Vitamin E Enhances Immune Function and the Intestinal Histological Structure by Regulating the Nodal-Mediated Signaling Pathway: A Case Study on the Sea Cucumber Apostichopus japonicus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Construction of the L4440-dsRNA-Nodal Expression System
2.3. Diet Preparation
2.4. Feeding Experiment
2.5. Sample Collection
2.6. Physiological and Chemical Analysis
2.6.1. Intestinal Histological Analysis
2.6.2. Enzyme Activity Analysis
2.6.3. Collagen Content Analysis
2.7. Real-Time Quantitative PCR
2.8. Formulas for Calculation
2.9. Data Analysis
3. Results
3.1. Growth Performance
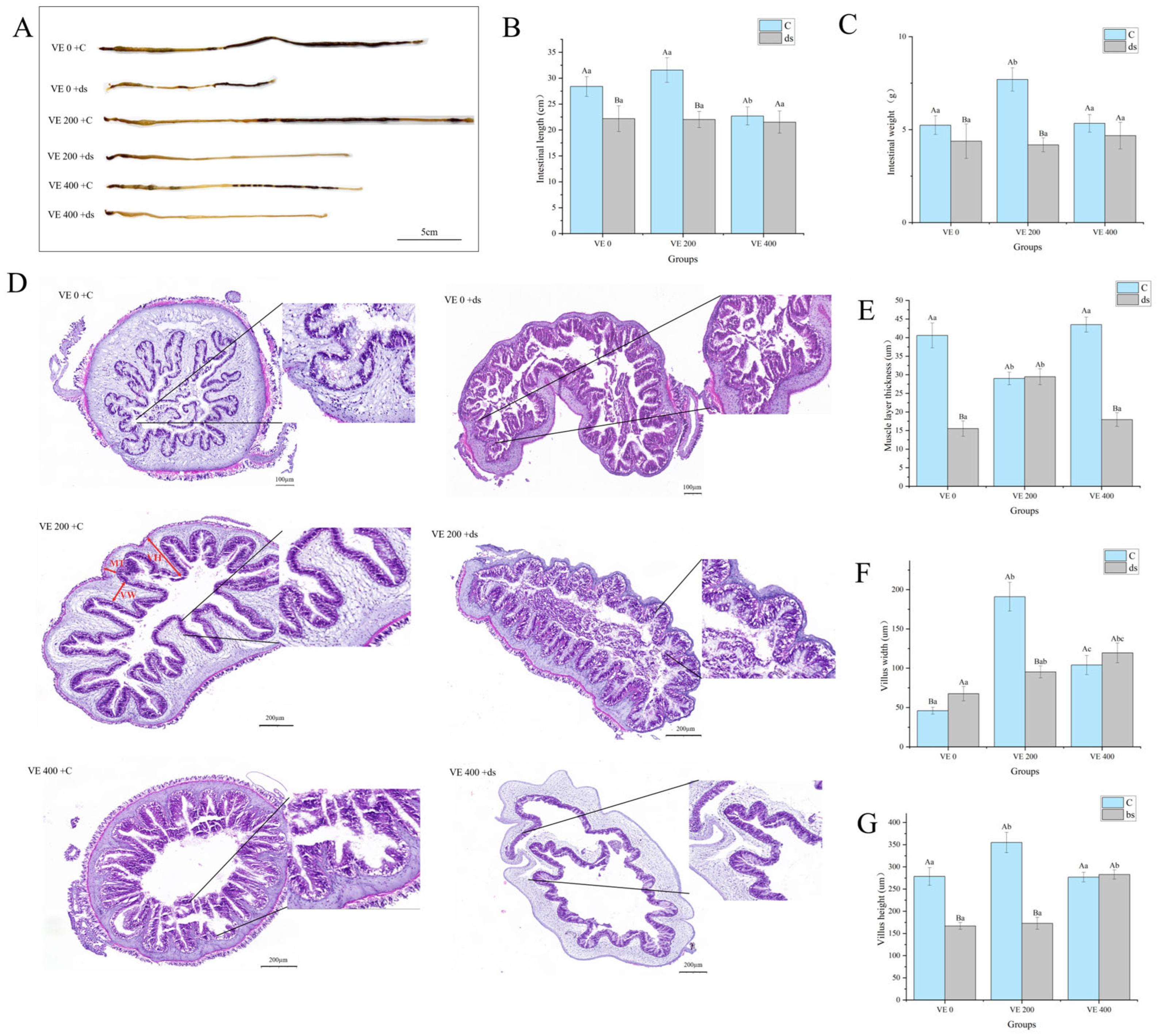
3.2. Intestinal Morphology
3.3. Immune Enzyme and Antioxidant Activities
3.3.1. Coelomic Fluid Immune Enzyme and Antioxidant Activities
3.3.2. Intestinal Immune Enzyme and Antioxidant Activities
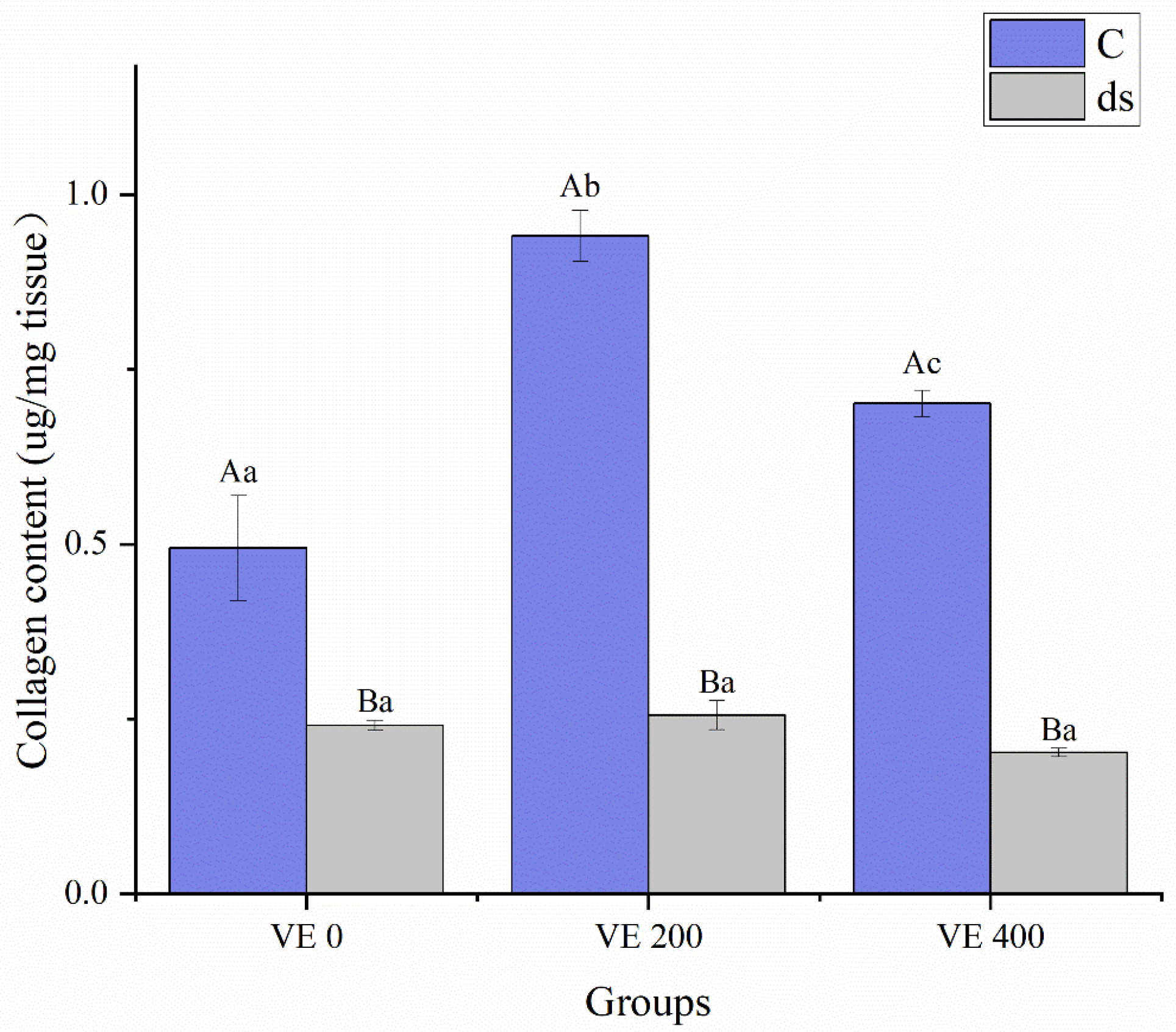
3.4. Intestinal Collagen Synthesis
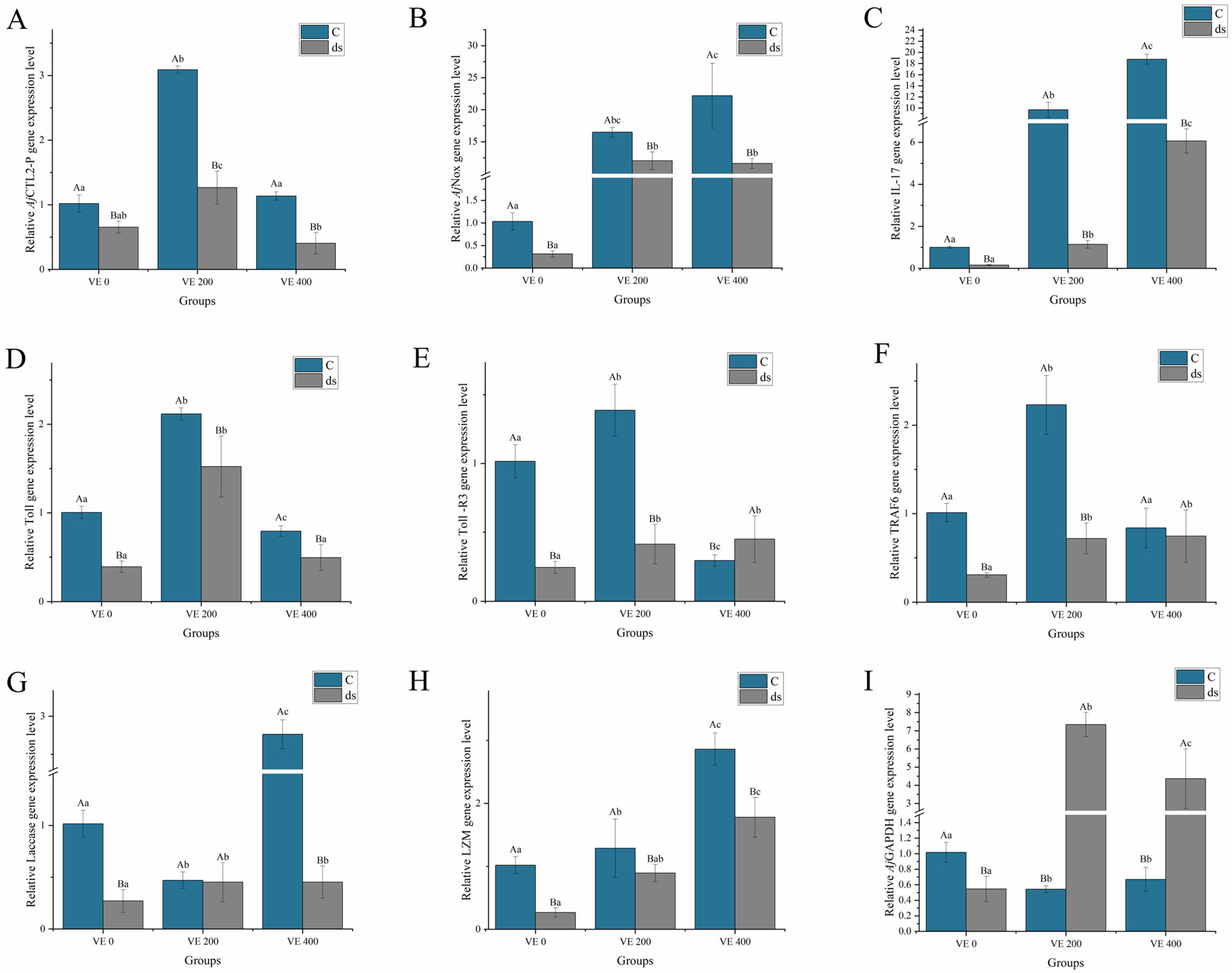
3.5. Gene Expression
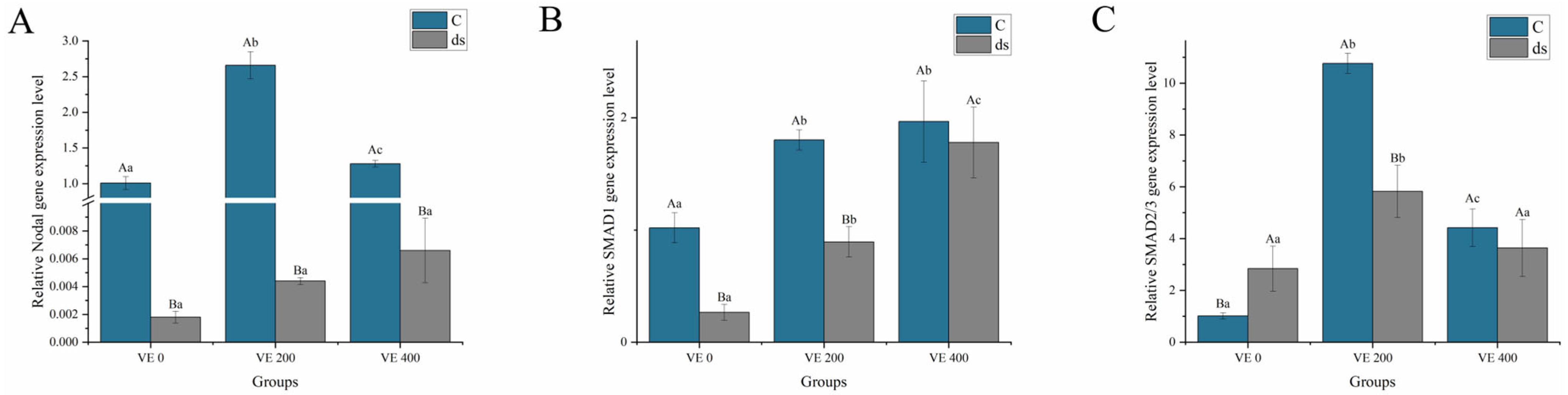
3.5.1. Nodal and SMAD Gene Expression
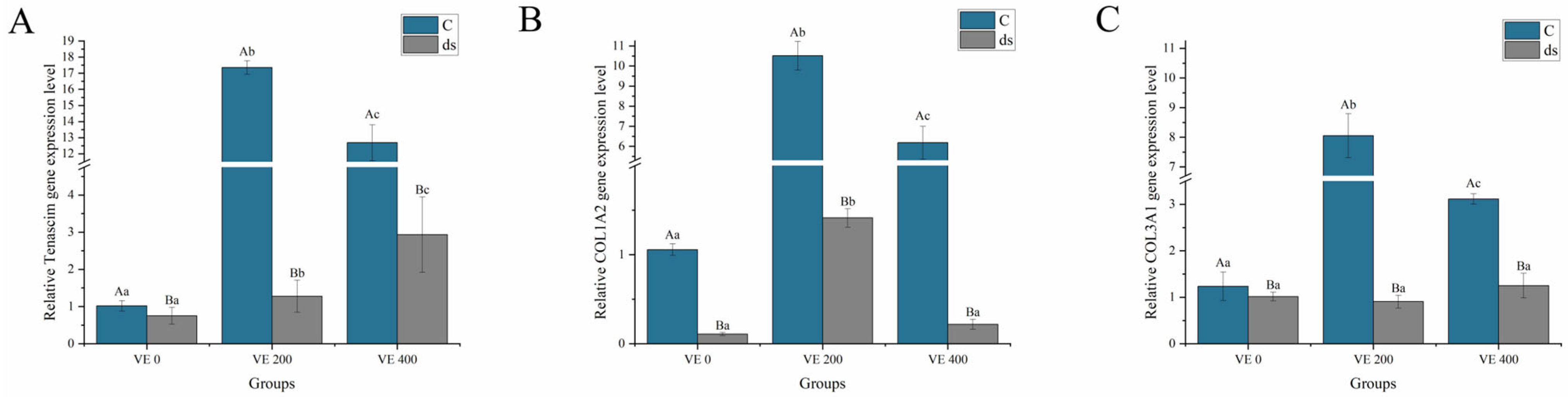
3.5.2. Immune-Related Gene Expression
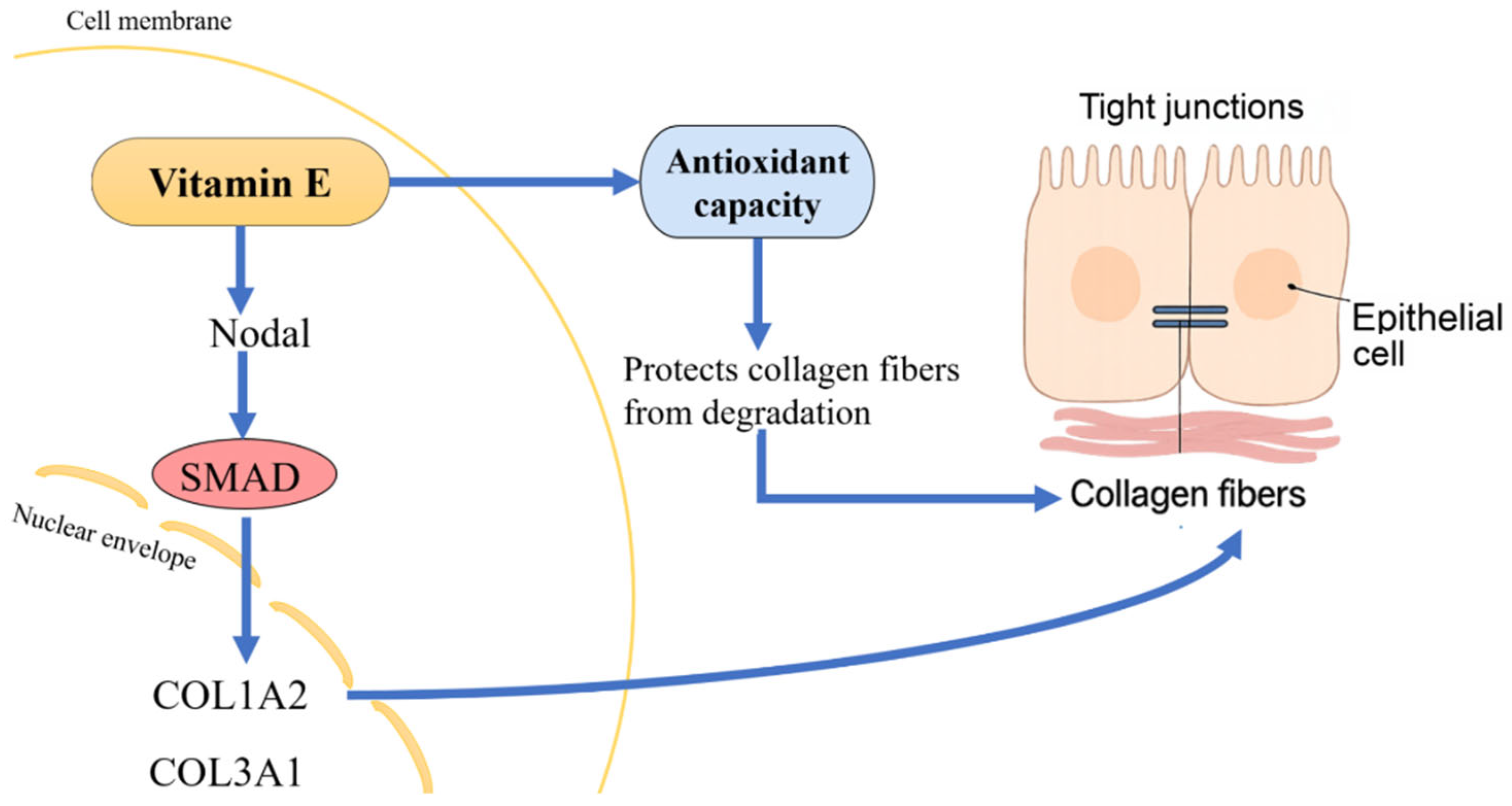
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Minami, K.; Masuda, R.; Takahashi, K.; Sawada, H.; Shirakawa, H.; Yamashita, Y. Seasonal and interannual variation in the density of visible Apostichopus japonicus (Japanese sea cucumber) in relation to sea water temperature. Estuar. Coast. Shelf Sci. 2019, 229, 106384. [Google Scholar] [CrossRef]
- Ciriminna, L.; Signa, G.; Cilluffo, G.; Rakaj, A.; Vizzini, S. Aquaculture of emerging species in North-Eastern Atlantic and Mediterranean Sea: A systematic review on sea cucumber farming and potential development. Front. Mar. Sci. 2024, 11, 1381836. [Google Scholar] [CrossRef]
- Liu, X.Y.; Hu, L.P.; Zhang, X.M.; Gao, Y.; Zhang, L.; Luan, M. Strategic thinking on the sustainable development of sea cucumber fingerling industry. Fish Inf. Strategy 2017, 32, 25–30. [Google Scholar] [CrossRef]
- Tian, R.H.; Wang, H.Y.; Wu, G.; Huang, X.Y.; Song, Q.Z.; Yang, Y.J.; Zhang, T.D.; Chang, Y.Q.; Zhao, C. Effects of Stocking Density on Behavior, Digestion, Gut Health, and Growth of Sea Cucumber Apostichopus japonicus. Dig. Gut Health Growth Sea Cucumber Apostichopus Jpn. 2024, 25, 4779116. [Google Scholar] [CrossRef]
- Ellis, R.; Parry, H.; Spicer, J.; Hutchinson, T.; Pipe, R.; Widdicombe, S. Immunological function in marine invertebrates: Responses to environmental perturbation. Fish Shellfish Immunol. 2011, 30, 1209–1222. [Google Scholar] [CrossRef]
