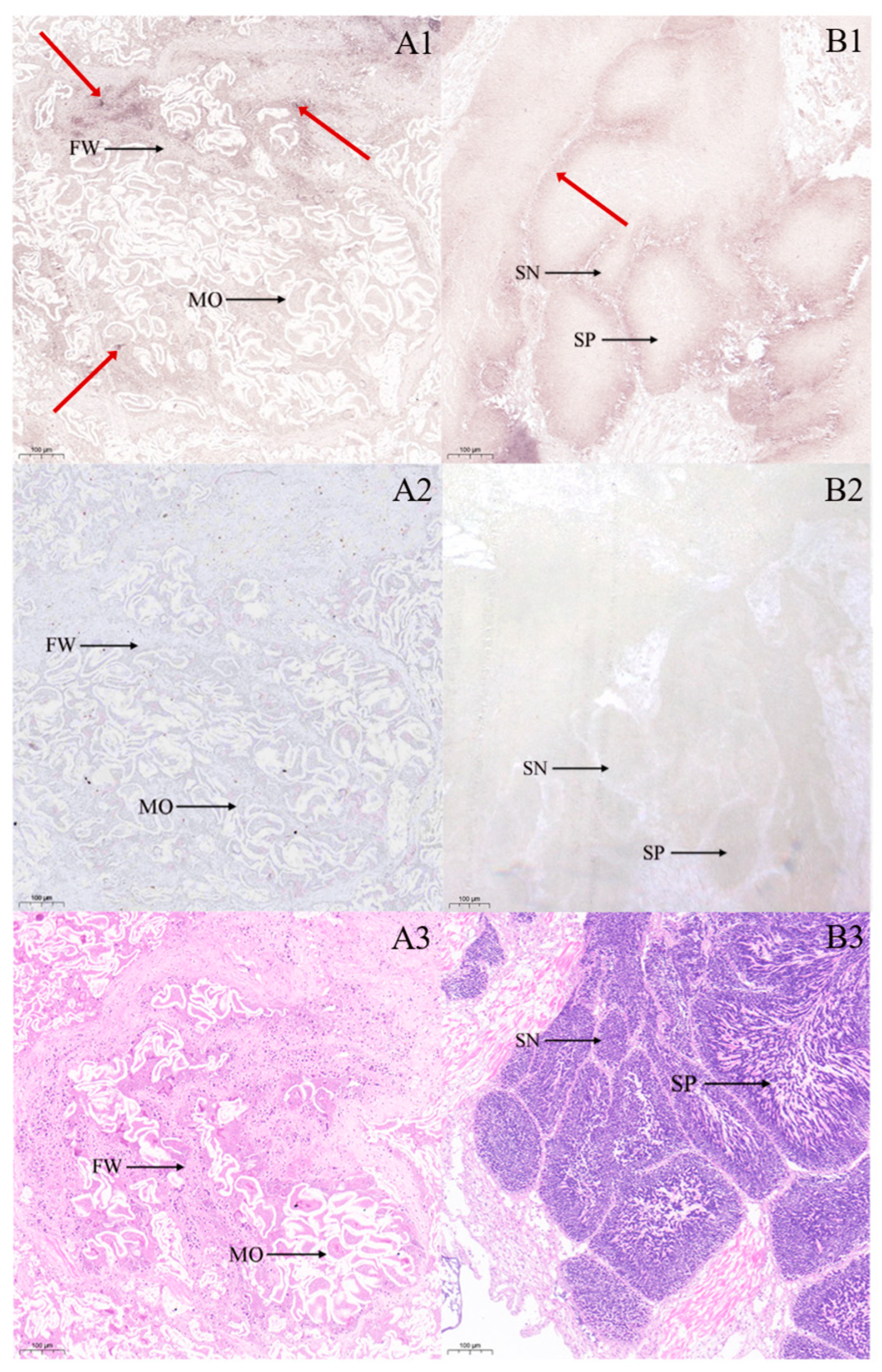
3.2. Cytological Mapping of StAR Gene mRNA in Seven Tissues of Ark Shell
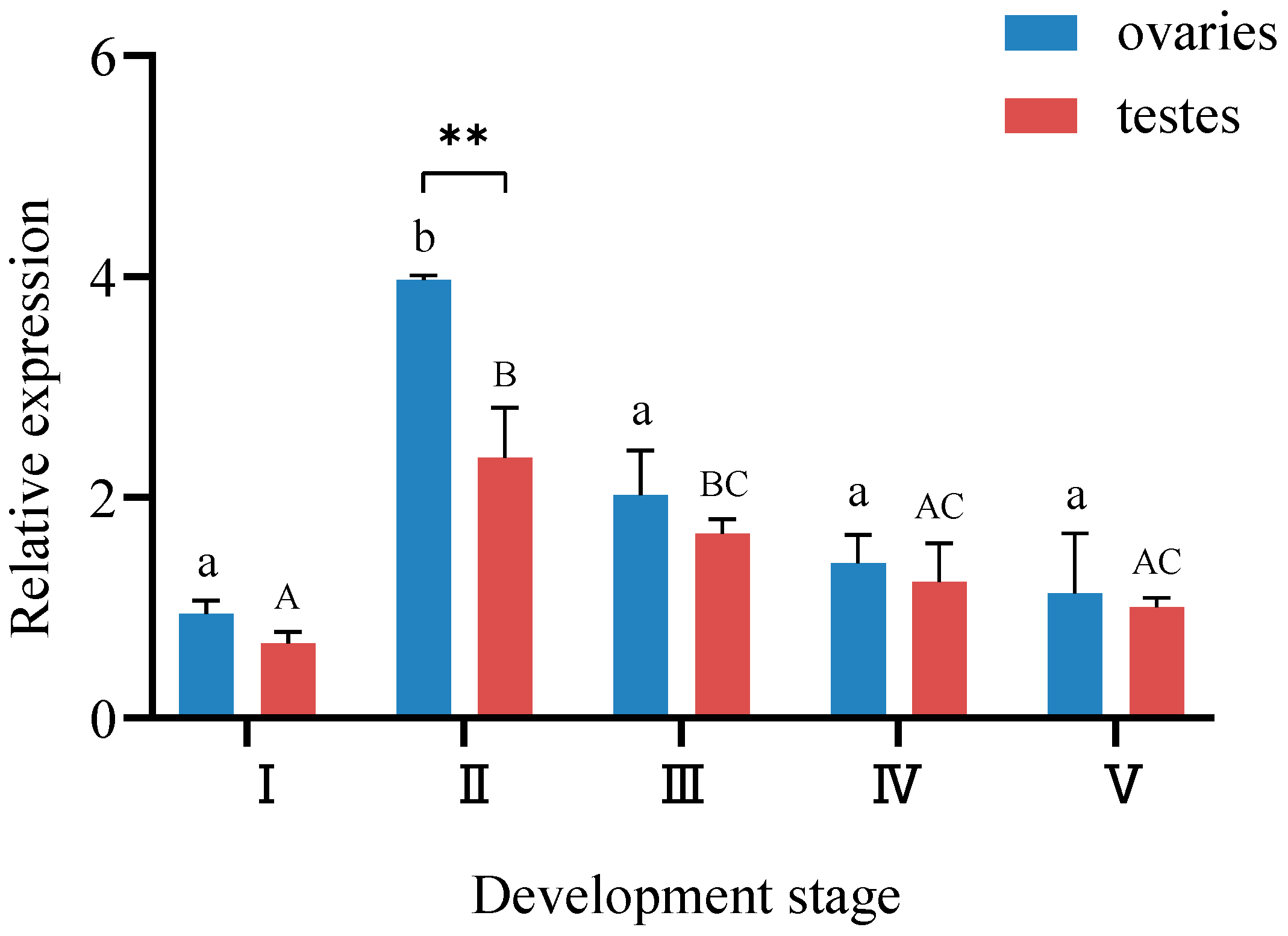
3.3. Analysis of StAR Gene Expression in Gonads at Different Developmental Stages
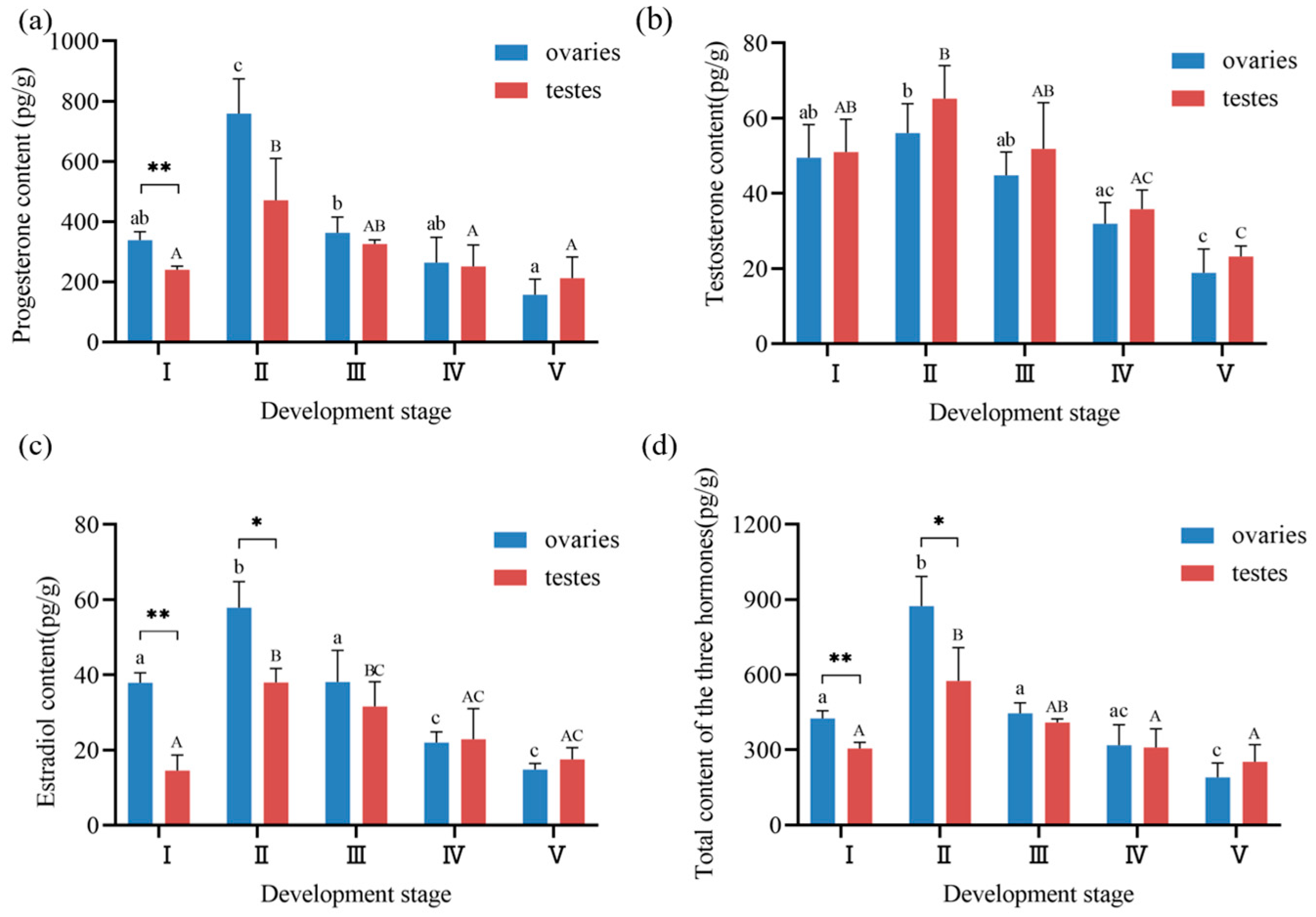
3.4. Steroid Hormone Profiles During Gonadal Development in Ark Shell
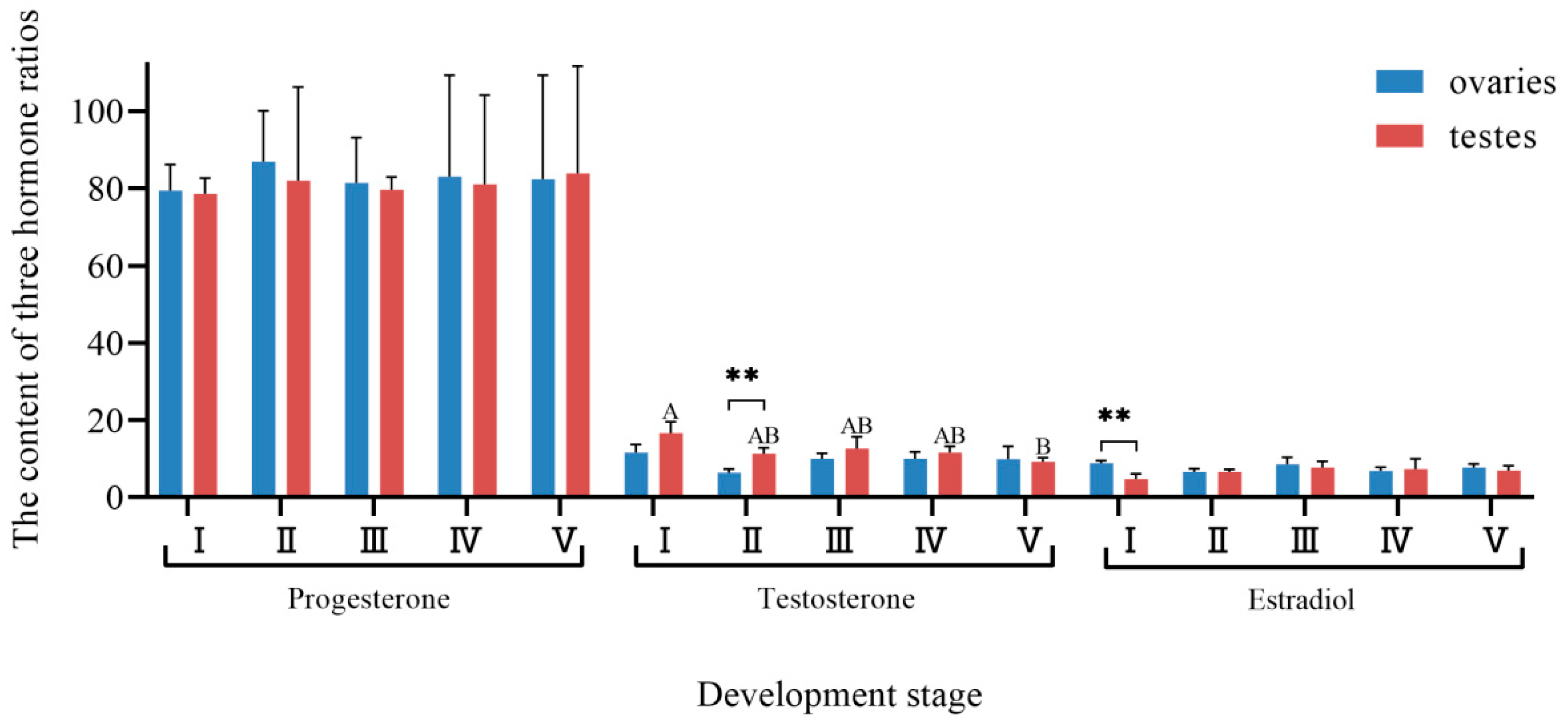
3.5. Changes in Hormone Proportion with Gonadal Development
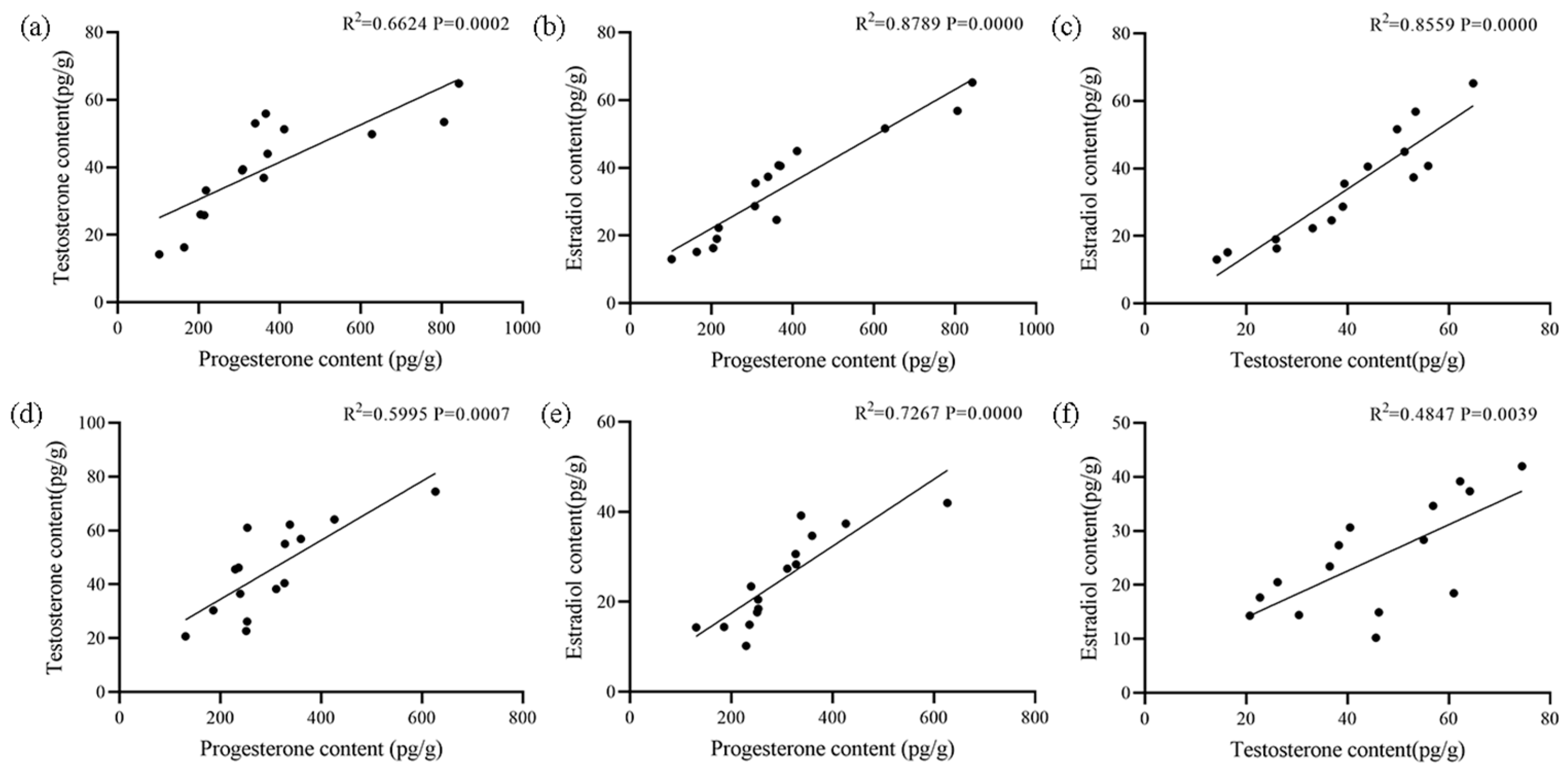
3.6. Correlation Among Hormones and Between Hormone and Gene Expression Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamaguchi, A.; Lee, K.H.; Fujimoto, H.; Kadomura, K.; Yasumoto, S.; Matsuyama, M. Expression of the DMRT gene and its roles in early gonadal development of the Japanese pufferfish Takifugu rubripes. Comp. Biochem. Physiol. Part D Genom. Proteom. 2006, 1, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Kazeto, Y.; Tosaka, R.; Matsubara, H.; Ijiri, S.; Adachi, S. Ovarian steroidogenesis and the role of sex steroid hormones on ovarian growth and maturation of the Japanese eel. J. Steroid Biochem. Mol. Biol. 2011, 127, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Lafont, R.; Mathieu, M. Steroids in aquatic invertebrates. Ecotoxicology 2007, 16, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Lupo, D.P.C.; Fulgheri, F.D.; Tomasucci, M. Identification and biosynthesis of steroids in the marine mollusc Aplysia depilans. Comp. Biochem. Physiol. Part B Comp. Biolchem. 1973, 45, 303–310. [Google Scholar] [CrossRef]
