Interaction Between Konjac Glucomannan and Gut Microbiota and Its Impact on Health
Simple Summary
Abstract
1. Introduction
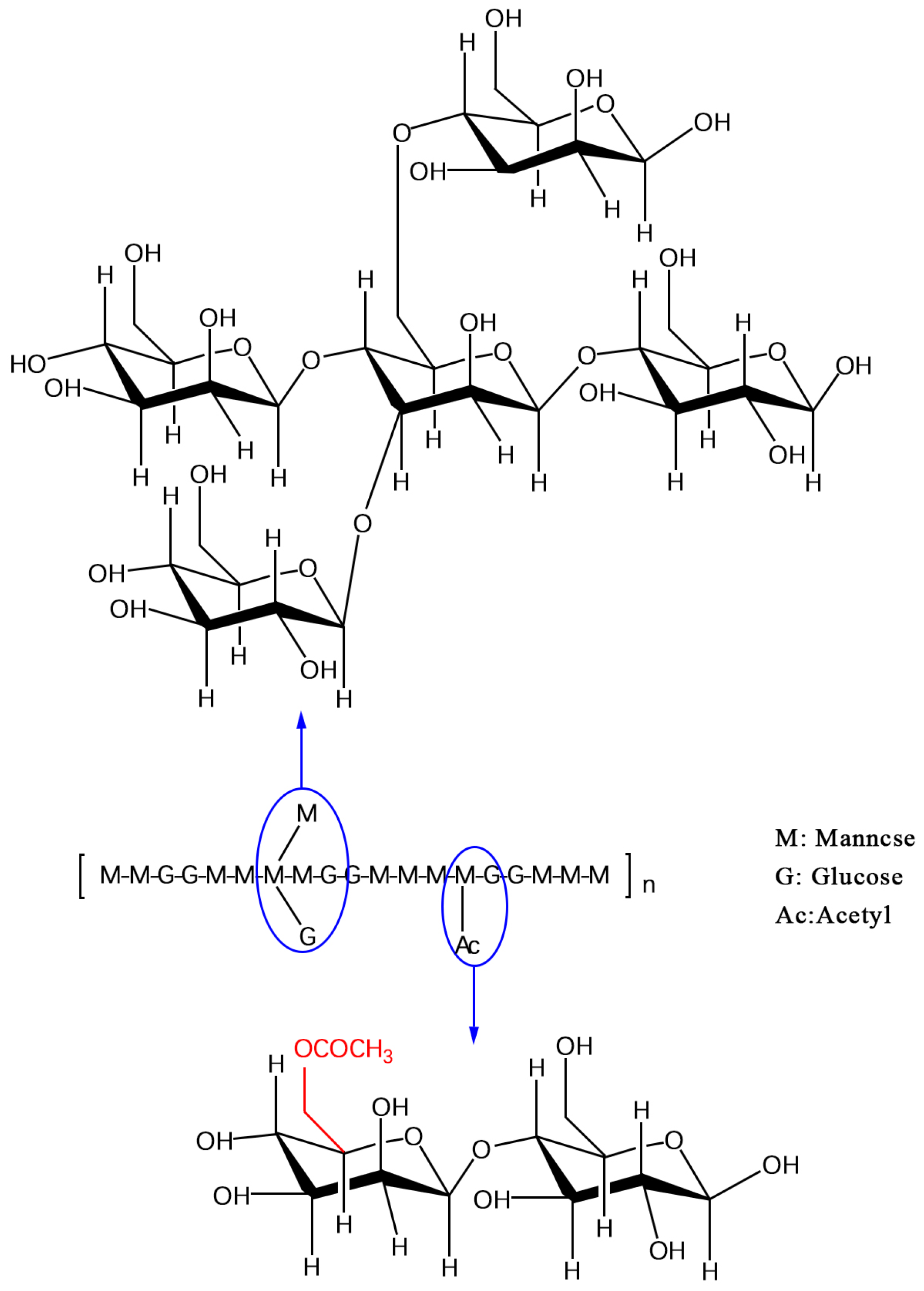
2. Chemical Composition and Structural Characteristics of KGM
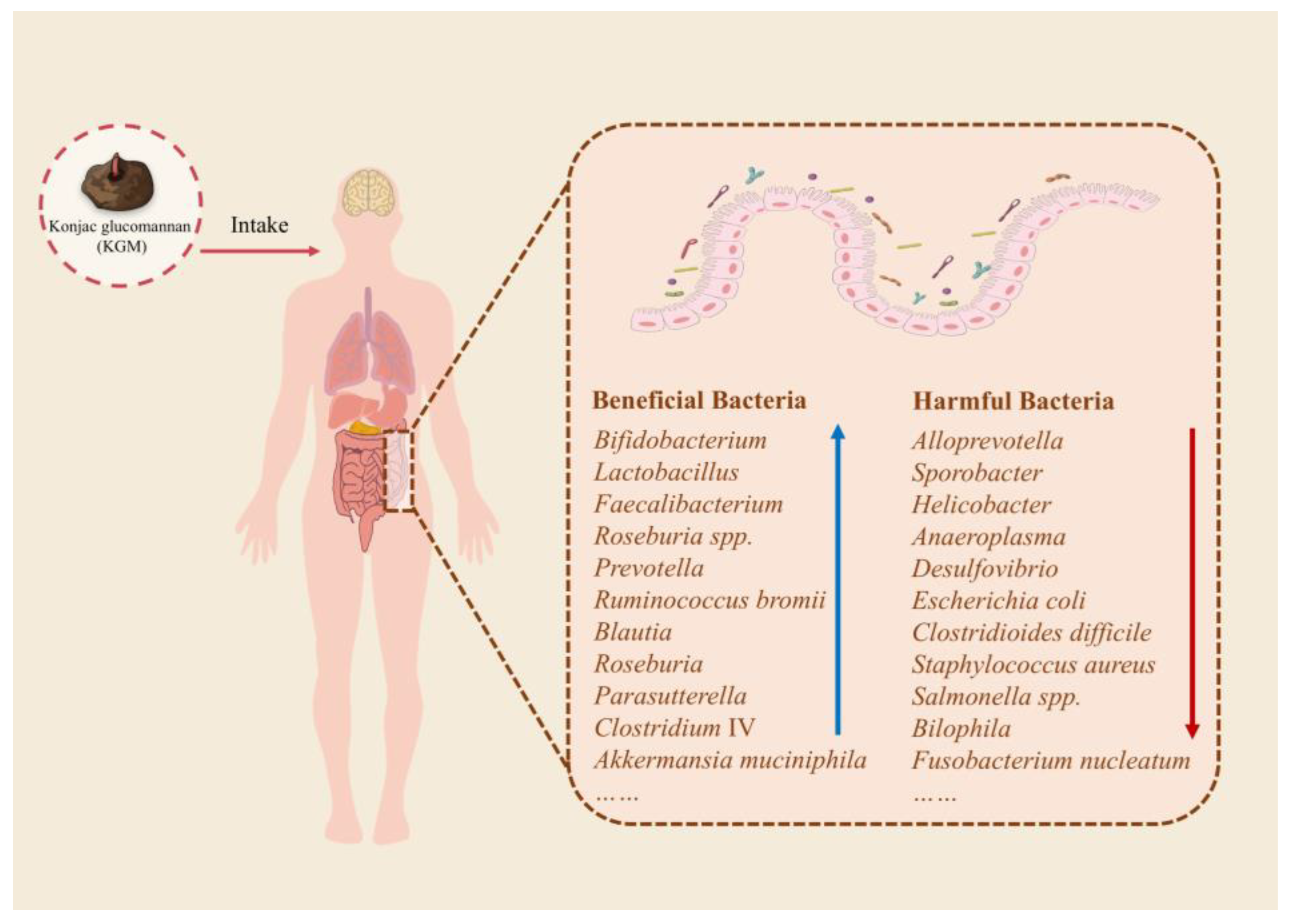
3. Regulatory Effects of KGM on Gut Microbiota
3.1. Changes in Gut Microbiota Composition
3.1.1. The Promoting Effect of KGM on Beneficial Bacteria
3.1.2. The Inhibitory Effect of KGM on Potentially Harmful Bacteria
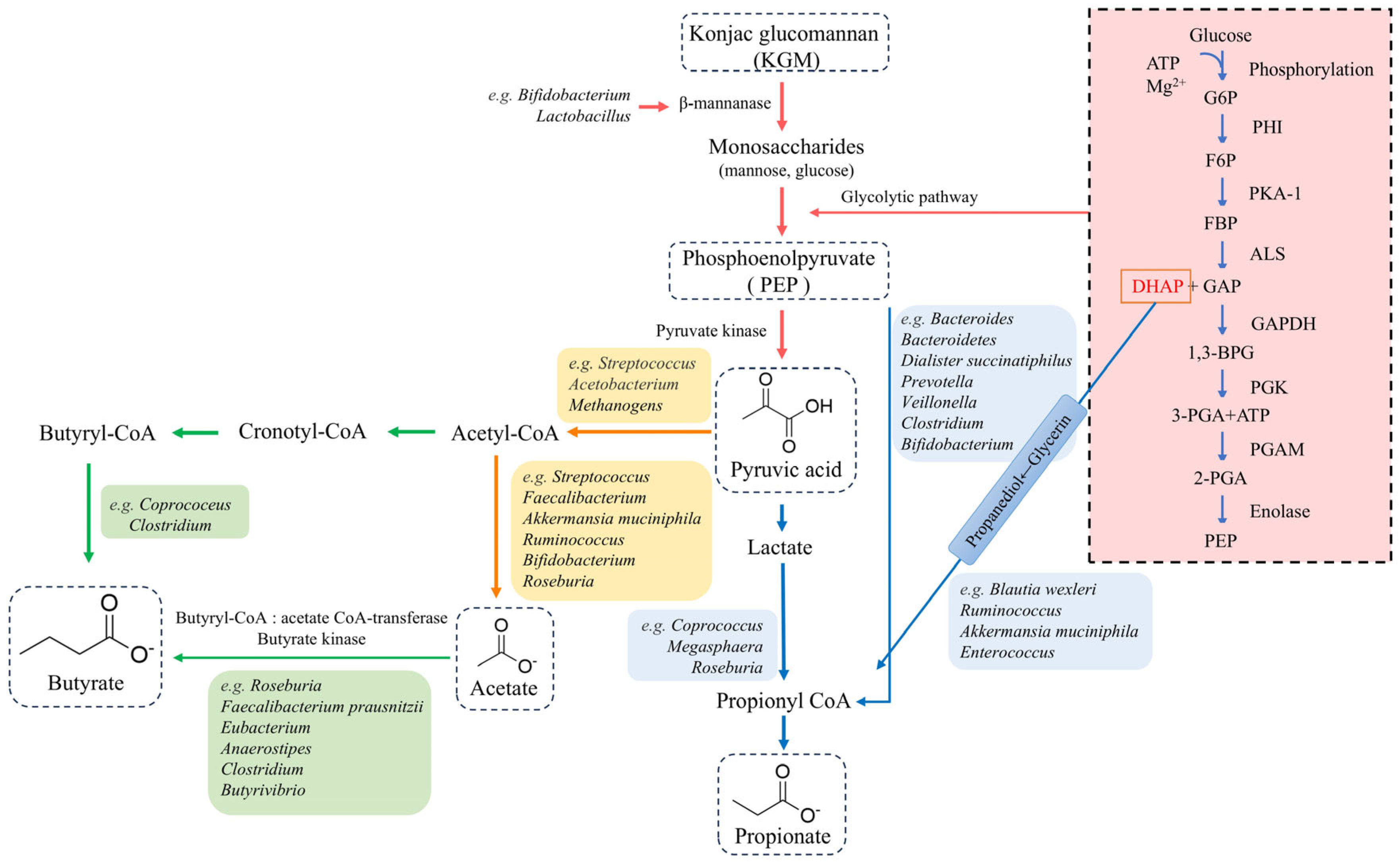
3.2. Metabolites of KGM Fermented by Gut Microbiota
3.2.1. Acetate
3.2.2. Propionate
3.2.3. Butyrate
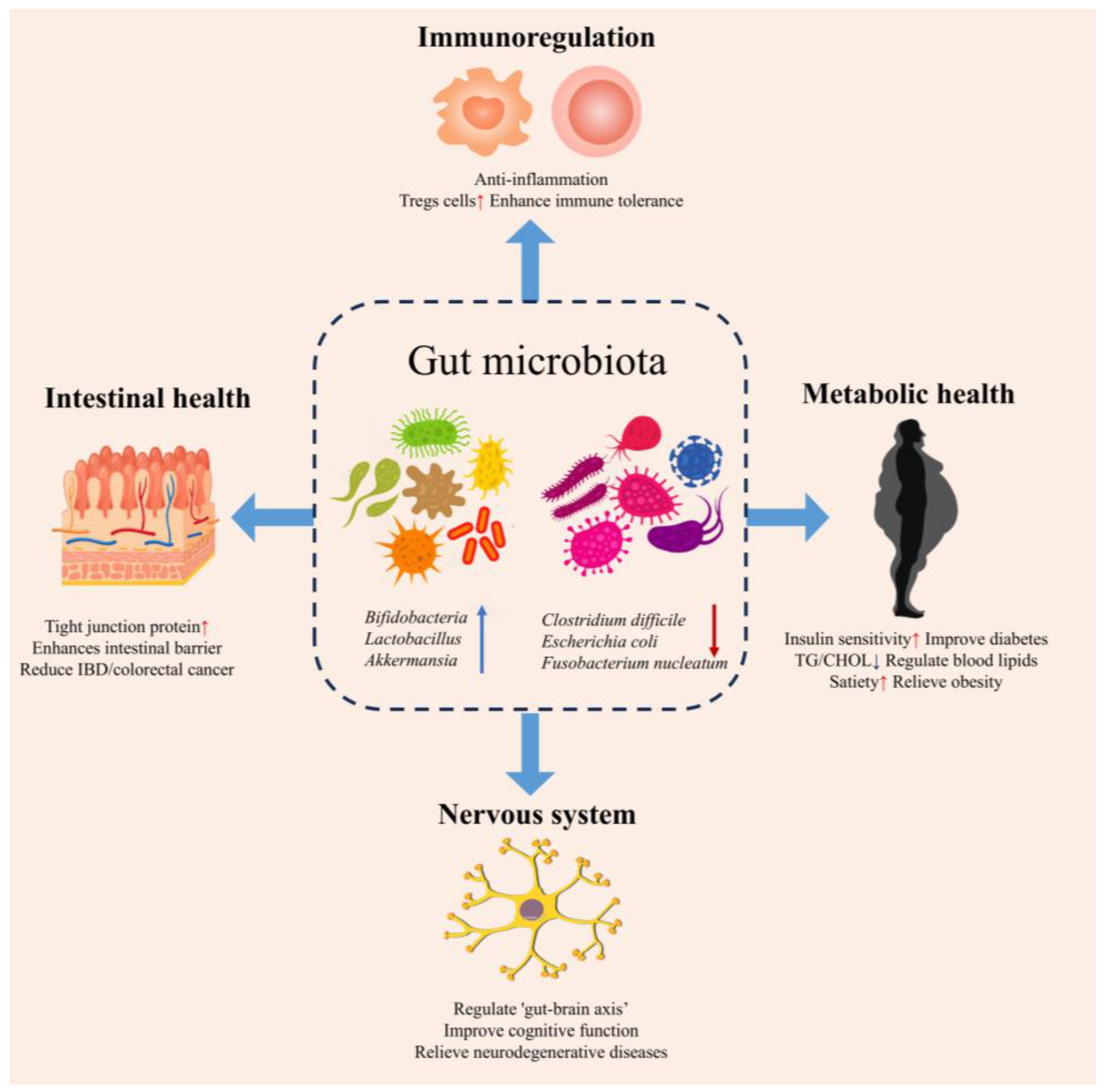
4. The Health Effects of KGM Mediated by Gut Microbiota
4.1. Metabolic Disorders
4.1.1. Obesity
4.1.2. Diabetes
4.1.3. Hyperlipidemia
4.2. Gastrointestinal Disease
4.2.1. Inflammatory Bowel Disease
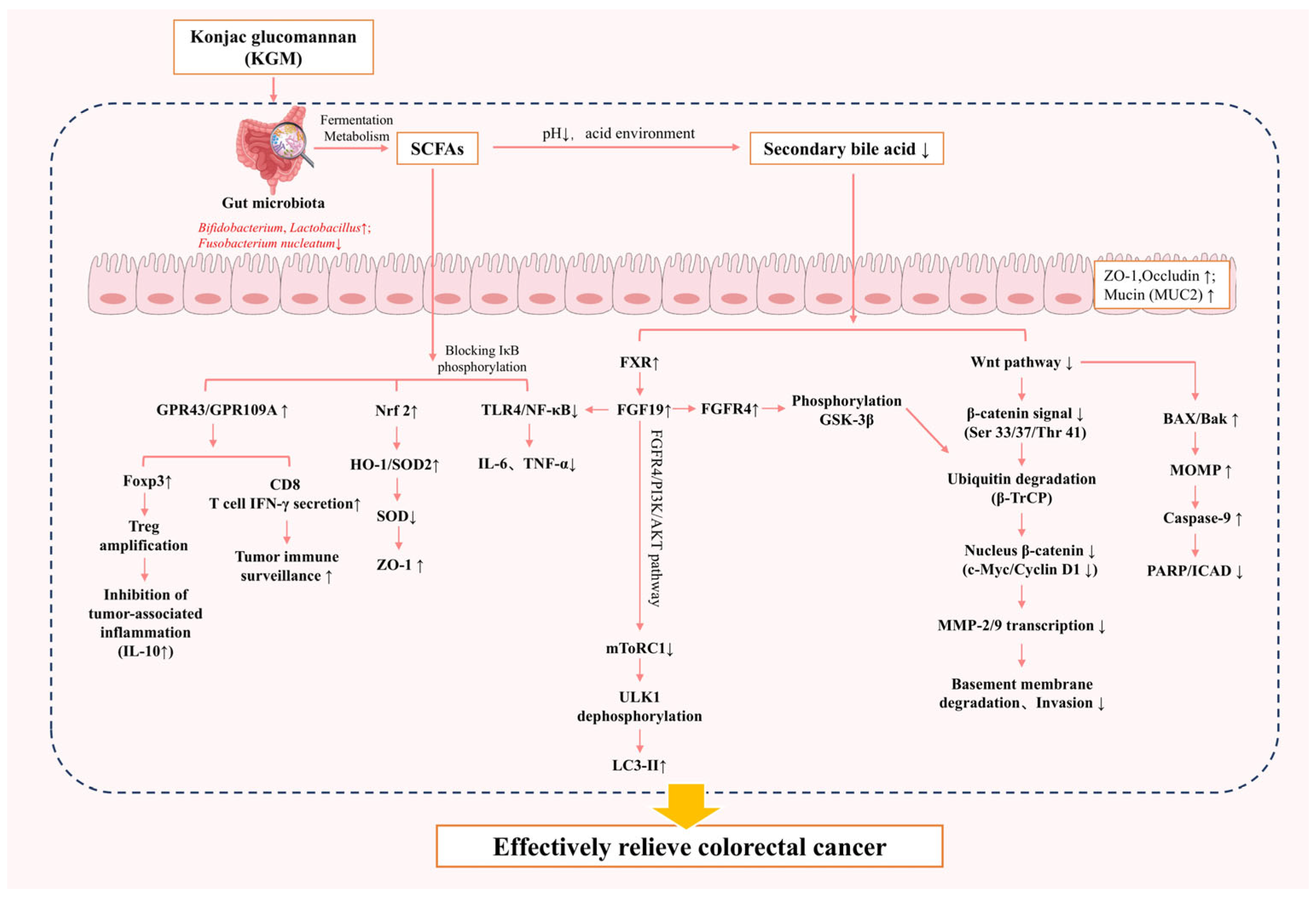
4.2.2. Colorectal Cancer
4.3. Immunoregulation
4.4. Nervous System Diseases
5. Limitations of KGM
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Ting, J.P.; Al-Azzam, S.; Ding, Y.; Afshar, S. Therapeutic advances in diabetes, autoimmune, and neurological diseases. Int. J. Mol. Sci. 2021, 22, 2805. [Google Scholar] [CrossRef] [PubMed]
- Montalto, M.; D’onofrio, F.; Gallo, A.; Cazzato, A.; Gasbarrini, G. Intestinal microbiota and its functions. Dig. Liver Dis. Suppl. 2009, 3, 30–34. [Google Scholar] [CrossRef]
- Pushpanathan, P.; Mathew, G.S.; Selvarajan, S.; Seshadri, K.G.; Srikanth, P. Gut microbiota and its mysteries. Indian. J. Med. Microbiol. 2019, 37, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Pai, L.; Patil, S. Unveiling the dynamics of gut microbial interactions: A review of dietary impact and precision nutrition in gastrointestinal health. Front. Nutr. 2024, 11, 1395664. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Scarano, F.; Nucera, S.; Scicchitano, M.; Oppedisano, F.; Bosco, F.; Ruga, S.; et al. The contribution of gut microbiota–brain axis in the development of brain disorders. Front. Neurosci. 2021, 15, 616883. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sarsaiya, S.; Gong, Q.; Wu, Q.; Shi, J. Amorphophallus konjac: Traditional uses, bioactive potential, and emerging health applications. Front. Plant Sci. 2025, 16, 1530814. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Ray, R.C. Nutritional and potential health benefits of konjac glucomannan, a promising polysaccharide of elephant foot yam, Amorphophallus konjac K. Koch: A review. Food Rev. Int. 2017, 33, 22–43. [Google Scholar] [CrossRef]
- Fang, Y.; Ma, J.; Lei, P.; Wang, L.; Qu, J.; Zhao, J.; Liu, F.; Yan, X.; Wu, W.; Jin, L.; et al. Konjac glucomannan: An emerging specialty medical food to aid in the treatment of type 2 diabetes mellitus. Foods 2023, 12, 363. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.; Jian, S.; Deng, B. Konjac glucomannan: A functional food additive for preventing metabolic syndrome. J. Funct. Foods 2024, 115, 106108. [Google Scholar] [CrossRef]
- Ye, S.; Zongo, A.W.-S.; Shah, B.R.; Li, J.; Li, B. Konjac glucomannan (KGM), deacetylated KGM (Da-KGM), and degraded KGM derivatives: A special focus on colloidal nutrition. J. Agric. Food Chem. 2021, 69, 12921–12932. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.-Y.; Ma, L.-Y.; Xie, M.-Y.; Nie, S.-P.; Wu, J.-Y. Molecular properties and gut health benefits of enzyme-hydrolyzed konjac glucomannans. Carbohydr. Polym. 2020, 237, 116117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Aldamarany, W.A.; Deng, L.; Zhong, G. Carbohydrate supplementation retains intestinal barrier and ameliorates bacterial translocation in an antibiotic-induced mouse model. Food Funct. 2023, 14, 8186–8200. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yokoyama, W.; Chen, M.; Zhong, F. Konjac glucomannan molecular and rheological properties that delay gastric emptying and improve the regulation of appetite. Food Hydrocoll. 2021, 120, 106894. [Google Scholar] [CrossRef]
- Kapoor, D.U.; Sharma, H.; Maheshwari, R.; Pareek, A.; Gaur, M.; Prajapati, B.G.; Castro, G.R.; Thanawuth, K.; Suttiruengwong, S.; Sriamornsak, P. Konjac glucomannan: A comprehensive review of its extraction, health benefits, and pharmaceutical applications. Carbohydr. Polym. 2024, 339, 122266. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.-J.; Ni, Y.; Wang, L.; Yuan, Y.; Yan, Z.; Pang, J.; Chen, S. Fabrication of ordered konjac glucomannan microfiber arrays via facile microfluidic spinning method. Mater. Lett. 2017, 196, 410–413. [Google Scholar] [CrossRef]
- Chua, M.; Baldwin, T.C.; Hocking, T.J.; Chan, K. Traditional uses and potential health benefits of Amorphophallus konjac K. Koch ex. NE Br. J. Ethnopharmacol. 2010, 128, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.; Chan, K.; Hocking, T.J.; Williams, P.A.; Perry, C.J.; Baldwin, T.C. Methodologies for the extraction and analysis of konjac glucomannan from corms of Amorphophallus konjac K. Koch. Carbohydr. Polym. 2012, 87, 2202–2210. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Do, T.T.; Nguyen, T.D.; Pham, L.D.; Du Nguyen, V. Isolation and characteristics of polysaccharide from Amorphophallus corrugatus in Vietnam. Carbohydr. Polym. 2011, 84, 64–68. [Google Scholar] [CrossRef]
- Albrecht, S.; van Muiswinkel, G.C.; Xu, J.; Schols, H.A.; Voragen, A.G.; Gruppen, H. Enzymatic production and characterization of konjac glucomannan oligosaccharides. J. Agric. Food Chem. 2011, 59, 12658–12666. [Google Scholar] [CrossRef] [PubMed]
- Chelliah, R.; Kim, N.H.; Park, S.; Park, Y.; Yeon, S.-J.; Barathikannan, K.; Vijayalakshmi, S.; Oh, D.-H. Revolutionizing renewable resources: Cutting-edge trends and future prospects in the valorization of oligosaccharides. Fermentation 2024, 10, 195. [Google Scholar] [CrossRef]
