Biocontrol Potential of Rhizosphere Bacteria Against Fusarium Root Rot in Cowpea: Suppression of Mycelial Growth and Conidial Germination
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Fungal Pathogens
2.2. Isolation and Identification of Rhizosphere Microbes
2.3. Screening for Antagonistic Activity
2.4. Broad-Spectrum Antifungal Activity Assay
2.5. Conidial Germination Assay
2.6. In Planta Biological Control
2.7. Statistical Analysis
3. Results
3.1. Isolation and Molecular Identification of Pathogenic Fusarium Strains
3.2. Isolation and Taxonomic Diversity of Rhizobacterial Strains
3.3. Antagonistic Activity of Rhizosphere Isolates
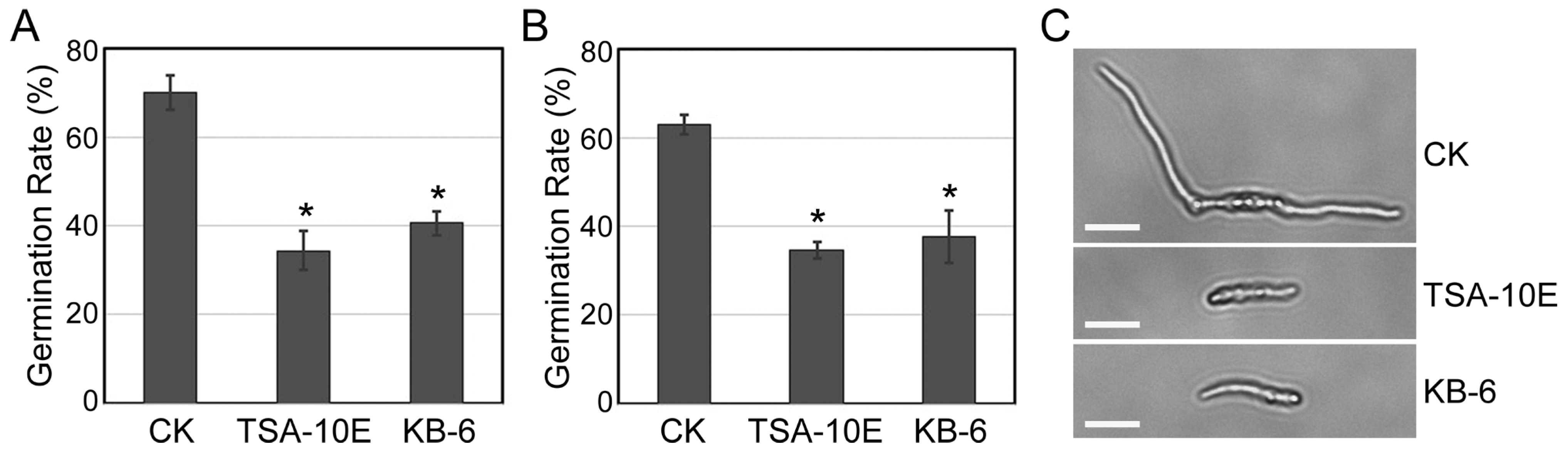
3.4. Inhibition of Conidial Germination by TSA-10E and KB-6 Filtrates
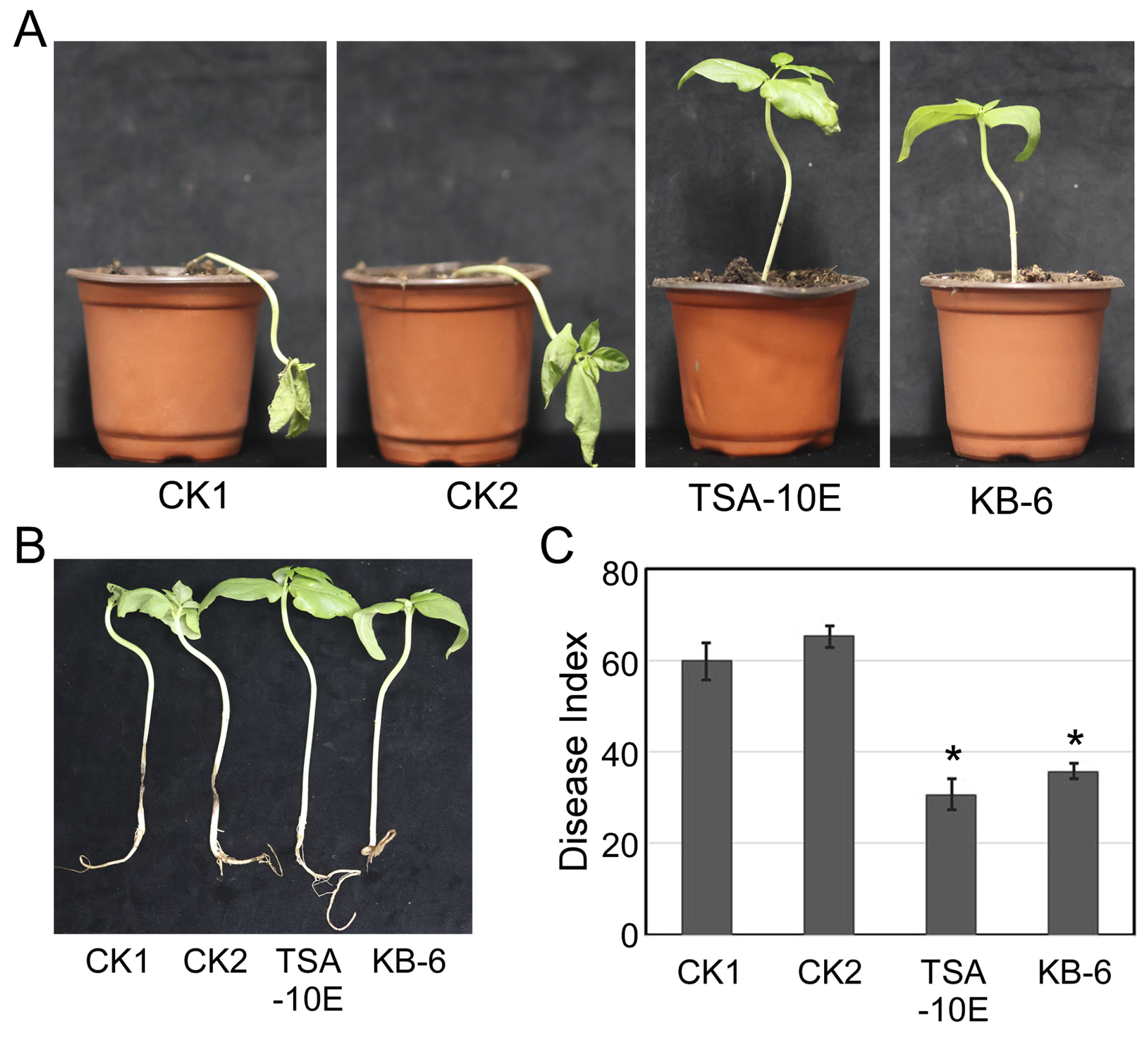
3.5. TSA-10E and KB-6 Can Effectively Control Cowpea Fusarium Root Rot in Greenhouse
3.6. Broad-Spectrum Antifungal Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jayathilake, C.; Visvanathan, R.; Deen, A. Cowpea: An overview on its nutritional facts and health benefits. J. Sci. Food Agric. 2018, 98, 4793–4806. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Wu, W.; Sun, S. Berkeleyomyces rouxiae is a causal agent of root rot complex on faba bean (Vicia faba L.). Front. Plant Sci. 2022, 13, 989517. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaradi, A.; Al-Mahmooli, I.; Janke, R. Isolation and identification of pathogenic fungi and oomycetes associated with beans and cowpea root diseases in Oman. PeerJ 2018, 6, e6064. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.S.; Manzar, N.; Ahamad, F. First report of root rot disease in green gram (Vigna radiata) caused by Ectophoma multirostrata in India. Plant Dis. 2022, 106, 2013–2025. [Google Scholar] [CrossRef]
- Yang, C.; Lin, L.; Bao, J. Genome sequence resource of Phytophthora vignae, the causal agent of stem and root rot of cowpea. Mol. Plant-Microbe Interact. 2021, 34, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, N.R.K.G. Evaluation of bioagents against Macrophomina phaseolina and its effect on root weight of cowpea (Vigna unguiculata (L.) Walp). J. Eco-Friendly Agric. 2022, 17, 166–168. [Google Scholar]
