Galangin and 1′-Acetoxychavicol Acetate from Galangal (Alpinia galanga) Suppress Lymphoma Growth via c-Myc Downregulation and Apoptosis Induction
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Crude Extracts of Five Zingiberaceae Plants
2.2. Cell Culture Condition for Lymphoma Cell Lines
2.3. Total Cell Number Count by Trypan Blue Exclusion Test
2.4. Cytotoxicity of Crude Extracts and Candidate Main Pure Compound from Zingiberacaeae on Lymphoma Cell Lines
2.5. Cytotoxicity of Pure Compounds from Zingiberaceae on PBMCs
2.5.1. PBMC Preparation by Ficoll-Hypaque Density Gradient Centrifugation Method
2.5.2. Cytotoxicity Effect of Major Pure Compound of Candidate Plant
2.6. Investigation of Candidate Zingiberaceae Plants and Major Pure Compounds on Cell Cycle Arrest in Lymphoma Cell Lines
2.7. Investigation of c-Myc Protein Expression by Western Blotting
2.8. Bioinformatics Analysis
2.9. Investigation of Candidate Zingiberaceae Plants and Major Pure Compounds on Apoptosis Induction in Lymphoma Cell Lines
2.10. Statistical Analysis
3. Results
3.1. Cytotoxicity of Crude Extracts from Zingiberacaeae on Lymphoma Cell Lines
3.2. Cytotoxicity of Major Compounds from Galangal on Lymphoma Cell Lines (Raji and Daudi) and Normal PBMCs
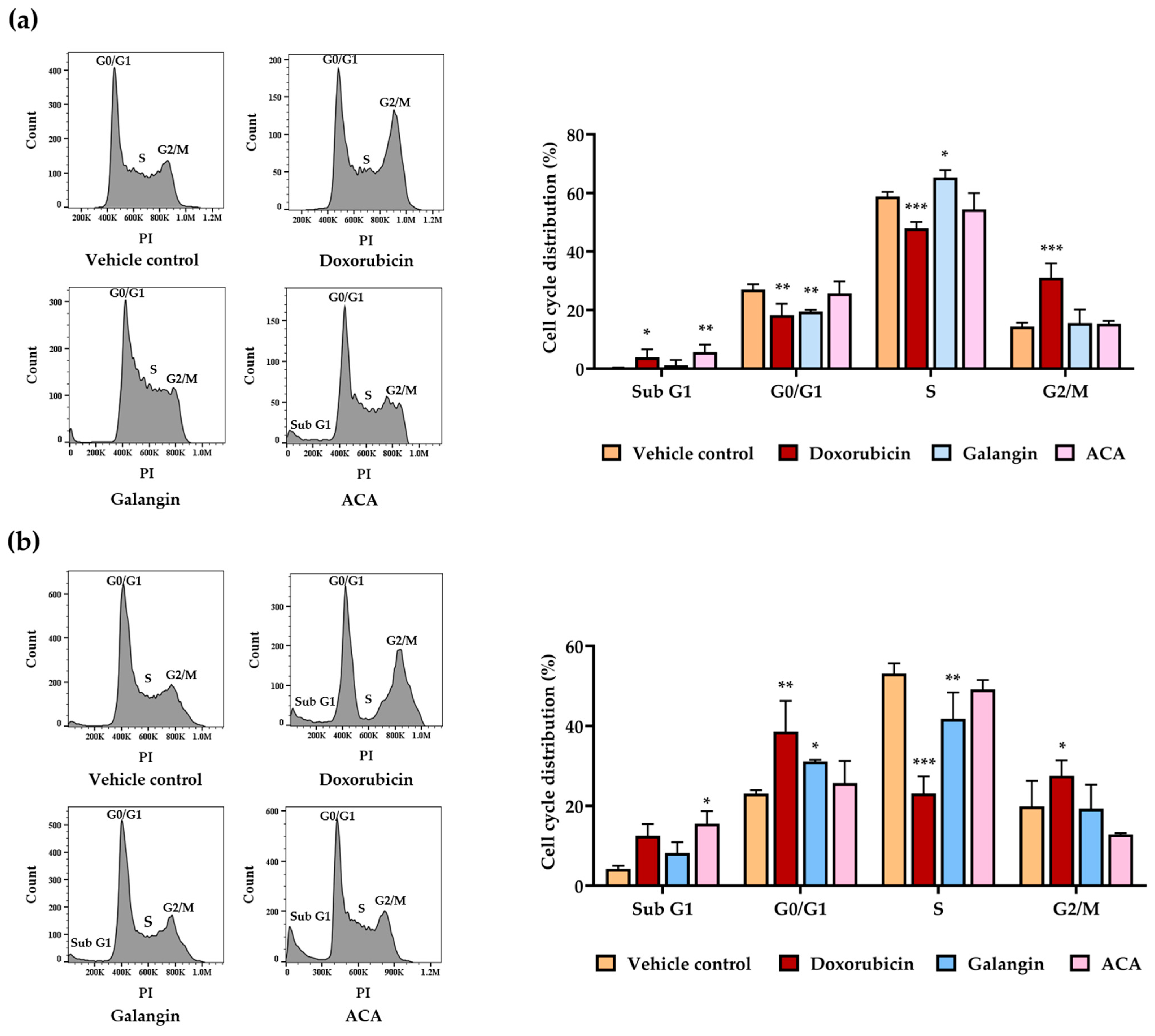
3.3. Effect of Galangin and ACA on Cell Cycle in Raji and Daudi Cells
3.4. Effects of Galangin and ACA on c-Myc and Phosphorylated c-Myc Protein Expressions in Raji and Daudi Cells
3.5. Effect of Galangin and ACA on Total Cell Number in Raji and Daudi Cells

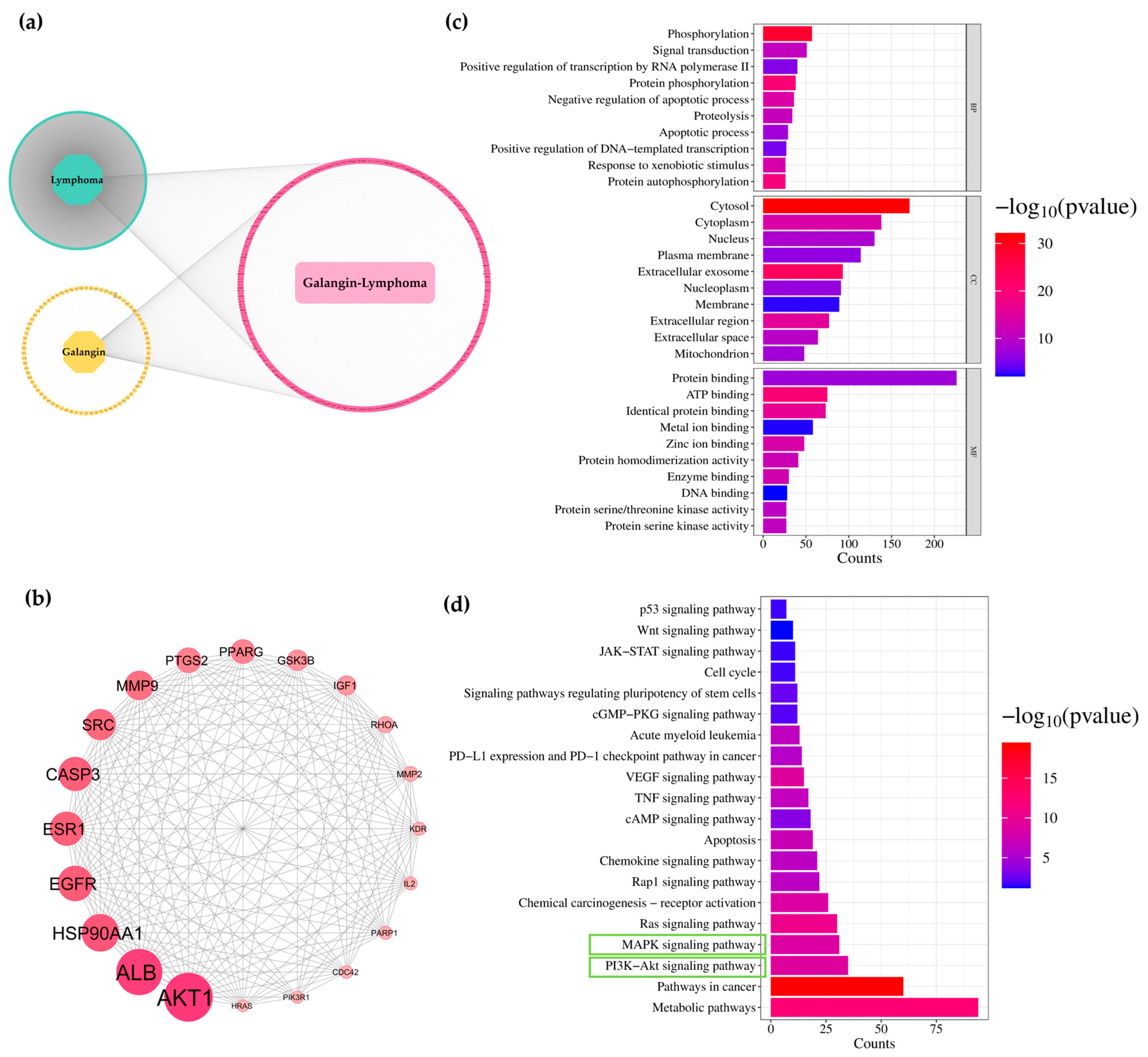
3.6. Network Analysis of Galangin and ACA for Predicting Targets Against Lymphoma
3.7. Effects of Galangin and ACA on Cleaved-Casp3 Expressions in Raji and Daudi Cells by Western Blotting Assay
3.8. Effects of Galangin and ACA on Apoptosis in Raji and Daudi Cells by Flow Cytometric Analysis

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACA | 1′-Acetoxychavicol acetate |
| Casp3 | Caspase-3 |
| cl-Casp3 | Cleaved caspase-3 |
| c-Myc | Cellular myelocytomatosis |
| p-c-Myc | Phosphorylated c-Myc |
| DLBCL | Diffuse large B-cell lymphoma |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| IC | Inhibitory concentration |
| KEGG | Kyoto Encycopedia of Gene and Genome |
| MTT | 3-(4,5-dimethythiazol-2-thizolyl)-2,5-diphenyl tetrazolium bromide |
| NHL | Non-Hodgkin lymphoma |
| PPI | Protein-protein interation |
| PBMC | Peripheral blood mononuclear cell |
| PKC | Protein kinase C |
| ROS | Reactive oxygen species |