- Hu, X.; Shi, Y.; Zhang, P.; Miao, M.; Zhang, T.; Jiang, B. D-Mannose: Properties, production, and applications: An overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Pokusaeva, K.; O’Connell-Motherway, M.; Zomer, A.; MacSharry, J.; Fitzgerald, G.F.; van Sinderen, D. Cellodextrin utilization by Bifidobacterium breve UCC2003. Appl. Environ. Microbiol. 2011, 77, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, Y.; Zhang, X.; Fu, B.; Xu, W.; Xue, D.; Chen, N.; Wang, X.; Xie, Q. Construction of sodium alginate/konjac glucomannan/chitosan oligosaccharide/Zeolite P hydrogel microspheres loaded with potassium diformate for sustained intestinal bacterial inhibition. Eur. Polym. J. 2022, 172, 111233. [Google Scholar] [CrossRef]
- Cordeiro, R.L.; Pirolla, R.A.S.; Persinoti, G.F.; Gozzo, F.C.; de Giuseppe, P.O.; Murakami, M.T. N-glycan utilization by bifidobacterium gut symbionts involves a specialist β-mannosidase. J. Mol. Biol. 2019, 431, 732–747. [Google Scholar] [CrossRef] [PubMed]
- Tailford, L.E.; Money, V.A.; Smith, N.L.; Dumon, C.; Davies, G.J.; Gilbert, H.J. Mannose foraging by bacteroides thetaiotaomicron: Structure and specificity of the β-mannosidase, BtMan2A. J. Biol. Chem. 2007, 282, 11291–11299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Yang, W.; Song, G.; Zhong, P.; Ren, Y.; Zhong, G. Structural complexity of Konjac glucomannan and its derivatives governs the diversity and outputs of gut microbiota. Carbohydr. Polym. 2022, 292, 119639. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhuang, B.; Wei, M.; Yuan, T.; Li, J.; Deng, P.; Du, L.; Yuan, B.; Jin, Y. Oral konjac glucomannan for prevention of ionizing radiation-induced injury by regulating gut microbiota and increasing short chain fatty acids. Int. J. Biol. Macromol. 2023, 240, 124402. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C. Genes and molecules of Lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef] [PubMed]
- Sanjiwani, M.I.D.; Aryadi, I.P.H.; Semadi, I.M.S. Review of literature on Akkermansia muciniphila and its possible role in the etiopathogenesis and therapy of type 2 diabetes mellitus. J. ASEAN Fed. Endocr. Soc. 2022, 37, 69. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, S.; Sheng, J.; Yang, X.; Li, J.; Li, B. Konjac Glucomannan and Its Degradation Products Inhibit Intestinal Lipid Absorption by Regulating Gut Microbiota and the Production of Short-Chain Fatty Acids. J. Agric. Food Chem. 2025, 73, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gong, T.; Lu, H.; Ma, S.; Liu, X. In vitro fermentation characteristics of oxidized konjac glucomannan and its modulation effects on gut microbiota. Food Hydrocoll. 2023, 141, 108693. [Google Scholar] [CrossRef]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Deng, J.; Wang, Z.; Zhang, M.; Sun, Y.; Li, M. Gut microbiota plays a predominant role in affecting hypolipidemic effect of deacetylated konjac glucomannan (Da-KGM). Int. J. Biol. Macromol. 2022, 208, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, W.; Cao, M.; Liu, X.; Han, T.; He, W.; Shi, B.; Gu, Z. Maternal supplementation with konjac glucomannan improves maternal microbiota for healthier offspring during lactation. J. Sci. Food Agric. 2024, 104, 3736–3748. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, C.; Fu, B.; Guo, H.; Chen, Y.; Xu, D. Impact of Ligilactobacillus salivarius Li01 on benzo [a] pyrene-induced colitis, based on host-microbiome interactions in mongolian gerbils. Front. Nutr. 2025, 12, 1494525. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Carroll-Portillo, A.; Lin, H.C. Desulfovibrio in the gut: The enemy within? Microorganisms 2023, 11, 1772. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, X.; Fei, W.; Ye, Y.; Zhao, M.; Zheng, C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hao, W.; He, Z.; Kwek, E.; Zhu, H.; Ma, N.; Ma, K.Y.; Chen, Z.-Y. Blueberry and cranberry anthocyanin extracts reduce bodyweight and modulate gut microbiota in C57BL/6 J mice fed with a high-fat diet. Eur. J. Nutr. 2021, 60, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Short-and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Fang, W.; Wu, P. Variations of konjac glucomannan (KGM) from Amorphophallus konjac and its refined powder in China. Food Hydrocoll. 2004, 18, 167–170. [Google Scholar] [CrossRef]
- Shi, X.-D.; Yin, J.-Y.; Cui, S.W.; Wang, Q.; Wang, S.-Y.; Nie, S.-P. Comparative study on glucomannans with different structural characteristics: Functional properties and intestinal production of short chain fatty acids. Int. J. Biol. Macromol. 2020, 164, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, T.-T.; Guo, R.-R.; Ye, Q.; Zhao, H.-L.; Huang, X.-H. The regulation of key flavor of traditional fermented food by microbial metabolism: A review. Food Chem. X 2023, 19, 100871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhong, D.; Ren, Y.-Y.; Meng, Z.-k.; Pegg, R.B.; Zhong, G. Effect of konjac glucomannan on metabolites in the stomach, small intestine and large intestine of constipated mice and prediction of the KEGG pathway. Food Funct. 2021, 12, 3044–3056. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, Y. Degradation of konjac glucomannan by enzymes in human feces and formation of short-chain fatty acids by intestinal anaerobic bacteria. J. Nutr. Sci. Vitaminol. 1998, 44, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Wang, B.; Zhou, X.; Liu, C.; Wang, C.; Bai, J. Fecal fermentation behaviors of Konjac glucomannan and its impacts on human gut microbiota. Food Chem. X 2024, 23, 101610. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, J.; McEwan, A.G.; Kappler, U. Bacterial acetate metabolism and its influence on human epithelia. Emerg. Top. Life Sci. 2023, 8, 1–13. [Google Scholar]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Bernal, V.; Castaño-Cerezo, S.; Cánovas, M. Acetate metabolism regulation in Escherichia coli: Carbon overflow, pathogenicity, and beyond. Appl. Microbiol. Biotechnol. 2016, 100, 8985–9001. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Bai, Y.; Zha, L.; Ullah, N.; Ullah, H.; Shah, S.R.H.; Sun, H.; Zhang, C. Mechanism of the gut microbiota colonization resistance and enteric pathogen infection. Front. Cell. Infect. Microbiol. 2021, 11, 716299. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.A.; McLeod, K.H.; McKenzie, C.I.; Gavin, P.G.; Davalos-Salas, M.; Richards, J.L.; Moore, R.J.; Lockett, T.J.; Clarke, J.M.; Eng, V.V.; et al. An acetate-yielding diet imprints an immune and anti-microbial programme against enteric infection. Clin. Transl. Immunol. 2021, 10, e1233. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Ishii, M.; Akagawa, M. Propionate suppresses hepatic gluconeogenesis via GPR43/AMPK signaling pathway. Arch. Biochem. Biophys. 2019, 672, 108057. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, A.; Chambers, E.S.; Hill, T.; Maldonado, I.R.; Liu, B.; Bewick, G.; Morrison, D.J.; Preston, T.; Wallis, G.A.; Tedford, C.; et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes. Metab. 2017, 19, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Pizarroso, N.A.; Fuciños, P.; Gonçalves, C.; Pastrana, L.; Amado, I.R. A review on the role of food-derived bioactive molecules and the microbiota–gut–brain axis in satiety regulation. Nutrients 2021, 13, 632. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, J.; Bayles, D.O.; Looft, T.; Levine, U.Y.; Allen, H.K.; Drake, H.L. Function and phylogeny of bacterial butyryl coenzyme a: Acetate transferases and their diversity in the proximal colon of swine. Appl. Environ. Microbiol. 2016, 82, 6788–6798. [Google Scholar] [CrossRef] [PubMed]
- van der Beek, C.M.; Bloemen, J.G.; van den Broek, M.A.; Lenaerts, K.; Venema, K.; Buurman, W.A.; Dejong, C.H. Hepatic uptake of rectally administered butyrate prevents an increase in systemic butyrate concentrations in humans. J. Nutr. 2015, 145, 2019–2024. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.; Brummer, R.J. The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.-H. Butyrate producers, “the sentinel of gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.; Cosgrove, L.; Lockett, T.; Head, R.; Topping, D.L. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br. J. Nutr. 2012, 108, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Meijer, K.; de Vos, P.; Priebe, M.G. Butyrate and other short-chain fatty acids as modulators of immunity: What relevance for health? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Pascal, V.; Pozuelo, M.; Borruel, N.; Casellas, F.; Campos, D.; Santiago, A.; Martinez, X.; Varela, E.; Sarrabayrouse, G.; Machiels, K. A microbial signature for crohn’s disease. Gut 2017, 66, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yang, J.; Xia, P.; Li, S.; Wang, Q.; Li, K.; Li, B.; Li, J. Effects of konjac glucomannan intake patterns on glucose and lipid metabolism of obese mice induced by a high fat diet. Food Funct. 2024, 15, 9116–9135. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yao, L.; Chen, W.; Hou, T.; Li, J.; Li, B. Konjac glucomannan inhibits appetite of obese mice by suppressing hypothalamic inflammatory response and Agrp/Npy Neuron expression. J. Agric. Food Chem. 2024, 72, 24489–24503. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zou, X.; Liang, Y.; Zhong, J.; Zhou, K.; Zhang, J.; Zhang, M.; Wang, Z.; Sun, Y.; Li, M. Hypoglycemic effects of different molecular weight konjac glucomannans via intestinal microbiota and SCFAs mediated mechanism. Int. J. Biol. Macromol. 2023, 234, 122941. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Goff, H.D.; Ding, C.; Liu, Q.; Zhao, S.; Tao, T.; Lu, R.; Gao, Y.; Wu, H.; Guo, L. Enhanced hypoglycemic effects of konjac glucomannan combined with Polygonatum cyrtonema Hua polysaccharide in complete nutritional liquid diet fed type 2 diabetes mice. Int. J. Biol. Macromol. 2024, 266, 131121. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Fu, H.; Yang, Y.; Wu, Z. An isofibrous diet with fiber konjac glucomannan ameliorates Salmonella typhimurium-induced colonic injury by regulating TLR2-NF-κB signaling and intestinal microbiota in mice. J. Agric. Food Chem. 2024, 72, 13415–13430. [Google Scholar] [CrossRef] [PubMed]
- Changchien, C.-H.; Wang, C.-H.; Chen, H.-L. Konjac glucomannan polysaccharide and inulin oligosaccharide ameliorate dextran sodium sulfate-induced colitis and alterations in fecal microbiota and short-chain fatty acids in C57BL/6J mice. BioMedicine 2021, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-T.; Chen, H.-L. Effects of konjac glucomannan on putative risk factors for colon carcinogenesis in rats fed a high-fat diet. J. Agric. Food Chem. 2011, 59, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Chen, J.; Ran, X.; Deng, L.; Deng, Y.; Liu, C.; Long, S.; Xie, J.; Peng, T.; Zhang, X. Konjac oligo-glucomannan ameliorate cognition impairments of Aβ1-42 induced alzheimer’s disease in mice by targeting microbiota-SCFAs-brain axis. J. Funct. Foods 2024, 122, 106469. [Google Scholar] [CrossRef]
- Liu, B.-N.; Liu, X.-T.; Liang, Z.-H.; Wang, J.-H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.-D.; Yang, F.-Q. Konjac glucomannan, a promising polysaccharide for OCDDS. Carbohydr. Polym. 2014, 104, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jayachandran, M.; Xu, B. In vivo antioxidant and anti-inflammatory effects of soluble dietary fiber konjac glucomannan in type-2 diabetic rats. Int. J. Biol. Macromol. 2020, 159, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Vasques, C.A.; Rossetto, S.; Halmenschlager, G.; Linden, R.; Heckler, E.; Fernandez, M.S.P.; Alonso, J.L.L. Evaluation of the pharmacotherapeutic efficacy of Garcinia cambogia plus Amorphophallus konjac for the treatment of obesity. Phytother. Res. 2008, 22, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Ciobârcă, D.; Cătoi, A.F.; Copăescu, C.; Miere, D.; Crișan, G. Bariatric surgery in obesity: Effects on gut microbiota and micronutrient status. Nutrients 2020, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yokoyama, W.; Chen, L.; Chen, M.; Zhong, F. Dynamic hydration and viscosity control of konjac glucomannan enhance long-term antiobesity effects by reducing food intake in high-fat-diet-fed mice. J. Agric. Food Chem. 2024, 72, 24561–24575. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab. 2018, 27, 1222–1235.e1226. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fang, J.; Huang, W.; Liu, S.; Zhang, X.; Gong, G.; Huang, L.; Lin, X.; Wang, Z. The intervention effects of konjac glucomannan with different molecular weights on high-fat and high-fructose diet-fed obese mice based on the regulation of gut microbiota. Food Res. Int. 2023, 165, 112498. [Google Scholar] [CrossRef] [PubMed]