- Reis-Henriques, M.A.; Guellec, D.L.; Remy-Martin, J.P.; Adessi, G.L. Studies of endogenous steroids from the marine mollusc Mytilus edulis L. By gas chromatography and mass spectrometry. Comp. Biochem. Physiol. Part B Comp. Biolchem. 1990, 95, 303–309. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, L.; Sun, H.; Zhang, Y.; Li, W.; Zhang, M.; Bao, Z. Distribution of sex steroids in the gonad of Patinopecten yessoensis During the Reproductive Cycle. J. Ocean Univ. China 2019, 49, 20–25. [Google Scholar]
- Liu, J.; Zhang, Z.; Zhang, L.; Liu, X.; Yang, D.; Ma, X. Variations of estradiol-17β and testosterone levels correlated with gametogenesis in the gonad of Zhikong scallop (Chlamys farreri) during annual reproductive cycle. Can. J. Zool. 2014, 92, 195–204. [Google Scholar] [CrossRef]
- Li, W. Dynamics of Foxl2, Dmrt1l and Sex Steroids on Gonadal Development in the Bay Scallops, Argopecten irradians. Master’s Thesis, Ocean University of China, Qingdao, China, 2019. [Google Scholar]
- Song, S. Preliminary Study on Roles of Sex Steroids, 5-HTcg, Cg-phb2 and Cg-IGFBP7 in Crassostrea gigas During Gonadal Development. Master’s Thesis, Ocean University of China, Qingdao, China, 2017. [Google Scholar]
- Zhu, Y.; Wang, W.; Xia, S.; Liu, Z.; Sun, X.; Zhou, L.; Zhang, X.; Wu, B. Distribution of sex steroid hormones in the gonad of Crassostrea ariakensis during the reproductive cycle. J. Ludong Univ. (Nat. Sci. Ed.) 2023, 39, 107–114. [Google Scholar]
- Gennotte, V.; Akonkwa, B.; Mélard, C.; Denoël, M.; Cornil, C.A.; Rougeot, C. Do sex reversal procedures differentially affect agonistic behaviors and sex steroid levels depending on the sexual genotype in Nile tilapia? J. Exp. Zool A Ecol. Integr. Physiol. 2017, 327, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed]