- Lamini, S.; Kusi, F.; Cornelius, E. Identification of sources of resistance in cowpea lines to Macrophomina root rot disease in northern Ghana. Heliyon 2022, 8, e12217. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.Q.; Li, Y.T.; Cai, Y.F. Isolation of Bacillus amyloliquefaciens JK6 and identification of its lipopeptides surfactin for suppressing tomato bacterial wilt. RSC Adv. 2015, 5, 82042–82049. [Google Scholar] [CrossRef]
- Olowe, O.M.; Nicola, L.; Asemoloye, M.D. Trichoderma: Potential bio-resource for the management of tomato root rot diseases in Africa. Microbiol. Res. 2022, 257, 126978. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Liu, N.; Lai, D.; Kang, X.H.; Pang, N.W.; Jiang, H.K.; Xu, H.H. A formulation of neem cake seeded with Bacillus sp. provides control over tomato Fusarium crown and root rot. Biocontrol Sci. Technol. 2017, 27, 393–407. [Google Scholar] [CrossRef]
- Zhong, J.; Sui, W.W.; Bai, X.Y. Characterization and biocontrol mechanism of Streptomyces olivoreticuli as a potential biocontrol agent against Rhizoctonia solani. Pestic. Biochem. Phys. 2023, 197, 105681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Qiang, R.; Zhou, Z.; Pan, Y.; Yu, S.; Yuan, W.; Cheng, J.; Wang, J.; Zhao, D.; Zhu, J.; et al. Biocontrol and action mechanism of Bacillus subtilis lipopeptides’ fengycins against Alternaria solani in potato as assessed by a transcriptome Analysis. Front. Microbiol. 2022, 13, 861113. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Li, C.; Wang, H. Community structures and antifungal activity of root-associated endophytic actinobacteria in healthy and diseased cucumber plants and Streptomyces sp. HAAG3-15 as a promising biocontrol agent. Microorganisms 2020, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Dastogeer, K.M.G.; Yasuda, M.; Okazaki, S. Microbiome and pathobiome analyses reveal changes in community structure by foliar pathogen infection in rice. Front. Microbiol. 2022, 13, 949152. [Google Scholar] [CrossRef] [PubMed]
- Dar, G.J.; Nazir, R.; Wani, S.A. Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification. Open Life Sci. 2025, 20, 20221006. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; American Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Wang, J.; Liu, Y.; Zhang, J. A Rapid Identification Method for Fusarium Species Using Specific Primer Combinations. Chinese Patent CN109504799A, 22 March 2019. [Google Scholar]
