Interannual Variations in Soil Bacterial Community Diversity and Analysis of Influencing Factors During the Restoration Process of Scirpus Mariqueter Wetlands
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sample Collection
2.3. Determination of Soil Environmental Factors
2.4. Soil DNA Extraction, PCR Amplification, and Illumina Sequencing
2.5. Statistical Analyses
3. Results
3.1. Soil Environmental Factors
3.2. Diversity and Composition of Soil Bacterial Communities
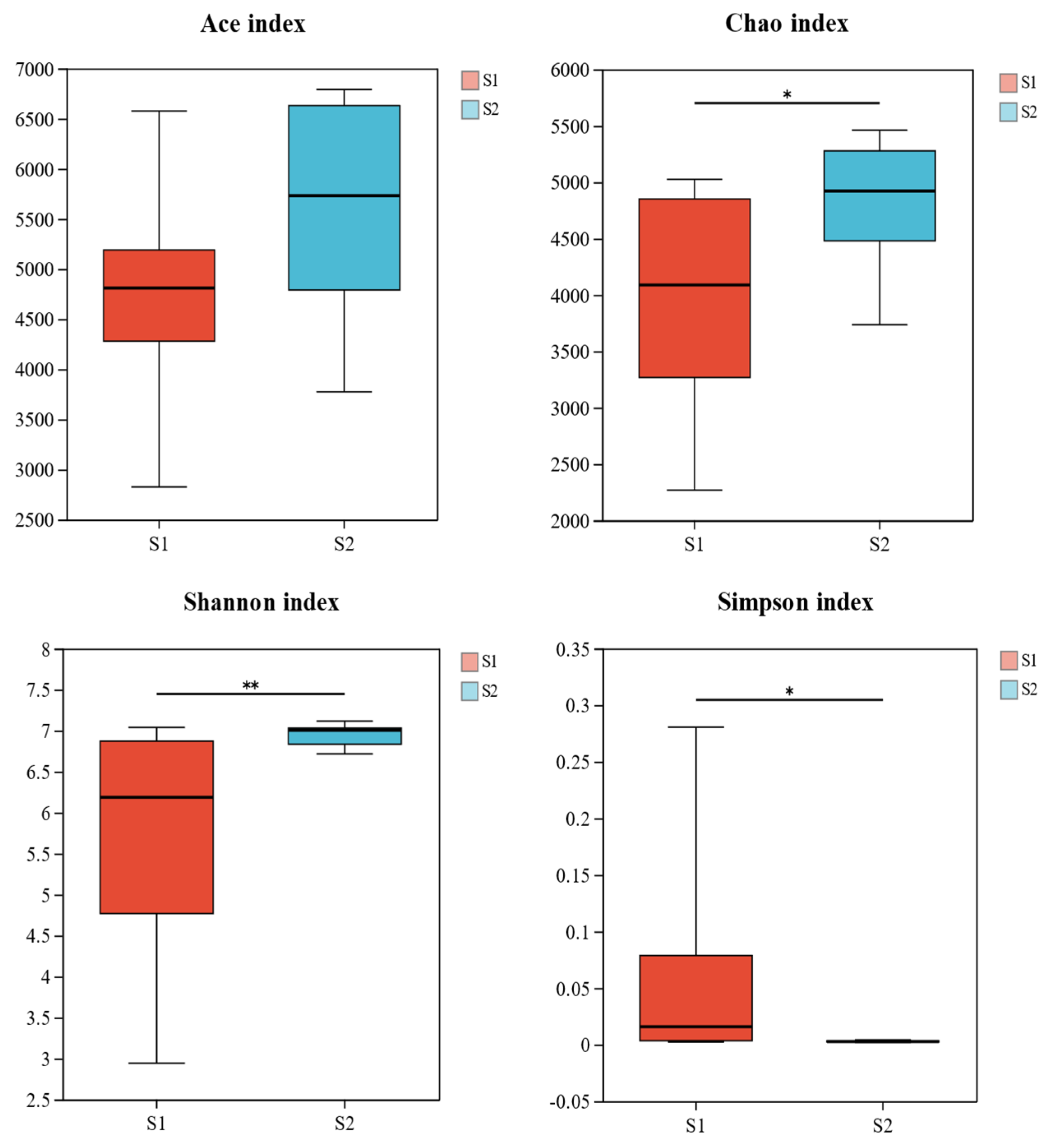
3.2.1. Analysis of Soil Bacterial Community Diversity
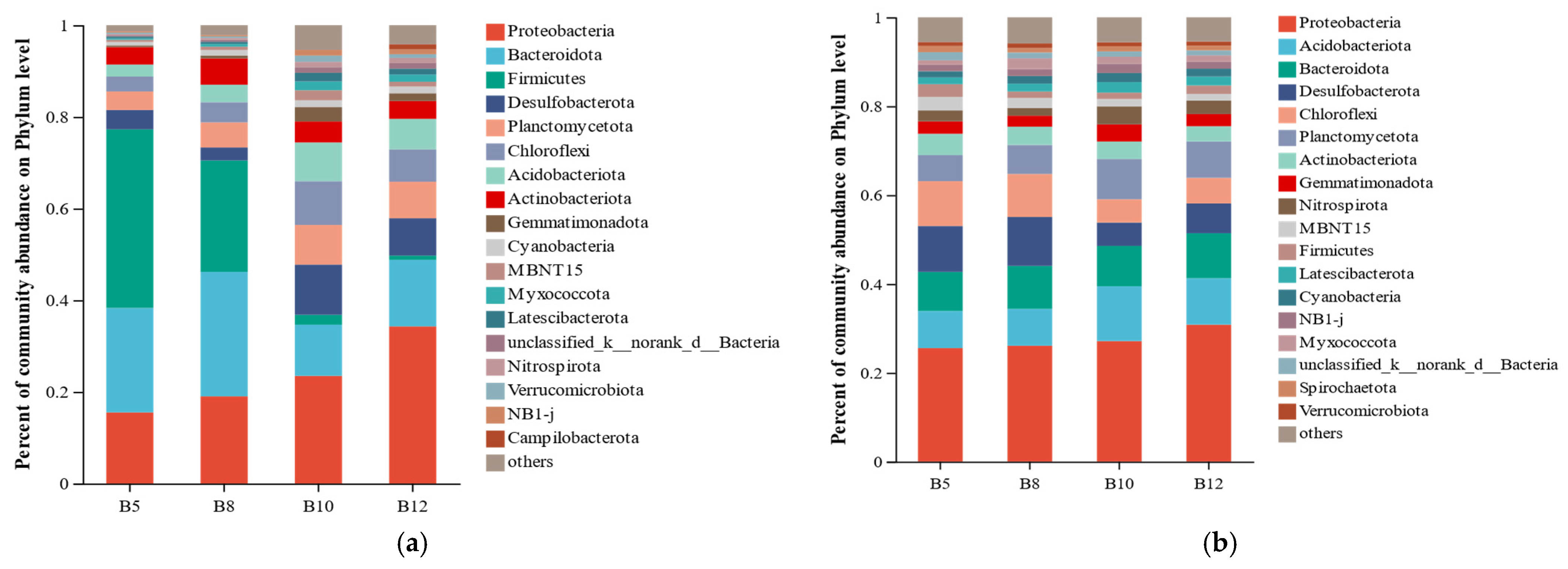
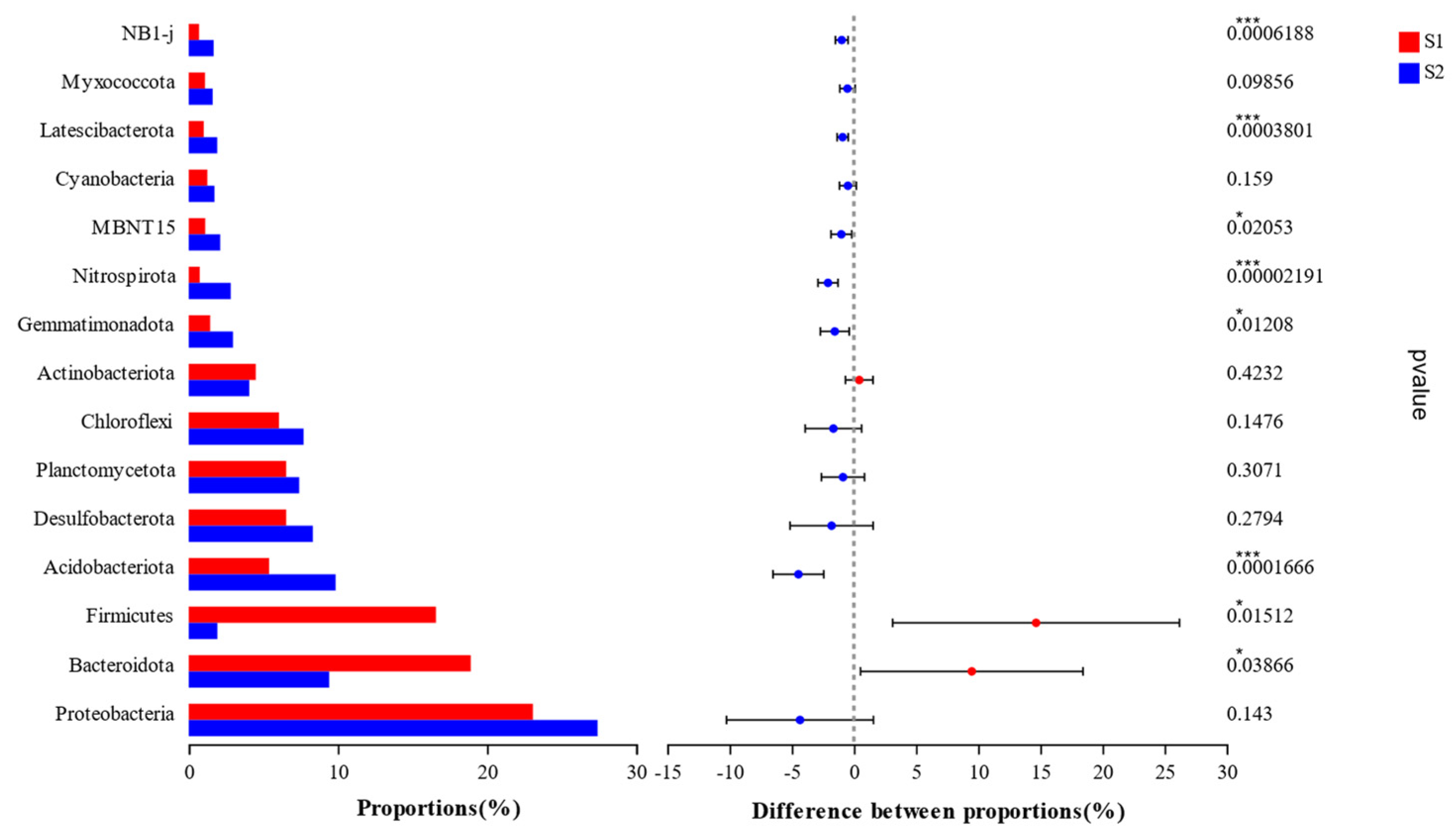
3.2.2. Analysis of Soil Bacterial Community Composition
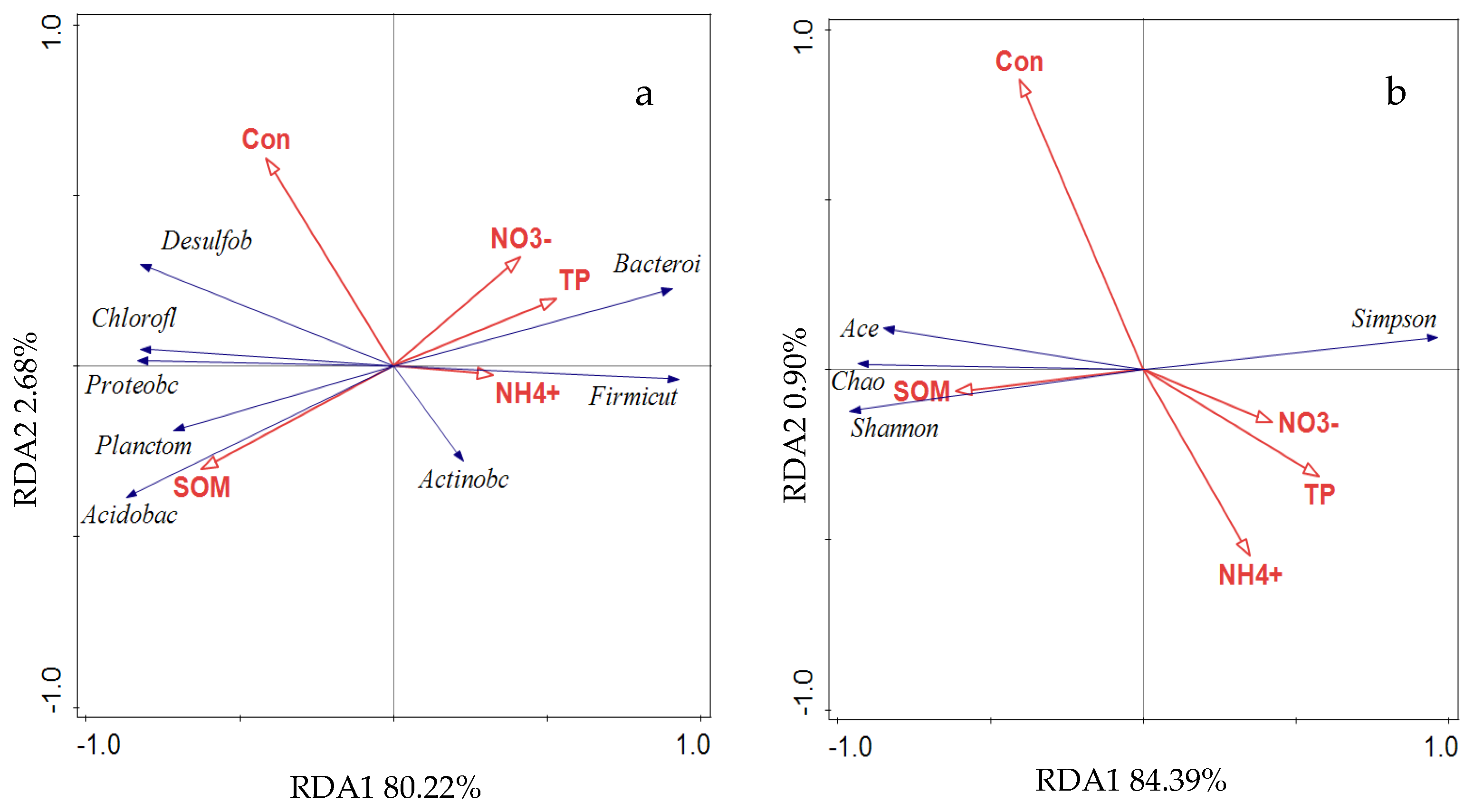
3.3. Correlation Between Soil Environmental Factors and Soil Bacterial Communities
4. Discussion
4.1. Characteristics of Changes in Soil Influencing Factors
4.2. The Diversity and Composition Structure Changes in Soil Bacterial Communities
4.3. Correlation Analysis of Soil Bacterial Communities and Soil Environmental Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, N.J.; Phinn, S.R.; Dewitt, M.; Ferrari, R.; Fuller, R.A. The global distribution and trajectory of tidal flats. Nature 2019, 565, 222–225. [Google Scholar] [CrossRef]
- Huang, L.B.; Bai, J.H.; Wen, X.J.; Zhang, G.L.; Zhang, C.D.; Cui, B.S.; Liu, X.H. Microbial resistance and resilience in response to environmental changes under the higher intensity of human activities than global average level. Glob. Change Biol. 2020, 26, 2377–2389. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, F.Z. Flora of China, 1st ed.; Science Press: Bejing, China, 1961; Volume 11, pp. 1–30. [Google Scholar]
- Yong, X.K.; Zhang, Q.L. Studies on Reproduction Ecology of Scirpus mariqueter Population. J. East China Norm. Univ. Nat. Sci. 1992, 4, 96–101. [Google Scholar]