- Tao, W.; Li, X.; Fu, X.; Shao, Y.; Guo, M.; Li, C. Akirin2 enhances antibacterial ability via interacting with 14-3-3ζ in V. splendidus-challenged Apostichopus japonicus. Fish Shellfish Immunol. 2024, 149, 109592. [Google Scholar] [CrossRef]
- Qian, Z.; Chen, K.; Yang, L.; Li, C. Apoptosis-inducing factor 1 mediates Vibrio splendidus-induced coelomocyte apoptosis via importin β dependent nuclear translocation in Apostichopus japonicus. Fish Shellfish Immunol. 2024, 148, 109491. [Google Scholar] [CrossRef]
- Watts, J.E.; Schreier, H.J.; Lanska, L.; Hale, M.S. The rising tide of antimicrobial resistance in aquaculture: Sources, sinks and solutions. Mar. Drugs 2017, 15, 158. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Esteban, M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Vijayaram, S.; Sun, Y.Z.; Zuorro, A.; Ghafarifarsani, H.; Van Doan, H.; Hoseinifar, S.H. Bioactive immunostimulants as health-promoting feed additives in aquaculture: A review. Fish Shellfish Immunol. 2022, 130, 294–308. [Google Scholar] [CrossRef]
- Yan, X.; Pan, S.; Dong, X.; Tan, B.; Li, T.; Huang, W.; Suo, X.; Li, Z.; Yang, Y. Vitamin E amelioration of oxidative stress and low immunity induced by high-lipid diets in hybrid grouper (♀ Epinephelus fuscoguttatus × ♂ E. lanceolatu). Fish Shellfish Immunol. 2022, 124, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Ye, J.; Wang, Y.; Chu, K.; Péré, M.; Xu, M.; Tang, X.; Fu, J. Vitamin E performs antioxidant effect via PAP retrograde signaling pathway in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2022, 127, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.; Zommara, M.; Eweedah, N.M.; Helal, A.I. Synergistic effects of selenium nanoparticles and vitamin E on growth, immune-related gene expression, and regulation of antioxidant status of Nile tilapia (Oreochromis niloticus). Biol. Trace Elem. Res. 2020, 195, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Qiang, J.; Wasipe, A.; He, J.; Tao, Y.F.; Xu, P.; Bao, J.W.; Chen, D.j.; Zhu, J.H. Dietary vitamin E deficiency inhibits fat metabolism, antioxidant capacity, and immune regulation of inflammatory response in genetically improved farmed tilapia (GIFT, Oreochromis niloticus) fingerlings following Streptococcus iniae infection. Fish Shellfish Immunol. 2019, 92, 395–404. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, H.; Chen, S.; Lin, Y.; Peng, J.; Hu, J.; Wang, Y. The Effects of Different Concentrations of Hydrogen-Rich Water on the Growth Performance, Digestive Ability, Antioxidant Capacity, Glucose Metabolism Pathway, mTOR Signaling Pathway, and Gut Microbiota of Largemouth Bass (Micropterus salmoides). Fishes 2024, 9, 210. [Google Scholar] [CrossRef]