References
- Institute, T.N.C. Cancer in Thailand Vol.X, 2016–2018; Rojanamatin, J., Ukranun, W., Supaattagorn, P., Chiawiriyabunya, I., Wongsena, M., Chaiwerattana, A., Laowahutanont, P., Chitapanarux, I., Vatanasapt, P., Greater, S.L., et al., Eds.; Ministry of Public Health: Talat Khwan, Thailand, 2021; Volume 10, p. 9. [Google Scholar]
- Armitage, J.O.; Gascoyne, R.D.; Lunning, M.A.; Cavalli, F. Non-Hodgkin lymphoma. Lancet 2017, 390, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Intragumtornchai, T.; Bunworasate, U.; Wudhikarn, K.; Lekhakula, A.; Julamanee, J.; Chansung, K.; Sirijerachai, C.; Norasetthada, L.; Nawarawong, W.; Khuhapinant, A.; et al. Non-Hodgkin lymphoma in South East Asia: An analysis of the histopathology, clinical features, and survival from Thailand. Hematol. Oncol. 2018, 36, 28–36. [Google Scholar] [CrossRef]
- Nguyen, L.; Papenhausen, P.; Shao, H. The role of c-MYC in B-cell lymphomas: Diagnostic and molecular Aspects. Genes 2017, 8, 116. [Google Scholar] [CrossRef]
- Spender, L.C.; Inman, G.J. Developments in Burkitt’s lymphoma: Novel cooperations in oncogenic MYC signaling. Cancer Manag. Res. 2014, 6, 27–38. [Google Scholar] [CrossRef]
- Rodríguez-Garza, N.E.; Quintanilla-Licea, R.; Romo-Sáenz, C.I.; Elizondo-Luevano, J.H.; Tamez-Guerra, P.; Rodríguez-Padilla, C.; Gomez-Flores, R. In vitro biological activity and lymphoma cell growth inhibition by selected mexican medicinal plants. Life 2023, 13, 958. [Google Scholar] [CrossRef]
- Ni, F.; Huang, X.; Chen, Z.; Qian, W.; Tong, X. Shikonin exerts antitumor activity in Burkitt’s lymphoma by inhibiting C-MYC and PI3K/AKT/mTOR pathway and acts synergistically with doxorubicin. Sci. Rep. 2018, 8, 3317. [Google Scholar] [CrossRef]
- Qiao, Q.; Jiang, Y.; Li, G. Curcumin improves the antitumor effect of X-ray irradiation by blocking the NF-κB pathway: An in-vitro study of lymphoma. Anti-Cancer Drugs 2012, 23, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-x.; Ouyang, K.-q.; Jiang, X.; Wang, D.; Hu, Y. Curcumin induces apoptosis and inhibits growth of human Burkitt’s lymphoma in xenograft mouse model. Mol. Cells 2009, 27, 283–289. [Google Scholar] [CrossRef]
- Anuchapreeda, S.; Limtrakul, P.; Thanarattanakorn, P.; Sittipreechacharn, S.; Chanarat, P. Inhibitory effect of curcumin on WT1 gene expression in patient leukemic cells. Arch. Pharmacal Res. 2006, 29, 80–87. [Google Scholar] [CrossRef]