- Kreutzer, C.; Peters, S.; Schulte, D.M.; Fangmann, D.; Türk, K.; Wolff, S.; van Eimeren, T.; Ahrens, M.; Beckmann, J.; Schafmayer, C.; et al. Hypothalamic inflammation in human obesity is mediated by environmental and genetic factors. Diabetes 2017, 66, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Yu, Y.; Zhang, H.; Du, J.; Sun, J.; Chen, L.; Feng, F.; Guan, R. Lactiplantibacillus plantarum N1 derived lipoteichoic acid alleviates insulin resistance in association with modulation of gut microbiota and amino acids metabolism. Food Funct. 2025, 16, 1371–1388. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Shan, Y.; Song, W. Targeting gut microbiota as a possible therapy for diabetes. Nutr. Res. 2015, 35, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ma, C.; Han, L.; Nawaz, M.; Gao, F.; Zhang, X.; Yu, P.; Zhao, C.A.; Li, L.; Zhou, A.; et al. Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr. Microbiol. 2010, 61, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Furet, J.-P.; Kong, L.-C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.-L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C. Differential adaptation of human gut microbiota to bariatric surgery–induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Lee, J.E. Dietary patterns related to triglyceride and high-density lipoprotein cholesterol and the incidence of type 2 diabetes in Korean men and women. Nutrients 2018, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Lee, A.R.; Kim, H.; Jhun, J.; Lee, S.-Y.; Choi, J.W.; Jeong, Y.; Park, M.S.; Ji, G.E.; Cho, M.-L.; et al. Faecalibacterium prausnitzii alleviates inflammatory arthritis and regulates IL-17 production, short chain fatty acids, and the intestinal microbial flora in experimental mouse model for rheumatoid arthritis. Arthritis Res. Ther. 2023, 25, 130. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Nie, Q.; Hu, J.; Huang, X.; Zhang, K.; Pan, S.; Nie, S. Hypoglycemic and hypolipidemic effects of glucomannan extracted from konjac on type 2 diabetic rats. J. Agric. Food Chem. 2019, 67, 5278–5288. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Sheu, W.H.-H.; Tai, T.-S.; Liaw, Y.-P.; Chen, Y.-C. Konjac supplement alleviated hypercholesterolemia and hyperglycemia in type 2 diabetic subjects—A randomized double-blind trial. J. Am. Coll. Nutr. 2003, 22, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.-J.; Lee, J.; Shin, N.-R.; Kim, M.-S.; Hyun, D.-W.; Yun, J.-H.; Kim, P.S.; Whon, T.W.; Bae, J.-W. Chronic repression of mTOR complex 2 induces changes in the gut microbiota of diet-induced obese mice. Sci. Rep. 2016, 6, 30887. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Y.; Fu, J. Advances in antiobesity mechanisms of capsaicin. Curr. Opin. Pharmacol. 2021, 61, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, C.; You, N.; Zhang, J. Relationship between Helicobacter pylori and glycated hemoglobin: A cohort study. Front. Cell. Infect. Microbiol. 2023, 13, 1196338. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Zhou, M.; Zogona, D.; Xing, Z.; Wu, T.; Chen, R.; Cui, D.; Liang, F.; Xu, X. Akkermansia muciniphila: A potential candidate for ameliorating metabolic diseases. Front. Immunol. 2024, 15, 1370658. [Google Scholar] [CrossRef] [PubMed]
- Mangiafico, S.; Costello-Boerrigter, L.C.; Andersen, I.A.; Cataliotti, A.; Burnett, J.C., Jr. Neutral endopeptidase inhibition and the natriuretic peptide system: An evolving strategy in cardiovascular therapeutics. Eur. Heart J. 2013, 34, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Roso, S.; Samuel, M.; Pladevall-Vila, M. Unmet need in the hyperlipidaemia population with high risk of cardiovascular disease: A targeted literature review of observational studies. BMC Cardiovasc. Disord. 2016, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Wang, Y.; Ren, Y.; Ai, T.; Zhou, P.; Hu, L.; Wang, L.; Li, J.; Li, B. In vitro gastric emptying characteristics of konjac glucomannan with different viscosity and its effects on appetite regulation. Food Funct. 2020, 11, 7596–7610. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.; Canfora, E.; Theis, S.; Frost, G.; Groen, A.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; Van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Haeri, M.; Parham, M.; Habibi, N.; Vafaeimanesh, J. Effect of Helicobacter pylori infection on serum lipid profile. J. Lipids 2018, 2018, 6734809. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wan, H.; Fan, H.; Zhang, Z.; Dai, H.; He, H. Complexation of starch and konjac glucomannan during screw extrusion exhibits obesity-reducing effects by modulating the intestinal microbiome and its metabolites. Food Funct. 2025, 16, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.; Maurya, A.K.; Mohanty, S.; Kumar, M.; Virdi, J.S. Evaluation of bile salt hydrolases, cholesterol-lowering capabilities, and probiotic potential of Enterococcus faecium isolated from rhizosphere. Front. Microbiol. 2019, 10, 1567. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Okpara, E.S.; Hu, W.; Yan, C.; Wang, Y.; Liang, Q.; Chiang, J.Y.; Han, S. Interactive relationships between intestinal flora and bile acids. Int. J. Mol. Sci. 2022, 23, 8343. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Kanai, T. The gut microbiota and inflammatory bowel disease. In the Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 47–55. [Google Scholar]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of disease: Inflammatory bowel diseases. In the Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2019; pp. 155–165. [Google Scholar]