- Yang, S.L.; Shi, Z.; Zhao, Q.Y. Influence of tidal marsh vegetations on hydrodynamics and sedimentation in the Changjiang Estuary. Acta Oceanol. Sin. 2001, 23, 75–80. [Google Scholar]
- Yang, Z.H.; Tong, C.F.; Lu, J.J. Effects of saltmarsh on the bentthic macroinvertebrate community in Yangtze Estuary. Acta Ecol. Sin. 2007, 27, 4387–4393. [Google Scholar]
- Zhang, Q.; Li, X.Z.; Zhang, Y.Q.; Sun, X.L.; Xu, Y.; Yan, Z.Z. Effects of nitrogen addition on growth and physiological characters of Scirpus mariqueter in the Yangtze River Estuary. Acta Agrestia Sin. 2017, 25, 115–121. [Google Scholar]
- Cao, H.B.; Ge, Z.M.; Zhu, Z.C.; Zhang, L.Q. The expansion pattern of saltmarshes at Chongming Dongtan and its underlying mechanism. Acta Ecol. Sin. 2014, 34, 3944–3952. [Google Scholar] [CrossRef]
- Chen, H.; Wang, D.Q.; Chen, Z.L.; Wang, J.; Xu, S.Y. The variation of sediments organic carbon content in Chongming east tidal flat during Scirpus mariqueter growing stage. J. Geographi. Sci. 2005, 15, 500–508. [Google Scholar]
- Zeller, V.; Bardgett, R.D.; Tappeiner, U. Site and management effects on soil microbial properties of subalpine meadows: A study of land abandonment along a north–south gradient in the European Alps. Soil Biol. Biochem. 2001, 33, 639–649. [Google Scholar] [CrossRef]
- Mohammad, B.; Falk, H.; Kristoffer, F.; Jennifer, A.; Nadejda, A.; Soudzilovskaia, P.M.; Van, B.; Johan, B.P.; Sten, A.; Luís, P.C.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef]
- Xie, X.F.; Xiang, Q.; Wu, T. Soil microorganisms and their influencing factors in coastal wetland ecosystem. Acta Ecol. Sin. 2021, 41, 1–12. [Google Scholar] [CrossRef]