- Heuer, H.; Krsek, M.; Baker, P. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoresis separation in denaturing gradients. Appl. Environ. Microbiol. 1997, 63, 3233–3241. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, M.; Germanier, F.; Vuille, N. Combining Different Potato-Associated Pseudomonas Strains for Improved Biocontrol of Phytophthora infestans. Front. Microbiol. 2018, 9, 2573. [Google Scholar] [CrossRef] [PubMed]
- Alisaac, E.; Mahlein, A.K. Fusarium Head Blight on Wheat: Biology, Modern Detection and Diagnosis and Integrated Disease Management. Toxins 2023, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R. Plant health: Feedback effect of root exudates-rhizobiome interactions. Appl. Microbiol. Biotechnol. 2019, 103, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Babich, O.; Sukhikh, S.; Dyshlyuk, L. Evaluation of biocompatibility and antagonistic properties of microorganisms isolated from natural sources for obtaining biofertilizers using microalgae hydrolysate. Microorganisms 2021, 9, 1667. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Ugolini, L.; D’Aquino, S.; Pagnotta, E.; Mari, M. Biocontrol of Monilinia laxa by Aureobasidium pullulans strains: Insights on competition for nutrients and space. Int. J. Food Microbiol. 2017, 248, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Pandin, C.; Darsonval, M.; Mayeur, C. Biofilm formation and synthesis of antimicrobial compounds by the biocontrol agent Bacillus velezensis QST713 in an Agaricus bisporus compost micromodel. Appl. Environ. Microbiol. 2019, 85, e00327-19. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Hu, L.; Zeng, L. Isolation and characterization of Priestia megaterium KD7 for the biological control of pear fire blight. Front. Microbiol. 2023, 14, 1099664. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-López, A.M.; Cordero-Ramírez, J.D.; Martínez-Álvarez, J.C. Rhizospheric bacteria of maize with potential for biocontrol of Fusarium verticillioides. SpringerPlus 2016, 5, 330. [Google Scholar] [CrossRef] [PubMed]
- Bhargavi, G.; Arya, M.; Jambhulkar, P.P. Evaluation of biocontrol efficacy of rhizosphere dwelling bacteria for management of Fusarium wilt and Botrytis gray mold of chickpea. BMC Genom. Data 2024, 25, 7. [Google Scholar] [CrossRef] [PubMed]