- Lavelle, A.; Hoffmann, T.W.; Pham, H.-P.; Langella, P.; Guédon, E.; Sokol, H. Baseline microbiota composition modulates antibiotic-mediated effects on the gut microbiota and host. Microbiome 2019, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Luo, X.; Abdullah; Liu, X.; Hussain, M.; Guan, R. Nano-targeted delivery system: A promising strategy of anthocyanin encapsulation for treating intestinal inflammation. Crit. Rev. Food Sci. Nutr. 2025, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; He, C.; Cong, Y.; Liu, Z. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol. 2015, 8, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fu, R.; Xu, D.; Chen, Y.; Yue, S.; Zhang, S.; Tang, Y. Traditional chinese medicine: A promising strategy to regulate the imbalance of bacterial flora, impaired intestinal barrier and immune function attributed to ulcerative colitis through intestinal microecology. J. Ethnopharmacol. 2024, 318, 116879. [Google Scholar] [CrossRef] [PubMed]
- Changchien, C.-H.; Han, Y.-C.; Chen, H.-L. Konjac glucomannan polysaccharide and inulin oligosaccharide enhance the colonic mucosal barrier function and modulate gut-associated lymphoid tissue immunity in C57BL/6J mice. Br. J. Nutr. 2020, 123, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Cheng, H.-C.; Liu, Y.-J.; Liu, S.-Y.; Wu, W.-T. Konjac acts as a natural laxative by increasing stool bulk and improving colonic ecology in healthy adults. Nutrition 2006, 22, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Zhao, M.; Jin, H.; Hou, T.; Deng, Z.; Zhang, M.; Zhou, Q.; Zhan, F.; Li, B.; Li, J. Konjac glucomannan-assisted curcumin alleviated dextran sulfate sodium-induced mice colitis via regulating immune response and maintaining intestinal barrier integrity. Food Funct. 2023, 14, 8747–8760. [Google Scholar] [CrossRef] [PubMed]
- Osman, N.; Adawi, D.; Molin, G.; Ahrne, S.; Berggren, A.; Jeppsson, B. Bifidobacterium infantis strains with and without a combination of oligofructose and inulin (OFI) attenuate inflammation in DSS-induced colitis in rats. BMC Gastroenterol. 2006, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M.H. Clostridium difficile infection and pseudomembranous colitis. Best Pract. Res. Clin. Gastroenterol. 2003, 17, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-B.; Wang, P.-Y.; Wang, X.; Wan, Y.-L.; Liu, Y.-C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Li, J.; Huang, R. The immunoregulatory role of gut microbiota in the incidence, progression, and therapy of breast cancer. Front. Cell. Infect. Microbiol. 2024, 14, 1411249. [Google Scholar] [CrossRef] [PubMed]
- Gasaly, N.; De Vos, P.; Hermoso, M.A. Impact of bacterial metabolites on gut barrier function and host immunity: A focus on bacterial metabolism and its relevance for intestinal inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Jiang, J.; Hussain, M.; Zhang, H.; Chen, L.; Guan, R. The encapsulation strategies for targeted delivery of probiotics in preventing and treating colorectal cancer: A review. Adv. Sci. 2025, 12, 2500304. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Coker, O.O.; Nakatsu, G.; Wu, W.K.; Zhao, L.; Chen, Z.; Chan, F.K.; Kristiansen, K.; Sung, J.J.; Wong, S.H.; et al. Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome 2018, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Liang, H.; Ji, X.; Zhou, Z.; Liu, Y.; Sun, T.; Zhang, L. Effects of fucoidan on gut flora and tumor prevention in 1, 2-dimethylhydrazine-induced colorectal carcinogenesis. J. Nutr. Biochem. 2020, 82, 108396. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-T.; Cheng, H.-C.; Chen, H.-L. Ameliorative effects of konjac glucomannan on human faecal β-glucuronidase activity, secondary bile acid levels and faecal water toxicity towards Caco-2 cells. Br. J. Nutr. 2011, 105, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Fini, A.; Roda, A. Chemical properties of bile acids. IV. Acidity constants of glycine-conjugated bile acids. J. Lipid Res. 1987, 28, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, S.; Zhou, W.; Hu, D.; Xu, H.; Ji, G. Secondary bile acids and tumorigenesis in colorectal cancer. Front. Oncol. 2022, 12, 813745. [Google Scholar] [CrossRef] [PubMed]
- Caliceti, C.; Punzo, A.; Silla, A.; Simoni, P.; Roda, G.; Hrelia, S. New insights into bile acids related signaling pathways in the onset of colorectal cancer. Nutrients 2022, 14, 2964. [Google Scholar] [CrossRef] [PubMed]
- Sarathy, J.; Detloff, S.J.; Ao, M.; Khan, N.; French, S.; Sirajuddin, H.; Nair, T.; Rao, M.C. The yin and yang of bile acid action on tight junctions in a model colonic epithelium. Physiol. Rep. 2017, 5, e13294. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Leghari, A.; Li, X.; Su, Y.; Gu, L.; Yang, Y.; Li, J. Konjac gum and maltodextrin compound tablets as carriers of IgY for sustained release in stomach. Int. Food Res. J. 2023, 30, 1297–1303. [Google Scholar] [CrossRef]
- Du, X.; Yang, L.; Ye, X.; Li, B. Antibacterial activity of konjac glucomannan/chitosan blend films and their irradiation-modified counterparts. Carbohydr. Polym. 2013, 92, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, M.; Salehi, R.; Haghjooy Javanmard, S.; Rafiee, L.; Faraji, H.; Jafarpor, S.; Ferns, G.A.; Ghayour-Mobarhan, M.; Manian, M.; Nedaeinia, R. The dysbiosis signature of Fusobacterium nucleatum in colorectal cancer-cause or consequences? A systematic review. Cancer Cell Int. 2021, 21, 194. [Google Scholar] [CrossRef] [PubMed]