- Thitiphuree, T.; Nagasawa, K.; Osada, M. Molecular identification of steroidogenesis-related genes in scallops and their potential roles in gametogenesis. J. Steroid Biochem. Mol. Biol. 2019, 186, 22–23. [Google Scholar] [CrossRef] [PubMed]
- He, F. Transcriptome Analysis of Male and Female Gonads and Study on Sex Steroid Hormone in Spotted Scat (Scatophagus argus). Master’s Thesis, Guangdong Ocean University, Zhanjiang, China, 2019. [Google Scholar]
- Lehoux, J.G.; Sandor, T. The occurrence of steroids and steroid metabolizing enzyme systems in invertebrates. A review. Steroids 1970, 16, 141–171. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Manna, P.R.; Stocco, D.M.; Chakrabarti, G.; Mukhopadhyay, A.K. Stimulatory effect of progesterone on the expression of steroidogenic acute regulatory protein in MA-10 Leydig cells. Biol. Reprod. 2003, 68, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Zempo, B.; Kanda, S.; Okubo, K.; Akazome, Y.; Oka, Y. Anatomical distribution of sex steroid hormone receptors in the brain of female medaka. J. Comp. Neurol. 2013, 521, 1760–1780. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, A.J.; Williams, S.C.; Clark, B.J.; Stocco, D.M. SF-1 (steroidogenic factor-1) and C/EBP beta (CCAAT/enhancer binding protein-beta) cooperate to regulate the murine StAR (steroidogenic acute regulatory) promoter. Mol. Endocrinol. 1999, 13, 729–741. [Google Scholar] [PubMed]