- Rigert, K.S.; Foster, K.W. Inheritance of Resistance to Two Races of Fusarium Wilt in Three Cowpea Cultivars. Crop Sci. 1987, 27, 220–224. [Google Scholar] [CrossRef]
- Al-Mutar, D.M.K.; Noman, M.; Abduljaleel Alzawar, N.S. Cyclic lipopeptides of Bacillus amyloliquefaciens DHA6 are the determinants to suppress watermelon Fusarium wilt by direct antifungal activity and host defense modulation. J. Fungi 2023, 9, 687. [Google Scholar] [CrossRef] [PubMed]
- Assena, M.W.; Pfannstiel, J.; Rasche, F. Inhibitory activity of bacterial lipopeptides against Fusarium oxysporum f.sp. Strigae. BMC Microbiol. 2024, 24, 227. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, M.; Tang, L. Screening and characterization of biocontrol bacteria isolated from Ageratum conyzoides against Collectotrichum fructicola causing Chinese plum (Prunus salicina Lindl.) anthracnose. Front. Microbiol. 2023, 14, 1296755. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.; Nasser, R.; Hafeez, R.; Ogunyemi, S.O.; Abdallah, Y.; Khattak, A.A.; Shou, L.; Zhang, Y.; Ahmed, T.; Atef Hatamleh, A.; et al. Biocontrol Efficacy of Endophyte Pseudomonas poae to Alleviate Fusarium Seedling Blight by Refining the Morpho-Physiological Attributes of Wheat. Plants 2023, 12, 2277. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Jeffries, P.; Pautasso, M. Combined use of biocontrol agents to manage plant diseases in theory and practice. Phytopathology 2011, 101, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.A.; Saravanan, R.S. Polymicrobial multi-functional approach for enhancement of crop productivity. Adv. Appl. Microbiol. 2013, 82, 53–113. [Google Scholar] [PubMed]
- Sarma, B.K.; Yadav, S.K.; Singh, S. Microbial consortium-mediated plant defense against phytopathogens: Readdressing for 22enhancing efficacy. Soil Biol. Biochem. 2015, 87, 25–33. [Google Scholar] [CrossRef]
- Parnell, J.J.; Berka, R.; Young, H. From the lab to the farm: An industrial perspective of plant beneficial microorganisms. Front. Plant Sci. 2016, 7, 1110. [Google Scholar] [CrossRef] [PubMed]


| Genus | Total of Isolates | Total of Species | Species | Quantity |
|---|---|---|---|---|
| Bacillus | 31 | 14 | Bacillus anthracis | 1 |
| Bacillus aryabhattai | 11 | |||
| Bacillus bataviensis | 1 | |||
| Bacillus bingmayongensis | 1 | |||
| Bacillus cereus | 2 | |||
| Bacillus ferrooxidans | 3 | |||