- Qin, H.; Long, Z.; Ma, J.; Kong, L.; Lin, H.; Zhou, S.; Lin, Y.; Huang, Z.; Liu, L.; Li, Z. Growth performance, digestive capacity and intestinal health of juvenile spotted seabass (Lateolabrax maculatus) fed dietary laminarin supplement. Front. Mar. Sci. 2023, 10, 1242175. [Google Scholar] [CrossRef]
- De Marco, G.; Cappello, T.; Maisano, M. Histomorphological Changes in Fish Gut in Response to Prebiotics and Probiotics Treatment to Improve Their Health Status: A Review. Animals 2023, 13, 2860. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, K.Y.; Liang, X.X.; Fang, J.; Geng, Y.; Chen, Z.L.; Pu, H.B.; Hu, Y.D.; Li, X.; Liu, L. Effects of dietary vitamin E on growth performance as well as intestinal structure and function of channel catfish (Ictalurus punctatus, Rafinesque 1818). Exp. Ther. Med. 2017, 14, 5703–5710. [Google Scholar] [CrossRef][Green Version]
- Lozano, A.R.; Borges, P.; Robaina, L.; Betancor, M.; Hernández-Cruz, C.M.; García, J.R.; Caballero, M.J.; Vergara, J.M.; Izquierdo, M. Effect of different dietary vitamin E levels on growth, fish composition, fillet quality and liver histology of meagre (Argyrosomus regius). Aquaculture 2017, 468, 175–183. [Google Scholar] [CrossRef]
- Do-Huu, H.; Thuy, N.T.T.; Ky, P.X. The ameliorative roles of dietary mannan oligosaccharides and vitamin E on growth performance, intestinal microbes, and structure, flesh quality, nutrient efficacy and stress resistance of pompano, Trachinotus ovatus. Aquac. Rep. 2024, 37, 102233. [Google Scholar] [CrossRef]
- Gao, X.; Yu, B.; Yu, J.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; Zheng, P.; He, J.; Chen, D. Influences of dietary starch structure on intestinal morphology, barrier functions, and epithelium apoptosis in weaned pigs. Food Funct. 2020, 11, 4446–4455. [Google Scholar] [CrossRef]
- Schneeberger, K.; Roth, S.; Nieuwenhuis, E.E.; Middendorp, S. Intestinal epithelial cell polarity defects in disease: Lessons from microvillus inclusion disease. Dis. Models Mech. 2018, 11, dmm031088. [Google Scholar] [CrossRef]
- Rodriguez-Boulan, E.; Macara, I.G. Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 2014, 15, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Cereijido, M.; Contreras, R.G.; Shoshani, L.; Flores-Benitez, D.; Larre, I. Tight junction and polarity interaction in the transporting epithelial phenotype. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 770–793. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, S.; Sailer, A.; Sadiq, K.; Iqbal, N.; Ahmed, K.; Kabir, F.; Ali, S.A.; Turner, J.R. Abnormal transporter and tight junction protein expression in environmental enteric dysfunction. FASEB J. 2022, 36, R5615. [Google Scholar] [CrossRef]