- Galasso, L.; Termite, F.; Mignini, I.; Esposto, G.; Borriello, R.; Vitale, F.; Nicoletti, A.; Paratore, M.; Ainora, M.E.; Gasbarrini, A.; et al. Unraveling the role of Fusobacterium nucleatum in colorectal cancer: Molecular mechanisms and pathogenic insights. Cancers 2025, 17, 368. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, A.A.; Lyra, A.; Ouwehand, A.C.; Rolny, P.; Lindegren, H.; Cedgård, L.; Wettergren, Y. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017, 4, e000145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhong, D.; Sun, R.; Zhang, Y.; Pegg, R.B.; Zhong, G. Prevention of loperamide induced constipation in mice by KGM and the mechanisms of different gastrointestinal tract microbiota regulation. Carbohydr. Polym. 2021, 256, 117418. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Giardino, G.; Gallo, V.; Prencipe, R.; Gaudino, G.; Romano, R.; De Cataldis, M.; Lorello, P.; Palamaro, L.; Di Giacomo, C.; Capalbo, D.; et al. Unbalanced immune system: Immunodeficiencies and autoimmunity. Front. Pediatr. 2016, 4, 107. [Google Scholar] [CrossRef] [PubMed]
- Kato, L.M.; Kawamoto, S.; Maruya, M.; Fagarasan, S. The role of the adaptive immune system in regulation of gut microbiota. Immunol. Rev. 2014, 260, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Zhu, H.; Bao, Z.; Han, D.; Du, J.; Zhao, M.; Feng, F.; He, G.; Mo, Q. Lactiplantibacillus plantarum T34 alleviates constipation by enhancing intestinal barrier and modulating gut homeostasis. Food Biosci. 2025, 66, 106195. [Google Scholar] [CrossRef]
- Cervantes, J.L.; Hong, B.-Y. Dysbiosis and immune dysregulation in outer space. Int. Rev. Immunol. 2016, 35, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.; Romero, E.; Garrido-Sanchez, L.; Alcain-Martinez, G.; Andrade, R.J.; Taminiau, B.; Daube, G.; Garcia-Fuentes, E. Microbiota insights in Clostridium difficile infection and inflammatory bowel disease. Gut Microbes 2020, 12, 1725220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, D.; Jiang, C.; Li, Z.; Wang, X.; Peng, Y. Insight into alteration of gut microbiota in Clostridium difficile infection and asymptomatic C. difficile colonization. Anaerobe 2015, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, Z.; Yang, Z.; Qiao, X. Microbiota-derived short-chain fatty acids and modulation of host-derived peptides formation: Focused on host defense peptides. Biomed. Pharmacother. 2023, 162, 114586. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Sun, Y.; Hu, W.; Chen, C.; Lin, Q.; Nie, S. Glucomannan promotes Bacteroides ovatus to improve intestinal barrier function and ameliorate insulin resistance. Imeta 2024, 3, e163. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Niculescu, A.-G.; Roza, E.; Vladâcenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters—Key factors in neurological and neurodegenerative disorders of the central nervous system. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wu, B.; Liang, J.; He, F.; Gu, W.; Li, K.; Luo, Y.; Chen, J.; Gao, Y.; Wu, Z.; et al. Altered gut microbiota and mucosal immunity in patients with schizophrenia. Brain Behav. Immun. 2020, 85, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.-D.; Xiao, Q. Alteration of the fecal microbiota in chinese patients with parkinson’s disease. Brain Behav. Immun. 2018, 70, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schreiber IV, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial genes, brain & behaviour–epigenetic regulation of the gut–brain axis. Genes Brain Behav. 2014, 13, 69–86. [Google Scholar] [PubMed]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.M.; Spiegel, J.; Dillmann, K.-U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.-H. Short chain fatty acids and gut microbiota differ between patients with parkinson’s disease and age-matched controls. Park. Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.M.; Sharrett, A.R.; Schneider, A.L.; Coresh, J.; Albert, M.; Couper, D.; Griswold, M.; Gottesman, R.F.; Wagenknecht, L.E.; Windham, B.G. Diabetes in midlife and cognitive change over 20 years: A cohort study. Ann. Intern. Med. 2014, 161, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Willette, A.A.; Bendlin, B.B.; Starks, E.J.; Birdsill, A.C.; Johnson, S.C.; Christian, B.T.; Okonkwo, O.C.; La Rue, A.; Hermann, B.P.; Koscik, R.L.; et al. Association of insulin resistance with cerebral glucose uptake in late middle–aged adults at risk for alzheimer disease. JAMA Neurol. 2015, 72, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, R.D.; Reddy, C.K.; Xu, B. Health-promoting effects of konjac glucomannan and its practical applications: A critical review. Int. J. Biol. Macromol. 2019, 126, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, M.; Subramanian, U.; Venkidasamy, B.; Thirupathi, P.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Chung, I.-M.; Rengasamy, K.R. Emerging role of nutritional short-chain fatty acids (SCFAs) against cancer via modulation of hematopoiesis. Crit. Rev. Food Sci. Nutr. 2023, 63, 827–844. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.-H.; Xie, R.-Y.; Liu, X.-L.; Chen, S.-D.; Tang, H.-D. Mechanisms of short-chain fatty acids derived from gut microbiota in alzheimer’s disease. Aging Dis. 2022, 13, 1252. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.E.; Triscott, J.; Emerling, B.M.; Hammond, G.R. Beyond PI3Ks: Targeting phosphoinositide kinases in disease. Nat. Rev. Drug Discov. 2023, 22, 357–386. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, L.; Shen, Y.; Wang, L.; Lai, X.; Hu, H. Qingxin Kaiqiao Fang decreases Tau hyperphosphorylation in Alzheimer’s disease via the PI3K/Akt/GSK3β pathway in vitro and in vivo. J. Ethnopharmacol. 2024, 318, 117031. [Google Scholar] [CrossRef] [PubMed]