- Li, X.F.; Hou, L.J.; Liu, M.; Lin, X.B.; Li, Y.; Li, S.W. Primary effects of extracellular enzyme activity and microbial community on carbon and nitrogen mineralization in estuarine and tidal wetlands. Appl. Microbiol. Biotechnol. 2015, 99, 2895–2909. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.D.; Zhong, S.C.; Li, C.W.; Zhao, M.; Ding, P.Z.; Fang, S.B.; He, P.M.; Yu, K.F. A Study on the Effect of Ecological Restoration and Reconstruction of Scirpus mariqueter Community: A case of Nanhui Coasts. Trans. Oceanol. Limnol. 2018, 5, 40–49. [Google Scholar]
- He, N.; Hu, Y.; Wu, M.X.; Zhang, B.L.; Zhang, S.L.; Wu, P.L.; Wang, M.Q.; Wang, Q.Y.; He, P.M.; Fang, S.B. Nitrogen allocation strategy of Scirpus mariqueter based on 15N tracing analysis on Nanhui coasts and restoration suggestions. Chin. J. Ecol. 2023, 42, 635–642. [Google Scholar]
- Zhong, S.C.; Yu, K.F.; Li, C.W.; Wu, M.X.; Wu, P.L.; He, P.M.; Fang, S.B. Variation and the Associated Affecting Factors of Benthic Biodiversity in Wetlands of Scirpus mariqueter on Remediated Nanhui Coasts. Resour. Environ. Yangtze Basin. 2020, 29, 889–899. [Google Scholar]
- Wang, M.Q.; Hu, Y.; He, N.; Wu, M.X.; Wu, P.L.; Wang, Q.Y.; Zhang, B.L.; Zhang, S.L.; Gao, M.H.; Fang, S.B. The Spatio-Temporal Patterns of Macro Benthos Functional Groups and the Associated Factors Affecting Them After Wetland Restoration. J. Res. Ecol. 2022, 13, 1152–1164. [Google Scholar]
- Li, H.; Chi, Z.F.; Li, J.L.; Wu, H.T.; Yan, B.X. Bacterial community structure and function in soils from tidal freshwater wetlands in a Chinese delta: Potential impacts of salinity and nutrient. Sci. Total Environ. 2019, 696, 134029. [Google Scholar] [CrossRef]
- Gu, G.H.; Li, M.S. Soil microbial functional diversity of different coastal wetland types. Jiangsu Agri. Sci. 2018, 46, 355–358. [Google Scholar]
- Guo, X.P.; Niu, Z.S.; Lu, D.P.; Feng, J.N.; Chen, Y.R.; Tou, F.Y.; Yang, Y. Bacterial community structure in the intertidal biofilm along the Yangtze Estuary, China. Mar. Pollut. Bull. 2017, 124, 314–320. [Google Scholar] [CrossRef]