- Sutherland, T.E.; Dyer, D.P.; Allen, J.E. The extracellular matrix and the immune system: A mutually dependent relationship. Science 2023, 379, eabp8964. [Google Scholar] [CrossRef]
- Aidos, L.; Mirra, G.; Pallaoro, M.; Herrera Millar, V.R.; Radaelli, G.; Bazzocchi, C.; Modina, S.C.; Di Giancamillo, A. How Do Alternative Protein Resources Affect the Intestine Morphology and Microbiota of Atlantic Salmon? Animals 2023, 13, 1922. [Google Scholar] [CrossRef]
- Elkhatib, N.; Bresteau, E.; Baschieri, F.; Rioja, A.L.; van Niel, G.; Vassilopoulos, S.; Montagnac, G. Tubular clathrin/AP-2 lattices pinch collagen fibers to support 3D cell migration. Science 2017, 356, eaal4713. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Teixeira, L.B.; Kiessling, L.L.; McAnulty, J.F.; Raines, R.T. Bifunctional peptide that anneals to damaged collagen and clusters TGF-β receptors enhances wound healing. ACS Chem. Biol. 2022, 17, 314–321. [Google Scholar] [CrossRef]
- Jabaji, Z.; Brinkley, G.J.; Khalil, H.A.; Sears, C.M.; Lei, N.Y.; Lewis, M.; Stelzner, M.; Martín, M.G.; Dunn, J.C. Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium. PLoS ONE 2014, 9, e107814. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Niu, C.; Ai, H.; Li, X.; Zhang, L.; Lang, Y.; Wang, S.; Gao, F.; Mei, X.; Yu, C. TSP50 Attenuates DSS-Induced Colitis by Regulating TGF-β Signaling Mediated Maintenance of Intestinal Mucosal Barrier Integrity. Adv. Sci. 2024, 11, 2305893. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.T.; Xu, R.J.; Yang, H.B.; Li, R.X.; Ding, J.; Chang, Y.Q.; Zuo, R.T. Vitamin E Regulates the Collagen Contents in the Body Wall of Sea Cucumber (Apostichopus japonicus) via Its Antioxidant Effects and the TGF-β/Smads Pathway. Antioxidants 2024, 13, 847. [Google Scholar] [CrossRef]
- Wang, T.; Liu, F.; Hu, Y.; Secombes, C.J.; Wang, T. The transforming growth factor (TGF)-Β family in rainbow trout (Oncorhynchus mykiss): Characterization and expression analysis. Fish Shellfish Immunol. 2019, 91, 448. [Google Scholar] [CrossRef]
- Bodenstine, T.M.; Chandler, G.S.; Seftor, R.E.; Seftor, E.A.; Hendrix, M.J. Plasticity underlies tumor progression: Role of Nodal signaling. Cancer Metastasis Rev. 2016, 35, 21–39. [Google Scholar] [CrossRef]
- Ayash, T.A.; Starr, L.M.; Dufort, D. Nodal is required to maintain the uterine environment in an anti-inflammatory state during pregnancy. Biol. Reprod. 2020, 102, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Starr, L.M.; Ayash, T.A.; Dufort, D. Evidence of a gene–environment interaction of Nodal variants and inflammation in preterm birth. J. Perinatol. 2018, 38, 482–488. [Google Scholar] [CrossRef]