| Bacillus mycoides | 3 | |||
| Bacillus niacini | 1 | |||
| Bacillus pseudomycoides | 3 | |||
| Bacillus pumilus | 1 | |||
| Bacillus safensis | 1 | |||
| Bacillus sporothermodurans | 1 | |||
| Bacillus subtilis | 1 | |||
| Bacillus zanthoxyli | 1 | |||
| Dyella | 1 | 1 | Dyella thiooxydans | 1 |
| Falsibacillus | 1 | 1 | Falsibacillus pallidus | 1 |
| Fictibacillus | 6 | 1 | Fictibacillus barbaricus | 6 |
| Gottfriedia | 1 | 1 | Gottfriedia acidiceleris | 1 |
| Heyndrickxi | 1 | 1 | Heyndrickxia oleronia | 1 |
| Neobacillus | 10 | 4 | Neobacillus citreus | 1 |
| Neobacillus drentensis | 2 | |||
| Neobacillus ginsengisoli | 6 | |||
| Neobacillus niacini | 1 | |||
| Paenibacillus | 4 | 4 | Paenibacillus cellulositrophicus | 1 |
| Paenibacillus pabuli | 1 | |||
| Paenibacillus septentrionalis | 1 | |||
| Paenibacillus silvae | 1 | |||
| Priestia | 23 | 2 | Priestia aryabhattai | 11 |
| Priestia megaterium | 12 | |||
| Ralstonia | 1 | 1 | Ralstonia pickettii | 1 |
| Rossellomorea | 1 | 1 | Rossellomorea marisflavi | 1 |
| Sinomonas | 2 | 1 | Sinomonas atrocyanea | 2 |
| Streptomyces | 7 | 3 | Streptomyces anandii | 5 |
| Streptomyces geysiriensis | 1 | |||
| Streptomyces triostinicus | 1 | |||
| Trinickia | 1 | 1 | Trinickia diaoshuihuensis | 1 |
| Isolate | Species | Inhibition Rate (%) | ||
|---|---|---|---|---|
| F. falciforme HKFf | F. incarnatum HKFi | F. oxysporum HKFo | ||
| R2A-7 | Sinomonas atrocyanea | 21.88 ± 0.022 cd | 33.75 ± 0.007 de | 21.26 ± 0.017 e |
| KB-5 | Paenibacillus silvae | 16.20 ± 0.055 d | 31.94 ± 0.042 e | 29.14 ± 0.009 d |
| TSA-10E | Priestia megaterium | 50.93 ± 0.029 a | 55.16 ± 0.001 ab | 63.21 ± 0.022 a |
| TSA-1 | Bacillus pumilus | 21.12 ± 0.090 cd | 39.32 ± 0.008 cd | 36.73 ± 0.025 c |
| TSA-6E | Bacillus subtilis | 46.27 ± 0.035 a | 58.54 ± 0.001 a | 49.12 ± 0.034 b |
| YG-2C | Rossellomorea marisflavi | 33.51 ± 0.036 b | 43.10 ± 0.011 cd | 40.95 ± 0.029 c |
| KB-6 | Bacillus cereus | 28.56 ± 0.021 bc | 47.93 ± 0.006 bc | 42.39 ± 0.015 bc |
| Isolate | Species | Inhibition Rate (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C. gloeosporioides | S. rolfsii | P. capsici | M. oryzae | E. turcicum | H. maydis | S. sclerotiorum | F. solani | ||
| R2A-7 | Sinomonas atrocyanea | 20.6 ± 0.038 d | 22.24 ± 0.018 e | 24.82 ± 0.028 d | 38.86 ± 0.018 e | 73.25 ± 0.030 a | 12.78 ± 0.021 d | 15.75 ± 0.038 cd | 8.93 ± 0.011 d |