- Piccini, C.; Garcia, A.J. Bacterial diversity patterns of the intertidal biofilm in urban beaches of Rio de la Platat. Mar. Pollut. Bull. 2015, 91, 476–482. [Google Scholar] [CrossRef]
- He, S.B.; Hu, W.G.; Jin, X.T.; Zhou, T.T.; Zhong, Z.T.; Wang, Y.E. Diversity and community structure of ammonia-oxidizing archaea in reed rhizosphere soil in Ebi Lake Wetland. Acta Microbiol. Sin. 2019, 59, 1576–1585. [Google Scholar]
- Liu, W.; Liu, J.L.; Zhang, M.J.; Zhang, J.L.; Sun, B.; He, C.Q.; He, P.M.; Zhang, W.T. 1 + 1 < 2: Combined effect of low temperature stress and salt stress on Sesuvium portulacastrum L. Plant Physiol. Bioch. 2025, 219, 109404. [Google Scholar]
- Lammel, D.R.; Nüsslein, K.; Tsai, S.M.; Cerri, C.C. Land use, soil and litter chemistry drive bacterial community structures in samples of the rainforest and Cerrado (Brazilian Savannah) biomes in Southern Amazonia. Eur. J. Soil Biol. 2015, 66, 32–39. [Google Scholar] [CrossRef]
- Zhang, A.D.; Zheng, Y.X.; Wu, B.S.; Huang, D.B. Soil Microbial Community Diversity and Its Influencing Factors in Coastal Wetland. Res. Soil Water Conserv. 2020, 27, 8–14. [Google Scholar]
- Moss, J.A.; Nocker, A.; Lepo, J.E.; Snyder, R.A. Stability and change in estuarine biofilm bacterial community diversity. Appl. Environ. Microbiol. 2006, 72, 5679–5688. [Google Scholar] [CrossRef] [PubMed]
- Li, J.N.; Chen, Q.F.; Li, Q. Microbial diversity and its driving factors in coastal wetlands of the Yellow River Delta. Acta Ecol. Sin. 2021, 41, 6103–6114. [Google Scholar] [CrossRef]
- Webster, G.; O’Sullivan, L.A.; Meng, Y.Y.; Williams, A.S.; Sass, A.M.; Watkins, A.J.; Weightman, A.J. Archaeal community diversity and abundance changes along a natural salinity gradient in estuarine sediments. FEMS Microbiol. Ecol. 2015, 91, 1–18. [Google Scholar] [CrossRef]