- Yu, X.; Wu, L.; Xie, L.; Yang, S.; Charkraborty, T.; Shi, H.; Wang, D.; Zhou, L. Characterization of two paralogous StAR genes in a teleost, Nile tilapia (Oreochromis niloticus). Mol. Cell. Endocrinol. 2014, 392, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Thongbuakaew, T.; Suwansa-Ard, S.; Chaiyamoon, A.; Cummins, S.F.; Sobhon, P. Sex steroids and steroidogenesis-related genes in the sea cucumber, Holothuria scabra and their potential role in gonad maturation. Sci. Rep. 2021, 11, 2194. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, X. Molecular Identification and Functional Study of Sex Steroid Hormone Synthesis Related Genes in Hyriopsis cumingii. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2022. [Google Scholar]
- Huang, B.; Zhang, X.; Wang, C.; Bai, C.; Li, C.; Li, C.; Xin, L. Isolation and Characterization of Vibrio kanaloae as a Major Pathogen Associated with Mass Mortalities of Ark Clam, Scapharca broughtonii, in Cold Season. Microorganisms 2021, 9, 2161. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Huang, B.; Bai, C.; Wang, C. Validation of housekeeping genes for quantitative mRNA expression analysis in OsHV-1 infected ark clam, Scapharca broughtonii. J. Invertebr. Pathol. 2018, 155, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; An, L.; Chang, H.; Liu, Y.; Jiang, Z. Evidence for the presence of sex steroid hormones in Zhikong scallop, Chlamys farreri. J. Steroid Biochem. Mol. Biol. 2014, 143, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Maheswarudu, G.; Rajkumar, U.; Sreeram, M.P.; Chakravarty, M.S.; Sajeev, C.K. Effect of testosterone hormone on performance of male broodstock of black tiger shrimp Penaeus monodon Fabricius, 1798. J. Vet. Sci. Photon. 2015, 116, 446–456. [Google Scholar]