- Pang, T.; Yin, X.Y.; Luo, T.H.; Lu, Z.M.; Nie, M.M.; Yin, K.; Xue, X.C. Cancer-associated fibroblasts promote malignancy of gastric cancer cells via Nodal signalling. Cell Biochem. Funct. 2020, 38, 4–11. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Huang, Y.F.; Kang, L.T. Persistence of RSV promotes proliferation and epithelial-mesenchymal transition of bronchial epithelial cells through Nodal signaling. J. Med. Microbiol. 2017, 66, 1499–1505. [Google Scholar] [CrossRef]
- Pan, J.; Chen, Z.; Yu, G.; Kong, Y.; Ai, Q.; Mai, K.; Zhang, Y. The supplementation of mannan oligosaccharide in diet promotes the skin wound healing of juvenile turbot, Scophthalmus maximus. Fish Shellfish Immunol. 2024, 154, 109953. [Google Scholar] [CrossRef]
- Gao, X.Y.; Yang, R.Y.; Song, W.H.; Shen, Y.Y.; Sun, H.; Nie, T.C.; Yue, X.L.; Song, Z.C.; Qi, J.; Zhang, Q.Q. Non-invasive dsRNA delivery via feeding for effective gene silencing in teleost fish: A novel approach in the study of gene function analysis. Aquaculture 2024, 586, 740763. [Google Scholar] [CrossRef]
- Bento, F.M.; Marques, R.N.; Campana, F.B.; Demétrio, C.G.; Leandro, R.A.; Parra, J.R.P.; Figueira, A. Gene silencing by RNAi via oral delivery of dsRNA by bacteria in the South American tomato pinworm. Pest Manag. Sci. 2020, 76, 287–295. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z.; Gui, Z.; Yang, B.; You, F.; Yang, G.; Zhang, X.; Chang, X.; Meng, X. Supplementation with Akkermansia muciniphila improved intestinal barrier and immunity in zebrafish (Danio rerio). Fish Shellfish Immunol. 2024, 154, 109935. [Google Scholar] [CrossRef]
- Gao, J.; Li, W.; Lin, J.; Han, Y.; Ji, G.; Liu, Z. Galnt3, an enzyme engaged in protein glycosylation modification, is essential for the maintaining of intestinal health in zebrafish. Fish Shellfish Immunol. 2025, 163, 110373. [Google Scholar] [CrossRef]
- Yao, H.; Hu, Y.; Tong, H.B.; Shi, S.R. Dimethylglycine Alleviates Metabolic Dysfunction-Associated Fatty Liver Disease by Improving the Circulating Estrogen Level via Gut Staphylococcus. J. Agric. Food Chem. 2023, 72, 2708–2717. [Google Scholar] [CrossRef]
- Liang, W.; Hu, L.; Dai, F.; Shi, Y.; Yang, L.; Li, C. Calreticulin from Apostichopus japonicus relieves endoplasmic reticulum stress induced by Vibrio splendidus through autophagy. Fish Shellfish Immunol. 2024, 153, 109798. [Google Scholar] [CrossRef]
- Wang, H.; Xue, Z.; Liu, Z.; Wang, W.; Wang, F.; Wang, Y.; Wang, L.; Song, L. A novel C-type lectin from the sea cucumber Apostichopus japonicus (AjCTL-2) with preferential binding of D-galactose. Fish Shellfish Immunol. 2018, 79, 218–227. [Google Scholar] [CrossRef]