- Anuchapreeda, S.; Tima, S.; Duangrat, C.; Dejkriengkraikul, P. Effect of pure curcumin, demethoxycurcumin, and bisdemethoxycurcumin on WT1 gene expression in leukemic cell lines. Cancer Chemother. Pharmacol. 2008, 62, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Semsri, S.; Krig, S.R.; Kotelawala, L.; Sweeney, C.A.; Anuchapreeda, S. Inhibitory mechanism of pure curcumin on Wilms’ tumor 1 (WT1) gene expression through the PKCα signaling pathway in leukemic K562 cells. FEBS Lett. 2011, 585, 2235–2242. [Google Scholar] [CrossRef]
- Panyajai, P.; Tima, S.; Chiampanichayakul, S.; Anuchapreeda, S. Dietary turmeric bisdemethoxycurcumin suppresses Wilms’ Tumor 1 and CD34 protein expressions in KG-1a leukemic stem cells. Nutr. Cancer 2019, 71, 1189–1200. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, L.G.; Lee, L.T.; Yang, L.L. Effects of 6-gingerol, an antioxidant from ginger, on inducing apoptosis in human leukemic HL-60 cells. In Vivo 2003, 17, 641–645. [Google Scholar]
- Rastogi, N.; Gara, R.K.; Trivedi, R.; Singh, A.; Dixit, P.; Maurya, R.; Duggal, S.; Bhatt, M.L.; Singh, S.; Mishra, D.P. (6)-Gingerolinduced myeloid leukemia cell death is initiated by reactive oxygen species and activation of miR-27b expression. Free Radic. Biol. Med. 2014, 68, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, N.; Nakamura, Y.; Ueda, Y.; Abe, M.; Ozawa, Y.; Uchida, K.; Osawa, T. Dietary ginger constituents, galanals A and B, are potent apoptosis inducers in Human T lymphoma Jurkat cells. Cancer Lett. 2003, 199, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Albaayit, S.F.A.; Khan, M.; Abdullah, R. Zerumbone induces growth inhibition of Burkitt’s lymphoma cell line via apoptosis. Nat. Volatiles Essent. Oils 2021, 8, 56–63. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y. The GeneCards suite: From gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinform. 2016, 54, 1–30. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar] [CrossRef]
- Lica, J.J.; Wieczor, M.; Grabe, G.J.; Heldt, M.; Jancz, M.; Misiak, M.; Gucwa, K.; Brankiewicz, W.; Maciejewska, N.; Stupak, A.; et al. Effective Drug Concentration and Selectivity Depends on Fraction of Primitive Cells. Int. J. Mol. Sci. 2021, 22, 4931. [Google Scholar] [CrossRef]
- Panyajai, P.; Viriyaadhammaa, N.; Chiampanichayakul, S.; Sakamoto, Y.; Okonogi, S.; Moroishi, T.; Anuchapreeda, S. Anticancer and cancer preventive activities of shogaol and curcumin from Zingiberaceae family plants in KG-1a leukemic stem cells. BMC Complement. Med. Ther. 2025, 25, 87. [Google Scholar] [CrossRef]