- Dvoretsky, A.G.; Tipisova, E.V.; Elfimova, A.E.; Alikina, V.A.; Dvoretsky, V.G. Sex hormones in hemolymph of red king crabs from the Barents Sea. Animals. 2021, 11, 2149. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.F., 3rd; Kallen, C.B.; Christenson, L.K.; Watari, H.; Devoto, L.; Arakane, F.; Kiriakidou, M.; Sugawara, T. The steroidogenic acute regulatory protein (StAR): A window into the complexities of intracellular cholesterol trafficking. Recent Prog. Horm. Res. 1999, 54, 369–394. [Google Scholar] [PubMed]
- Clark, B.J.; Wells, J.; King, S.R.; Stocco, D.M. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J. Biol. Chem. 1994, 269, 28314–28322. [Google Scholar] [CrossRef] [PubMed]
- Nunez, B.S.; Evans, A.N. Hormonal regulation of the steroidogenic acute regulatory protein (StAR) in gonadal tissues of the Atlantic croaker (Micropogonias undulatus). Gen. Comp. Endocrinol. 2007, 150, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M. Tracking the role of a star in the sky of the new millennium. Mol. Endocrinol. 2001, 15, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Garmey, J.C.; Guthrie, H.D.; Garrett, W.M.; Stoler, M.H.; Veldhuis, J.D. Localization and expression of low-density lipoprotein receptor, steroidogenic acute regulatory protein, cytochrome P450 side-chain cleavage and P450 17-alpha-hydroxylase/C17-20 lyase in developing swine follicles: In situ molecular hybridization and immunocytochemical studies. Mol. Cell. Endocrinol. 2000, 170, 57–65. [Google Scholar] [PubMed]