- Grünert, S.C.; Wendel, U.; Lindner, M.; Leichsenring, M.; Schwab, K.O.; Vockley, J.; Lehnert, W.; Ensenauer, R. Clinical and neurocognitive outcome in symptomatic isovaleric acidemia. Orphanet J. Rare Dis. 2012, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Missiego-Beltrán, J.; Olalla-Álvarez, E.M.; González-Brugera, A.; Beltrán-Velasco, A.I. Implications of butyrate signaling pathways on the motor symptomatology of parkinson’s disease and neuroprotective effects—Therapeutic approaches: A systematic review. Int. J. Mol. Sci. 2024, 25, 8998. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Wang, M.; Zheng, L.; Cen, Q.; Wang, F.; Zhu, L.; Pang, R.; Zhang, A. Bifidobacterium: A probiotic for the prevention and treatment of depression. Front. Microbiol. 2023, 14, 1174800. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, R.; Chen, Y.; Wang, Z.; Dong, Y. 5-HT attenuates chronic stress-induced cognitive impairment in mice through intestinal flora disruption. J. Neuroinflamm. 2023, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Lee, J.-W.; Lim, S.-D. The probiotic characteristics and GABA production of Lactobacillus plantarum K154 isolated from kimchi. Food Sci. Biotechnol. 2014, 23, 1951–1957. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Choi, T.G.; Kim, S.S. Role of short chain fatty acids in epilepsy and potential benefits of probiotics and prebiotics: Targeting “health” of epileptic patients. Nutrients 2022, 14, 2982. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Dong, H.; Li, X.; Hu, C.; Cui, F.; Li, S.; Zhang, X.; Du, Y.; Yang, P.; Ji, W.; et al. L-arabinose alleviates functional constipation in mice by regulating gut microbiota and metabolites. Foods 2025, 14, 900. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Nakamura, T.; Aoyama, N.; Matsuura, M.; Kawara, A. Metabolic and nutritional effects of long-term use of konjac glucomannan in the treatment of obese or non-obese diabetics. In the Progress in Obesity Research, Proceedings of the Sixth International Congress on Obesity; John Libbey: London, UK, 1990; pp. 507–514. [Google Scholar]
- Onakpoya, I.; Posadzki, P.; Ernst, E. The efficacy of glucomannan supplementation in overweight and obesity: A systematic review and meta-analysis of randomized clinical trials. J. Am. Coll. Nutr. 2014, 33, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, X.; Zhang, Q.; Zhang, D.; Xie, X.; Zhou, H.; Wu, Z.; Liu, R.; Pang, J. Review of konjac glucomannan structure, properties, gelation mechanism, and application in medical biology. Polymers 2023, 15, 1852. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Matsuura, M.; Kawara, A.; Tanaka, T.; Baba, S. Influence of dietary fiber (konjac mannan) on absorption of vitamin B12 and vitamin E. Tohoku J. Exp. Med. 1983, 141, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Aldamarany, W.A.; Song, G.; Liu, J.; Liu, S.; Chen, Y.; Jiang, W.; Zhong, G. Influence of konjac glucomannan and its derivatives on the oral pharmacokinetics of antimicrobial agent in antibiotics cocktails: Keep vigilant on dietary fiber supplement. Int. J. Biol. Macromol. 2023, 251, 126306. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V. Can konjac (Amorphophallus konjac K. Koch) and it constituent glucomannan be useful for treatment of obesity? Obes. Med. 2021, 24, 100343. [Google Scholar] [CrossRef]
- Shima, K.; Tanaka, A.; Ikegami, H.; Tabata, M.; Sawazaki, N.; Kumahara, Y. Effect of dietary fiber, glucomannan, on absorption of sulfonylurea in man. Horm. Metab. Res. 1983, 15, 1–3. [Google Scholar] [CrossRef] [PubMed]
| Disease Type | Dosage | Experimental Subject | Microbiota Influence | Others’ Influence | Conclusions | Reference |
|---|---|---|---|---|---|---|
| Obesity | 400 mg/kg | C57Bl/6J | unclassified_f_Lachnospiraceae↑, norank_f_Lachnospiraceae↑, Blautia↑, Romboutsia↑, Colidextribacter↑, norank_f_Oscillospiraceae↑, Lachnospiraceae_NK4A136↑, Faecalibacterium↓, Muribaculaceae↓ | Body weight↓, fat mass↓, FBG↓, insulin resistance↓; appetite regulator GLP-1 and intestinal hormone PYY↑; blood lipid levels TG, TC and LDL-C↓, HDL-C↑; inflammatory factors TNF-α, IL-1β, and IL-6↓; expression of lipid metabolism genes LDLR, GCK, and G-6-pase mRNA↑; SCFAs↑ | Konjac dietary fiber (KGM) intake reduces body weight in obese mice, improves glucose and lipid metabolism homeostasis, and modulates gut microbiota composition. | [71] |
| Obesity | 100 g/kg | C57Bl/6J | Bifidobacterium↑, Lactobacillus↑, Alistipes↑, Clostridium_XlVa↑, Blautia↓, Allobaculum↓, Saccharibacteria↓, Enterorhabdus↓, Coprococcus↓ | Inguinal fat↓, brown fat↑; food intake and energy intake↓, energy consumption and heat production↑; leptin and adiponectin↓, GLP-1↑; inflammatory factors TNF-α, IL-6, and IL-1β↓; repair of intestinal epithelial barrier injury, Claudin-1 and Occludin protein expression↑; expression of Agrp, Npy, and Orx in hypothalamus of obese mice↓, Cart expression↑; SCFAs↑ | KGM modulates energy balance via the gut microbiota-brain axis, suppressing appetite and alleviating obesity. | [72] |
| Diabetes | Feed contains 5% KGM | Sprague Dawley male rats | Muribaculaceae↑, Ruminococcus↑, Lachnoclostridium↑, Romboutsia↓ | FBG, HOMA-IR↓; inflammatory factors TNF-α and IL-6↓; fecal SCFAs↑; expression of GPR41, GPR43, and GPR109A mRNA↑ | KGM effectively lowers blood glucose by modulating gut microbiota composition, increasing SCFA levels, and activating G protein-coupled receptors (GPRCs), thereby alleviating diabetes symptoms. | [73] |
| Diabetes | Liquid food contains 0.38% KGM | C57Bl/6J | Bifidobacterium↑, Allobaculum↑, S24-7↓, Helicobacter pylori↓ | Body weight, epididymal fat, and subcutaneous fat↓; FBG, serum insulin level, HOMA-IR index, and postprandial blood glucose↓; blood lipid levels TC, TG, and LDL-C↓, HDL-C↑; serum ALT, AST, ALP, and LDH levels↓, effectively reduced liver injury; hepatocellular lesions↓, liver lipid droplets | The KGM-PCP combination improved body weight, lipid homeostasis, and liver health in T2DM mice by lowering postprandial blood glucose and modulating gut microbiota composition and abundance in the intestinal environment. | [74] |