- Suciati, A. Systematic review: Anticancer potential of active compounds from galangal (Alpinia galanga). In Proceedings of the 4th International Conference Current Breakthrough in Pharmacy (ICB-Pharma 2022), Sukoharjo, Indonesia, 14–15 January 2022; pp. 269–282. [Google Scholar]
- Chouni, A.; Paul, S. A review on phytochemical and pharmacological potential of Alpinia galanga. Pharmacogn. J. 2018, 10, 9–15. [Google Scholar] [CrossRef]
- Eram, S.; Mujahid, M.; Bagga, P.; Ansari, V.; Ahmad, M.; Kumar, A.; Ahsan, F.; Akhter, M. A review on phytopharmacological activity of Alpinia galanga. Int. J. Pharm. Pharm. Sci. 2019, 11, 6–11. [Google Scholar] [CrossRef]
- Dang, C.V. MYC on the path to cancer. Cell 2012, 149, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, H.; Yeh, G. The flavonoid galangin is an inhibitor of CYP1A1 activity and an agonist/antagonist of the aryl hydrocarbon receptor. Br. J. Cancer 1999, 79, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.T.; Lamb, A.J. Detection of galangin-induced cytoplasmic membrane damage in Staphylococcus aureus by measuring potassium loss. J. Ethnopharmacol. 2005, 101, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, B.; Arshad, N.M.; Malagobadan, S.; Misran, M.; Nyamathulla, S.; Mun, K.S.; Nagoor, N.H. Development and evaluation of 1′-acetoxychavicol acetate (ACA)-Loaded nanostructured lipid carriers for prostate cancer therapy. Pharmaceutics 2021, 13, 439. [Google Scholar] [CrossRef]
- Ketkomol, P.; Songsak, T.; Jongrungruangchok, S.; Madaka, F.; Pradubyat, N. The effect of 1’-acetoxychavicol acetate on A549 human non-small cell lung cancer. J. Curr. Sci. Technol. 2024, 14, 43. [Google Scholar] [CrossRef]
- Liew, S.K.; Azmi, M.N.; In, L.L.; Awang, K.; Nagoor, N.H. Anti-proliferative, apoptotic induction, and anti-migration effects of hemi-synthetic 1′ S-1′-acetoxychavicol acetate analogs on MDA-MB-231 breast cancer cells. Drug Des. Dev. Ther. 2017, 11, 2763–2776. [Google Scholar] [CrossRef]
- Ito, K.; Nakazato, T.; Murakami, A.; Yamato, K.; Miyakawa, Y.; Yamada, T.; Hozumi, N.; Ohigashi, H.; Ikeda, Y.; Kizaki, M. Induction of apoptosis in human myeloid leukemic cells by 1′-acetoxychavicol acetate through a mitochondrial-and Fas-mediated dual mechanism. Clin. Cancer Res. 2004, 10, 2120–2130. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, M.Y.; Han, L.; Tai, Y.; Cao, S.; Li, J.; Zhao, H.; Wang, R.; Lv, B.; Shan, Z. Galangin inhibits programmed cell death-ligand 1 expression by suppressing STAT3 and MYC and enhances T cell tumor-killing activity. Phytomedicine 2023, 116, 154877. [Google Scholar] [CrossRef] [PubMed]