- Yang, L.; Sun, L.L.; Li, C.H. NOX-derived ROS modulates the antibacterial immune responses in coelomocytes of Apostichopus japonicus. Aquaculture 2024, 588, 740953. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The role of vitamin E in immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef]
- Jiang, M.; Ma, L.; Shao, H.; Wu, F.; Liu, W.; Tian, J.; Yu, L.J.; Lu, X.; Wen, H. Dietary vitamin E requirement of sub-adult genetically improved farmed tilapia strain of Nile tilapia (Oreochromis niloticus) reared in freshwater. Aquac. Nutr. 2020, 26, 233–241. [Google Scholar] [CrossRef]
- Liu, H.P.; Wen, B.; Chen, Z.Z.; Gao, J.Z.; Liu, Y.; Zhang, Y.C.; Wang, Z.X.; Peng, Y. Effects of dietary vitamin C and vitamin E on the growth, antioxidant defence and digestive enzyme activities of juvenile discus fish (Symphysodon haraldi). Aquac. Nutr. 2019, 25, 176–183. [Google Scholar] [CrossRef]
- Li, Y.M.; Huang, Y.H.; Zhang, M.; Chen, Q.; Fan, W.J.; Zhao, Y.L. Effect of dietary vitamin E on growth, immunity and regulation of hepatopancreas nutrition in male oriental river prawn, Macrobrachium nipponense. Aquac. Res. 2019, 50, 1741–1751. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.Q.; Li, X.Y.; Li, J.G.; Bao, P.Y.; Che, J.; Li, S.Y.; Jin, L.J. Vitamin E requirement of sea cucumber (Apostichopus japonicus) and its’ effects on nonspecific immune responses. Aquac. Res. 2015, 46, 1628–1637. [Google Scholar] [CrossRef]
- Yang, L.; Li, C. Guanylate-binding proteins protect the sea cucumbers Apostichopus japonicus against infection of Vibrio splendidus. Fish Shellfish Immunol. 2025, 162, 110320. [Google Scholar] [CrossRef]
- Chen, D.; Zhong, J.; Jiang, W.; Wu, P.; Ma, Y.; Liu, Y.; Ren, H.; Jin, X.; Zhou, X.; Feng, L. Dietary phytic acid damages the intestinal mucus barrier and structural integrity in the grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2025, 161, 110300. [Google Scholar] [CrossRef]
- Seo, K.; Seo, J.; Yeun, J.; Choi, H.; Kim, Y.L.; Chang, S.Y. The role of mucosal barriers in human gut health. Arch. Pharmacal Res. 2021, 44, 325–341. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhang, X.F.; Chen, J.J.; Jiang, S.C.; Han, Y.; Du, H.H. Maternal folic acid supplementation improves the intestinal health of offspring porcine by promoting the proliferation and differentiation of intestinal stem cells. Animals 2023, 13, 3092. [Google Scholar] [CrossRef]
- Ouyang, P.; Huang, S.H.; Wei, W.Y.; Wu, J.N.; Zhou, Y.H.; Li, S.H.; Li, Q.; Geng, Y.; Huang, X.L.; Chen, D.F. Cinnamaldehyde treats largemouth bass non-lethal bacterial enteritis by regulating gut morphology, barrier, inflammation, microbiota and serum biochemistry. Aquaculture 2024, 581, 740463. [Google Scholar] [CrossRef]