- Zhao, M.; Yin, C.S.; Li, C.W.; Zhong, S.C.; Yu, K.F.; Fang, S.B. Using Miseq sequencing to analyze seasonal soil microbial community dynamics in reclaimed Scirpus mariqueter coastal wetlands. J. Shanghai Ocean Univ. 2018, 27, 718–727. [Google Scholar]
- Chi, Z.F.; Wang, W.J.; Li, H.; Wu, H.T.; Yan, B.X. Soil organic matter and salinity as critical factors affecting the bacterial community and function of Phragmites australis dominated riparian and coastal wetlands. Sci. Total Environ. 2020, 762, 143156. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.J.; Hu, Z.Q.; Zhang, H.M.; Hou, Z.N.; Min, W. Soil Microbial Metabolic Activity and Community Structure in Drip-Irrigated Calcareous Soil as Affected by Irrigation Water Salinity. Water Air Soil Poll. 2019, 230, 44. [Google Scholar] [CrossRef]
- Lu, P.; Guo, J.X.; Zhu, L. Soil catalase activity of main plant communities in Leymus chinensis grassland in northeast China. Chin. J. Appl. Ecol. 2002, 6, 675–679. [Google Scholar]
- Tan, J.; Hu, S.F.; Wang, Q.; Wu, J.Q.; Wang, M. Ecosystem health assessment and causes analysis of tidal marshes in Shanghai. Resour. Environ. Yangtze Basin. 2014, 23, 1705. [Google Scholar]
- Balasooriya, W.K.; Denef, K.; Peters, J.; Verhoest, N.E.C.; Boeckx, P. Vegetation composition and soil microbial community structural changes along a wetland hydrological gradient. Hydrol. Earth Syst. Sci. 2008, 12, 277–291. [Google Scholar] [CrossRef]
- Che, J.F.; Li, Z.F.; Wang, G.J. Analysis on the functional diversity of biofilm community in mixed ponds. J. Shanghai Ocean Univ. 2017, 26, 862–871. [Google Scholar]
- Zhu, Y.; Tan, H.; Sun, D. Biolog-ECO analysis of functional diversity characteristics of filter material microbial communities in aquaculture system of circulating aquatic eel (Anguilla anguilla) under different biodegradation technologies. J. Shanghai Ocean Univ. 2017, 26, 64–74. [Google Scholar]