- Gu, Y.; Shangguan, X.; Mao, Y.; Li, W.; Ren, Y.; Li, Z.; Lv, H.; Wu, Y.; Wang, G. Molecular identification of a steroidogenesis-related gene, star3, and its function in gonadal development of Hyriopsis cumingii. Aquacult. Rep. 2024, 39, 102375. [Google Scholar] [CrossRef]
- Zheng, C.; Xue, C.; Zhao, Q.; Sun, S. Molecular cloning and expression analysis of StAR gene from oriental river pawn (Macrobrachium nipponense) in response to hypoxia. J. Fish. China 2024, 48, 66–77. [Google Scholar]
- Amenyogbe, E.; Chen, G.; Wang, Z.; Lu, X.; Lin, M.; Lin, A. A Review on Sex Steroid Hormone Estrogen Receptors in Mammals and Fish. Int. J. Endocrinol. 2020, 2020, 5386193. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Potential Roles of Sex Steroids and 17β-Hydroxysteroid Dehydrogenase 8 in Chlamys farreri During Gonadal Development. Master’s Thesis, Ocean University of China, Qingdao, China, 2014. [Google Scholar]
- Mouneyrac, C.; Linot, S.; Amiard, J.C.; Amiard-Triquet, C.; Métais, I.; Durou, C.; Minier, C.; Pellerin, J. Biological indices, energy reserves, steroid hormones and sexual maturity in the infaunal bivalve Scrobicularia plana from three sites differing by their level of contamination. Gen. Comp. Endocrinol. 2008, 157, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, W.; Liu, T.; Li, Y.; Liu, L.; Shu, Y.; Zhang, L.; Wang, S.; Xing, Q.; Zhang, L.; et al. Sexual Development of the Hermaphroditic Scallop Argopecten irradians Revealed by Morphological, Endocrine and Molecular Analysis. Front. Cell Dev. Biol. 2021, 9, 646754. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.R.; Stetson, C.L.; Slominski, A.T.; Pruitt, K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine. 2016, 51, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Zhao, J.; Tang, S.; Zhao, Y. Effects of Estradiol and Testosterone on the Expression of Growth-related Genes in Female and Male Nile Tilapia, Oreochromis niloticus. J. World Aquac. Soc. 2018, 49, 216–228. [Google Scholar] [CrossRef]


| Prime | Sequence (5′-3′) | Function |
|---|---|---|
| StAR-F | CGTGAGAGATGTTGCAAGCATTCA | validation |
| StAR-R | ATCACAATGTTCCATCCAATGGCA | validation |
| q-StAR-F | TTTAACGCCAGTAAAGCAGGAGGAG | qRT-PCR |
| q-StAR-R | CAAGTCTCTACCCACGCCAACAC | qRT-PCR |
| RL15-F | AGACCAGACAAAGCCAGAAGAC | qRT-PCR |
| RL15-R | GCTGAAGTAAGTCCACGCATT | qRT-PCR |
| StAR | UCUAAGAACCAAGUCUCUACCCACGCCAA | in situ hybridization |
| Taxonomic Status | Species Name | Sequence Number | Homology |
|---|---|---|---|
| Shellfish | Mizuhopecten yessoensis | XP_021369546.1 | 51.45% |
| Mimachlamys nobilis | AJM13632.1 | 51.24% | |
| Pecten maximus | XP_033733178.1 | 50.21% | |
| Hyriopsis cumingii | WEY07736.1 | 49.78% | |
| Mercenaria mercenaria | XP_053408879.1 | 46.17% | |
| Dreissena polymorpha | XP_052253900.1 | 51.85% | |
| Mya arenaria | XP_052804046.1 | 51.38% | |
| Ostrea edulis | XP_048727697.1 | 48.77% | |
| Crassostrea angulata | XP_052718357.1 | 47.51% | |
| Crassostrea gigas | XP_034305144.1 | 47.51% | |
| Fish | Salmo salar | ABD73012.1 | 33.19% |
| Danio rerio | AAG28593.1 | 35.68% | |
| Cynoglossus semilaevis | AIB06798.1 | 33.33% | |