- Ma, M.; Gui, Q.; Zheng, W.; Zhang, Y.; Wang, K. Nitrogen Removal Mechanism and Microbial Community Changes of the MBR Bioaugmented with Two Novel Fungi Pichia kudriavzevii N7 and Candida tropicalis N9. Water 2024, 16, 757. [Google Scholar] [CrossRef]
- Vargas-Albores, F.; Martinez-Cordova, L.R.; Hernandez-Mendoza, A.; Cicala, F.; Martinez-Porchas, M. Therapeutic modulation of fish gut microbiota, a feasible strategy for aquaculture? Aquaculture 2021, 544, 737050. [Google Scholar] [CrossRef]
- Monier, M.N.; Grana, Y.S.; Amer, A.A.; Abd El-Ghaffar, H.A.; Abd El-Naby, A.S.; Elmorshedy, E.; El-Saftawy, H.; Abdelhakim, T.M.N.; Gewaily, M.S.; El-Nagar, W.G. Dietary vitamin E (α-tocopherol acetate) modulates growth, digestive enzymes, histopathology, and vulnerability of Nile tilapia, Oreochromis niloticus to Aeromonas hydrophila infection. Anim. Feed Sci. Technol. 2024, 318, 116147. [Google Scholar] [CrossRef]
- Xu, H.; Gong, L.; Zhang, X.; Li, Z.; Fu, J.; Lv, Z.; Guo, Y. Effects of tannic acid on growth performance, intestinal health, and tolerance in broiler chickens. Poult. Sci. 2025, 104, 104676. [Google Scholar] [CrossRef]
- Niklasson, L.; Sundh, H.; Fridell, F.; Taranger, G.; Sundell, K. Disturbance of the intestinal mucosal immune system of farmed Atlantic salmon (Salmo salar), in response to long-term hypoxic conditions. Fish Shellfish Immunol. 2011, 31, 1072–1080. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.J.; Won, Y.S.; Kim, E.K.; Park, S.I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Zhan, W.; Peng, H.; Xie, S.; Deng, Y.; Zhu, T.; Cui, Y.; Cao, H.; Tang, Z.; Jin, M.; Zhou, Q. Dietary lauric acid promoted antioxidant and immune capacity by improving intestinal structure and microbial population of swimming crab (Portunus trituberculatus). Fish Shellfish Immunol. 2024, 151, 109739. [Google Scholar] [CrossRef]
- Eder, K.; Siebers, M.; Most, E.; Scheibe, S.; Weissmann, N.; Gessner, D.K. An excess dietary vitamin E concentration does not influence Nrf2 signaling in the liver of rats fed either soybean oil or salmon oil. Nutr. Metab. 2017, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, X.-F.; Tan, Q.; Yuan, X.; Liu, L.; Zhou, Y.; Li, B. Effects of vitamin E on growth performance and antioxidant status in juvenile grass carp Ctenopharyngodon idellus. Aquaculture 2014, 430, 21–27. [Google Scholar] [CrossRef]