- Liu, C.; Liu, Y.; Jin, G. Seasonal Variation of soil microbial biomass of six forest types in Lesser Khingan Mountains. Acta Ecol. Sin. 2014, 34, 451–459. [Google Scholar]
- Tong, C.F.; Zhang, F.J.; Lu, J.J. Variation characteristics of the macrobenthic fauna community in the Scirpus mariqueter zone of the Yangtze estuary during the growing seasons. Zool. Res. 2007, 28, 640–646. [Google Scholar]
- Chen, W.F.; Shi, Y.X. Distribution Characteristics of Microbes in New-born Wetlands of the Yellow River Delta. Acta Agrestia Sin. 2010, 18, 859–864. [Google Scholar]
- Sun, F.L.; Fan, L.L.; Xie, G.J. Effect of copper on the performance and bacterial communities of activated sludge using Illumina MiSeq platforms. Chemosphere 2016, 156, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Li, G.R.; Gong, G.N.; Zhang, S.L.; Gao, M.H.; Zhang, B.L.; Ma, Y.X.; He, P.M.; Fang, S.B. Observation of physical variables of coastal wetland and response of wetland system under the influence of typhoon process. Acta Oceanol. Sin. Chin. Ed. 2022, 44, 116–125. [Google Scholar]
- Zhang, H.X.; Zheng, S.L.; Ding, J.W.; Wang, O.M.; Liu, F.H. Spatial variation in bacterial community in natural wetland-river-sea ecosystems. J. Basic Microbiol. 2017, 57, 536–546. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Wang, G.H.; Liu, J.J.; Yu, Z.H.; Wang, X.Z.; Jin, J.; Liu, X.B. Research Progress of Acidobacteriota Ecology in Soils. Biotechnol. Bull. 2016, 32, 14–20. [Google Scholar]
- Rath, K.M.; Fierer, N.; Murphy, D.V.; Rousk, J. Linking bacterial community composition to soil salinity along environmental gradients. ISME J. 2019, 13, 836–846. [Google Scholar] [CrossRef]
- Han, G.M.; Lan, J.Y.; Chen, Q.Q.; Yu, C.; Bie, S. Response of soil microbial community to application of biochar in cotton soils with different continuous cropping years. Sci. Rep. 2017, 7, 10184. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, Y.; Tang, Z.R.; Huang, J.G.; Zhou, Q.; Vymazal, J. Effects of plant biomass on bacterial community structure in constructed wetlands used for tertiary wastewater treatment. Ecol. Eng. 2015, 84, 38–45. [Google Scholar] [CrossRef]