- Duthu, A.; Debuire, B.; Romano, J.; Ehrhart, J.; Fiscella, M.; May, E.; Appella, E.; May, P. p53 mutations in Raji cells: Characterization and localization relative to other Burkitt’s lymphomas. Oncogene 1992, 7, 2161–2167. [Google Scholar] [PubMed]
- Ito, K.; Nakazato, T.; Miyakawa, Y.; Xian, M.J.; Yamada, T.; Ohigashi, H.; Ikeda, Y.; Kizaki, M. 1′-Acetoxychavicol Acetate (ACA) Is a Novel NF-κB Inhibitor with Significant Activity Against Multiple Myeloma In Vitro and In Vivo. Blood 2004, 104, 765. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Y.; Qin, C.; Shi, Y.; Rong, G.; Yu, X. Growth inhibition and apoptosis of human B-cell lymphoma in vitro and in vivo by Bcl-2 short hairpin RNA. Oncol. Rep. 2013, 29, 244–252. [Google Scholar] [CrossRef]
- Zou, W.-W.; Xu, S.-P. Galangin inhibits the cell progression and induces cell apoptosis through activating PTEN and Caspase-3 pathways in retinoblastoma. Biomed. Pharmacother. 2018, 97, 851–863. [Google Scholar] [CrossRef]
- Song, W.; Yan, C.-y.; Zhou, Q.-q.; Zhen, L.-l. Galangin potentiates human breast cancer to apoptosis induced by TRAIL through activating AMPK. Biomed. Pharmacother. 2017, 89, 845–856. [Google Scholar] [CrossRef]
- Williams, M.; Tietzel, I.; Quick, Q.A. 1’-Acetoxychavicol acetate promotes caspase 3-activated glioblastoma cell death by overcoming enhanced cytokine expression. Oncol. Lett. 2013, 5, 1968–1972. [Google Scholar] [CrossRef]
- Pradubyat, N.; Giannoudis, A.; Elmetwali, T.; Mahalapbutr, P.; Palmieri, C.; Mitrpant, C.; Ketchart, W. 1′-Acetoxychavicol Acetate from alpinia galanga represses proliferation and invasion, and induces apoptosis via HER2-signaling in endocrine-resistant breast cancer cells. Planta Medica 2022, 88, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Kojima-Yuasa, A.; Matsui-Yuasa, I. Pharmacological effects of 1′-Acetoxychavicol acetate, a major constituent in the Rhizomes of Alpinia galanga and Alpinia conchigera. J. Med. Food 2020, 23, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Asri, A.; Winarko, S. Antiproliferative activity by ethanolic extract of red Alpinia galanga (L) Willd in inoculated breast carcinoma cells of C3H mice. J. Adv. Med. Pharm. Sci. 2016, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]


| Zingiberaceae Plant | IC50 Value (μg/mL) (Mean ± SD) | Selectivity Index (SI) | |||
|---|---|---|---|---|---|