| KB-5 | Paenibacillus silvae | 17.35 ± 0.007 d | 27.16 ± 0.034 d | 19.48 ± 0.018 e | 51.90 ± 0.005 c | 33.55 ± 0.108 c | 18.00 ± 0.009 c | 8.34 ± 0.004 d | 17.85 ± 0.025 cd |
| TSA-10E | Priestia megaterium | 64.76 ± 0.003 b | 46.72 ± 0.024 a | 43.81 ± 0.019 a | 75.91 ± 0.024 a | 72.76 ± 0.034 a | 29.09 ± 0.006 b | 26.22 ± 0.010 ab | 52.29 ± 0.01 a |
| TSA-1 | Bacillus pumilus | 46.27 ± 0.016 c | 34.30 ± 0.007 c | 36.61 ± 0.039 b | 66.23 ± 0.145 b | 35.18 ± 0.045 c | 26.83 ± 0.086 b | 12.93 ± 0.041 cd | 19.82 ± 0.015 bc |
| TSA-6E | Bacillus subtilis | 77.76 ± 0.016 a | 48.32 ± 0.018 a | 41.35 ± 0.028 a | 73.73 ± 0.041 a | 71.86 ± 0.015 a | 46.98 ± 0.018 a | 29.49 ± 0.055 a | 53.18 ± 0.020 a |
| YG-2C | Rossellomorea marisflavi | 59.69 ± 0.019 b | 37.48 ± 0.036 bc | 30.32 ± 0.043 c | 50.36 ± 0.038 cd | 57.01 ± 0.046 b | 29.50 ± 0.023 b | 19.52 ± 0.057 bc | 15.16 ± 0.081 cd |
| KB-6 | Bacillus cereus | 55.90 ± 0.050 bc | 40.54 ± 0.069 b | 36.06 ± 0.008 b | 44.55 ± 0.080 de | 64.17 ± 0.032 ab | 26.00 ± 0.007 b | 20.87 ± 0.025 bc | 15.60 ± 0.100 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Ma, Y.; Zhang, T.; Liu, W.; Zhang, S.; Chen, Y.; Peng, D.; Zhang, X. Biocontrol Potential of Rhizosphere Bacteria Against Fusarium Root Rot in Cowpea: Suppression of Mycelial Growth and Conidial Germination. Biology 2025, 14, 921. https://doi.org/10.3390/biology14080921
Zhu Q, Ma Y, Zhang T, Liu W, Zhang S, Chen Y, Peng D, Zhang X. Biocontrol Potential of Rhizosphere Bacteria Against Fusarium Root Rot in Cowpea: Suppression of Mycelial Growth and Conidial Germination. Biology. 2025; 14(8):921. https://doi.org/10.3390/biology14080921
Chicago/Turabian StyleZhu, Qinghua, Yixuan Ma, Tong Zhang, Weirong Liu, Songbai Zhang, Yue Chen, Di Peng, and Xin Zhang. 2025. "Biocontrol Potential of Rhizosphere Bacteria Against Fusarium Root Rot in Cowpea: Suppression of Mycelial Growth and Conidial Germination" Biology 14, no. 8: 921. https://doi.org/10.3390/biology14080921
APA StyleZhu, Q., Ma, Y., Zhang, T., Liu, W., Zhang, S., Chen, Y., Peng, D., & Zhang, X. (2025). Biocontrol Potential of Rhizosphere Bacteria Against Fusarium Root Rot in Cowpea: Suppression of Mycelial Growth and Conidial Germination. Biology, 14(8), 921. https://doi.org/10.3390/biology14080921