| Amphibian | Xenopus laevis | NP_001167502.1 | 35.15% |
| Andrias davidianus | AUS91513.1 | 32.70% | |
| Pelophylax nigromaculatus | AVP72471.1 | 38.61% | |
| Bird | Gallus gallus | AAG28594.1 | 33.01% |
| Taeniopygia guttata | AAR91038.1 | 32.31% | |
| Rissa tridactyla | XP_054081021.1 | 32.86% | |
| Mammal | Bos taurus | CAA76718.1 | 33.65% |
| Mus musculus | AAB94783.1 | 33.18% | |
| Homo sapiens | AAB88174.1 | 34.12% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Liu, Z.; Zhang, H.; Gao, Z.; Xia, S.; Sun, X.; Zhou, L.; Li, Z.; Ma, P.; Wu, B. Role of StAR Gene in Sex Steroid Hormone Regulation and Gonadal Development in Ark Shell Scapharca broughtonii. Biology 2025, 14, 925. https://doi.org/10.3390/biology14080925
Wang W, Liu Z, Zhang H, Gao Z, Xia S, Sun X, Zhou L, Li Z, Ma P, Wu B. Role of StAR Gene in Sex Steroid Hormone Regulation and Gonadal Development in Ark Shell Scapharca broughtonii. Biology. 2025; 14(8):925. https://doi.org/10.3390/biology14080925
Chicago/Turabian StyleWang, Wenjing, Zhihong Liu, Huaying Zhang, Zheying Gao, Sudong Xia, Xiujun Sun, Liqing Zhou, Zhuanzhuan Li, Peizhen Ma, and Biao Wu. 2025. "Role of StAR Gene in Sex Steroid Hormone Regulation and Gonadal Development in Ark Shell Scapharca broughtonii" Biology 14, no. 8: 925. https://doi.org/10.3390/biology14080925
APA StyleWang, W., Liu, Z., Zhang, H., Gao, Z., Xia, S., Sun, X., Zhou, L., Li, Z., Ma, P., & Wu, B. (2025). Role of StAR Gene in Sex Steroid Hormone Regulation and Gonadal Development in Ark Shell Scapharca broughtonii. Biology, 14(8), 925. https://doi.org/10.3390/biology14080925