| Raji | Daudi | PBMCs | Raji | Daudi | |
| Galangal (Alpinia galanga) | 31.52 ± 3.59 | 14.20 ± 2.34 | 43.18 ± 3.05 | 1.36 | 3.04 |
| Black turmeric (Curcuma aeroginosa) | 55.54 ± 5.74 | 35.48 ± 3.23 | 79.11 ± 3.92 # | 1.42 | 2.23 |
| Black ginger (Kaempferia parviflora) | 46.30 ± 3.97 | 17.47 ± 0.73 | 46.77 ± 1.48 # | 1.01 | 2.68 |
| Phlai lueang (Zingiber montanum) | 43.67 ± 5.25 | 22.00 ± 0.98 | 56.25 ± 3.41 # | 1.28 | 2.56 |
| Phlai dam (Zingiber ottensii) | 51.74 ± 6.77 | 32.65 ± 1.42 | >100 # | >1.93 | >3.06 |
| Compound | IC50 Value | Selectivity Index (SI) | |||
|---|---|---|---|---|---|
| Raji | Daudi | PBMCs | Raji | Daudi | |
| Galangin (μg/mL) | 21.00 ± 1.58 | 10.75 ± 1.29 | >100 | >4.76 | >9.30 |
| ACA (μg/mL) | 1.93 ± 0.26 | 1.74 ± 0.46 | 4.69 ± 0.25 | 2.43 | 2.70 |
| Doxorubicin (ng/mL) | 61.00 ± 12.86 | 20.79 ± 2.20 | >1000 | >16.39 | >48.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moakmamern, S.; Rueankham, L.; Viriyaadhammaa, N.; Panyakham, K.; Khakhai, N.; Khamphikham, P.; Duangmano, S.; Okonogi, S.; Chiampanichayakul, S.; Anuchapreeda, S. Galangin and 1′-Acetoxychavicol Acetate from Galangal (Alpinia galanga) Suppress Lymphoma Growth via c-Myc Downregulation and Apoptosis Induction. Biology 2025, 14, 1098. https://doi.org/10.3390/biology14081098
Moakmamern S, Rueankham L, Viriyaadhammaa N, Panyakham K, Khakhai N, Khamphikham P, Duangmano S, Okonogi S, Chiampanichayakul S, Anuchapreeda S. Galangin and 1′-Acetoxychavicol Acetate from Galangal (Alpinia galanga) Suppress Lymphoma Growth via c-Myc Downregulation and Apoptosis Induction. Biology. 2025; 14(8):1098. https://doi.org/10.3390/biology14081098
Chicago/Turabian StyleMoakmamern, Sirinya, Lapamas Rueankham, Natsima Viriyaadhammaa, Kittikawin Panyakham, Natnicha Khakhai, Pinyaphat Khamphikham, Suwit Duangmano, Siriporn Okonogi, Sawitree Chiampanichayakul, and Songyot Anuchapreeda. 2025. "Galangin and 1′-Acetoxychavicol Acetate from Galangal (Alpinia galanga) Suppress Lymphoma Growth via c-Myc Downregulation and Apoptosis Induction" Biology 14, no. 8: 1098. https://doi.org/10.3390/biology14081098
APA StyleMoakmamern, S., Rueankham, L., Viriyaadhammaa, N., Panyakham, K., Khakhai, N., Khamphikham, P., Duangmano, S., Okonogi, S., Chiampanichayakul, S., & Anuchapreeda, S. (2025). Galangin and 1′-Acetoxychavicol Acetate from Galangal (Alpinia galanga) Suppress Lymphoma Growth via c-Myc Downregulation and Apoptosis Induction. Biology, 14(8), 1098. https://doi.org/10.3390/biology14081098


_Kwok.png)