- Canfora, L.; Salvati, L.; Benedetti, A.; Francaviglia, R. Is soil microbial diversity affected by soil and groundwater salinity? Evidences from a coastal system in central Italy. Environ. Monit. Assess. 2017, 189, 319. [Google Scholar] [CrossRef] [PubMed]
- Canfora, L.; Bacci, G.; Pinzari, F.; Lo, P.G.; Dazzi, C.; Benedetti, A. Salinity and bacterial diversity: To what extent does the concentration of salt affect the bacterial community in a saline soil? PLoS ONE 2014, 9, e106662. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhou, X.S.; Hale, L.; Yuan, M.T.; Ning, D.L.; Feng, J.J.; Shi, Z.; Li, Z.X.; Feng, B.; Gao, Q.; et al. Climate warming accelerates temporal scaling of grassland soil microbial biodiversity. Nat. Ecol. Evol. 2019, 3, 612–619. [Google Scholar] [CrossRef]

| Anniversary Number | Sampling Time | Sample Number |
|---|---|---|
| S1 | May 2019 | 19-5 |
| August 2019 | 19-8 | |
| October 2019 | 19-10 | |
| December 2019 | 19-12 | |
| S2 | October 2020 | 20-10 |
| December 2020 | 20-12 | |
| May 2021 | 21-5 | |
| August 2021 | 21-8 |
| Year | Samples | SOM/ (g/kg) | NH4+/(mg/kg) | NO3−/(mg/kg) | TP/(g/kg) | Con/(µs/cm) |
|---|---|---|---|---|---|---|
| S1 | 19-5 | 3.99 | 16.13 | 8.31 | 0.61 | 900.40 |
| 19-8 | 2.45 | 14.24 | 4.95 | 0.52 | 602.20 | |
| 19-10 | 1.40 | 22.34 | 13.04 | 0.64 | 538.80 | |
| 19-12 | 7.93 | 6.91 | 1.49 | 0.38 | 1948.00 | |
| Mean | 3.94 | 14.91 | 6.95 | 0.54 | 997.35 | |
| S2 | 20-10 | 12.37 | 9.95 | 0.66 | 0.36 | 1201.33 |
| 20-12 | 11.00 | 12.54 | 1.71 | 0.47 | 1051.67 | |
| 21-5 | 10.94 | 13.39 | 0.70 | 0.47 | 758.11 | |
| 21-8 | 12.84 | 9.01 | 0.97 | 0.32 | 1026.39 | |