| Hyperlipidemia | 60 g/kg | Male Golden Syrian hamsters | Lachnospiraceae_UCG-006↑, Parasutterella↑, Lachnospiraceae_NK4A136↓, Lachnoclostridium↓, unclassified_f_Oscillospiraceae↓, Adlercreutzia↓, Eubacterium_brachy_group↓, Gordonibacter↓ | Body weight, liver lipid vacuoles, epididymal fat weight and adipocyte size↓; blood lipid levels TC, TG, and LDL-C↓; SCFAs↑; significantly changed bile acid composition, DCA/LCA↓, GCA/UDCA↑; liver CYP7A1 mRNA expression↑; FXR and sterol 12α-hydroxylase (CYP8B1) mRNA expression↓ | KGM primarily modulates gut microbiota activity, which in turn alters bile acid metabolism, ultimately improving blood lipid profiles. | [35] |
| Hyperlipidemia | 100 g/kg | C57Bl/6J | Akkermansia muciniphila↑, Alistipes↑, Olsenella↑, Bifidobacterium↑, Sporobacter↓, Allobaculum↓, Acetatifactor↓, Helicobacter pylori↓ | Lipid levels TC, TG, FAA, and LDL-C↓, HDL-C↑, insulin and leptin levels↓; liver function markers AST and ALT↓, SOD↑; lipid accumulation in IECs↓; fatty acid decomposition protein and gene (FABP1, PPARα, SREBP1 mRNA, and CD36 protein expression)↓; depth of crypt and the number of goblet cells↑; improved mucosal epithelial damage and disorderly loose arrangement of epithelial cells | KGM significantly reduced systemic and intestinal lipid accumulation in obese mice by altering microbial populations linked to lipid absorption and enhancing SCFA production, thereby inhibiting lipid absorption and output in HFD mice. | [32] |
| IBD | 70 g/kg | C57Bl/6J | norank_f__Muribaculaceae↑, Akkermansia muciniphila↑, Parabacteroides↑, Monoglobus↑, Bacteroides↓, Blautia↓, Escherichia-Shigella↓, Colidextribacter↓, Salmonella↓ | Serum MDA and H2O2↓; relative mRNA levels of Nqo1 and Nrf2↑; alleviated colonic oxidative stress; tight junction OCLN and ZO-2 protein expression↑; TNF-α, Ccl8, and Il-10 mRNA↑; INF-β mRNA↓; inflammatory factors Ccl2, Ccl3, Ccl8, and interleukin-1β (Il-1β) mRNA expression↓; reduced inflammatory response; Toll-like receptor (Tlr2, Tlr9) mRNA↓; ratio of phosphorylated (p) -NF-κB/NF-κB↓ | KGM alleviates colitis by modulating the gut microbiota and inhibiting the TLR2/NF-κB signaling pathway. | [75] |
| IBD | 25 g/kg | C57Bl/6J | Lactobacillus↑, Bifidobacterium↑, Clostridium↓ | Improved the distal colon cross-sectional tissue dysplasia; inflammatory factors TNF-α, IL-6↓, and IL-10↑; tight junction protein ZO-1 and occludin gene expression↑; improved the intestinal barrier damage; fecal SCFAs↑ | The KGM-inulin oligosaccharide combination prevents colitis by modulating gut microbiota, strengthening the intestinal barrier, and boosting SCFA production to suppress inflammation. | [76] |
| CRC | 62.5 g/kg | Sprague Dawley male rats | Bifidobacterium↑, Lactobacillus↑, Clostridium↓ | Energy intake and body weight↓; fecal β-glucuronidase and mucin activity↓; lithocholic acid ↓, fecal excretion↑; cecal i-butyrate↑ | The KGM-inulin combination enriched beneficial gut bacteria, altered microbiota composition, elevated SCFA levels, and improved the intestinal environment, thereby reducing fecal secondary bile acids and lowering CRC risk. | [77] |
| Immunoregulation | 0.4 g/kg | C57Bl/6J | Lactobacillus↑, Lachnoclostridium↑, Alloprevotella↑, Blautia↑, f_Lachnospiraceae↑, Akkermansia muciniphila↑ | Pulse oxygen saturation (SpO2) ↑; peripheral blood components RBC, WBC, PLT, HGB, and Lym↑; white pulp area of the spleen, femur, and spleen cells↑, improved ability of bone marrow to produce new blood cells damage; crypt and villus structure of mice were protected; DAO↓; improved intestinal permeability, relieved inflammation; SCFAs (acetate, propionate, and butyrate)↑ | KGM boosts gut microbiota abundance, probiotics, and SCFA production, maintains intestinal homeostasis, protects epithelial cells from apoptosis, and reduces inflammation while enhancing immune regulation. | [29] |
| AD | 800 mg/kg | C57Bl/6J | Prevotella sp. CAG:485↑, Muribaculaceae bacterium Isolate-114 (HZI) ↑, Parabacteroides distasonis↑, Duncaniella freteri↑, Alistipes sp. 56 11↑, Alistipes sp. Z76↑, Muribaculum sp. NM65 B17↑, Alistipes sp. HGB5↓, Alistipes sp. CAG:268↓, Bacterium 1XD8-76↓, Alistipes finegoldii↓, Alistipes onderdonkii↓, Muribaculaceae bacterium Isolate-104 (HZI) ↓, Odoribacter sp. Z80↓, Enterorhabdus caecimuris↓ | Identification index (RI)↑; central area crossing ability↑; average speed↓; effectively relieved anxiety; positive area of Aβ1-40 and Aβ1-42↓ effectively inhibited the accumulation of Aβ in hippocampus, and the hyperphosphorylation of Tau protein was inhibited; expression of bdnf↑; brain-derived neurotrophic factor (BDNF)↑; eelative expression of trkb, pi3k, and akt↑; relative expression of gsk3β↓; activated the BDNF/TrkB signaling pathway | KGM may ameliorate AD by modulating gut microbiota composition, elevating SCFA levels, activating the BDNF/PI3K/GSK3β pathway to enhance hippocampal neurogenesis, and reducing Aβ/Tau accumulation. | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Jin, S.; Yang, Y.; Han, X.; Guan, R.; Zhong, H. Interaction Between Konjac Glucomannan and Gut Microbiota and Its Impact on Health. Biology 2025, 14, 923. https://doi.org/10.3390/biology14080923
Yu Y, Jin S, Yang Y, Han X, Guan R, Zhong H. Interaction Between Konjac Glucomannan and Gut Microbiota and Its Impact on Health. Biology. 2025; 14(8):923. https://doi.org/10.3390/biology14080923
Chicago/Turabian StyleYu, Yufen, Shuo Jin, Yi Yang, Xiaodong Han, Rongfa Guan, and Hao Zhong. 2025. "Interaction Between Konjac Glucomannan and Gut Microbiota and Its Impact on Health" Biology 14, no. 8: 923. https://doi.org/10.3390/biology14080923
APA StyleYu, Y., Jin, S., Yang, Y., Han, X., Guan, R., & Zhong, H. (2025). Interaction Between Konjac Glucomannan and Gut Microbiota and Its Impact on Health. Biology, 14(8), 923. https://doi.org/10.3390/biology14080923