| Mean | 11.79 | 11.22 | 1.01 | 0.41 | 1009.37 | |
| p | 0.002 ** | 0.313 | 0.031 * | 0.107 | 0.973 |
| Year | Samples | Ace | Shannon | Simpson | Chao |
|---|---|---|---|---|---|
| S1 | 19-5 | 3877.225 | 3.983 | 0.173 | 2965.878 |
| 19-8 | 4724.559 | 4.711 | 0.113 | 3515.368 | |
| 19-10 | 5932.280 | 6.996 | 0.002 | 4922.999 | |
| 19-12 | 6385.339 | 6.833 | 0.004 | 5190.885 | |
| Mean | 5229.851 | 5.631 | 0.073 | 4148.783 | |
| S2 | 20-10 | 5091.273 | 6.956 | 0.003 | 4562.520 |
| 20-12 | 5908.541 | 6.963 | 0.003 | 4902.821 | |
| 21-5 | 6264.320 | 6.965 | 0.003 | 5109.963 | |
| 21-8 | 6889.622 | 7.060 | 0.002 | 5518.087 | |
| Mean | 6038.439 | 6.986 | 0.003 | 5023.348 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Fang, S.; Wang, Q.; Wu, P.; He, P.; Liu, W. Interannual Variations in Soil Bacterial Community Diversity and Analysis of Influencing Factors During the Restoration Process of Scirpus Mariqueter Wetlands. Biology 2025, 14, 1013. https://doi.org/10.3390/biology14081013
Li Y, Fang S, Wang Q, Wu P, He P, Liu W. Interannual Variations in Soil Bacterial Community Diversity and Analysis of Influencing Factors During the Restoration Process of Scirpus Mariqueter Wetlands. Biology. 2025; 14(8):1013. https://doi.org/10.3390/biology14081013
Chicago/Turabian StyleLi, Yaru, Shubo Fang, Qinyi Wang, Pengling Wu, Peimin He, and Wei Liu. 2025. "Interannual Variations in Soil Bacterial Community Diversity and Analysis of Influencing Factors During the Restoration Process of Scirpus Mariqueter Wetlands" Biology 14, no. 8: 1013. https://doi.org/10.3390/biology14081013
APA StyleLi, Y., Fang, S., Wang, Q., Wu, P., He, P., & Liu, W. (2025). Interannual Variations in Soil Bacterial Community Diversity and Analysis of Influencing Factors During the Restoration Process of Scirpus Mariqueter Wetlands. Biology, 14(8), 1013. https://doi.org/10.3390/biology14081013