- Ou, J.; Guan, X.; Wang, J.; Wang, T.; Zhang, B.; Li, R.; Xu, H.; Hu, X.; Guo, X.-K. Epithelial NELF guards intestinal barrier function to ameliorate colitis by maintaining junctional integrity. Mucosal Immunol. 2022, 15, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Yui, S.; Azzolin, L.; Maimets, M.; Pedersen, M.T.; Fordham, R.P.; Hansen, S.L.; Larsen, H.L.; Guiu, J.; Alves, M.R.; Rundsten, C.F. YAP/TAZ-dependent reprogramming of colonic epithelium links ECM remodeling to tissue regeneration. Cell Stem Cell 2018, 22, 35–49. [Google Scholar] [CrossRef]
- Stewart, D.C.; Brisson, B.K.; Yen, W.K.; Liu, Y.; Wang, C.; Ruthel, G.; Gullberg, D.; Mauck, R.L.; Maden, M.; Han, L. Type III collagen regulates matrix architecture and mechanosensing during wound healing. J. Investig. Dermatol. 2025, 145, 919–938.e914. [Google Scholar] [CrossRef] [PubMed]
- Liebing, E.; Krug, S.M.; Neurath, M.F.; Siegmund, B.; Becker, C. Wall of Resilience: How the intestinal epithelium prevents inflammatory Onslaught in the Gut. Cell. Mol. Gastroenterol. Hepatol. 2024, 19, 101423. [Google Scholar] [CrossRef]
- Varma, P.; Kandasubramanian, B. Exploring the Antiaging Properties of Curcumin, Flaxseed, and Collagen: A Comprehensive Review. ChemistrySelect 2024, 9, e202403395. [Google Scholar] [CrossRef]
- Damianos, J.; Abdelnaem, N.; Camilleri, M. Gut Goo: Physiology, Diet, and Therapy of Intestinal Mucus and Biofilms in Gastrointestinal Health and Disease. Clin. Gastroenterol. Hepatol. 2024, 23, 205–215. [Google Scholar] [CrossRef]
- Izu, Y.; Birk, D.E. Collagen XII mediated cellular and extracellular mechanisms in development, regeneration, and disease. Front. Cell Dev. Biol. 2023, 11, 1129000. [Google Scholar] [CrossRef]
- Huang, P.Y.; Tsai, M.C.; Kiu, K.T.; Yen, M.H.; Chang, T.C. Collagen patch cover facilitates recovery of bowel function after laparoscopic colectomy. BMC Surg. 2024, 24, 66. [Google Scholar] [CrossRef]
- Nathan, F.M.; Li, S. Environmental cues determine the fate of astrocytes after spinal cord injury. Neural Regen. Res. 2017, 12, 1964–1970. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Lin, H.H.; Tang, M.J. A tale of two collagen receptors, integrin β1 and discoidin domain receptor 1, in epithelial cell differentiation. Am. J. Physiol. Cell Physiol. 2012, 303, C1207–C1217. [Google Scholar] [CrossRef]
- Xiao, H.; Yu, J.; Song, L.; Hu, M.; Guo, H.; Xue, Y.; Xue, C. Characterization of flesh firmness and ease of separation in the fermentation of sea bass in terms of protein structure, texture, and muscle tissue structural changes. Food Res. Int. 2022, 162, 111965. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kobayashi, S.; Yamashita, M. Comparison of connective tissue structure and muscle toughness of spotted mackerel Scomber australasicus and Pacific mackerel S. japonicus during chilled and frozen storage. Fish. Sci. 2017, 83, 133–139. [Google Scholar] [CrossRef]
- Tak, L.J.; Kim, H.Y.; Ham, W.K.; Agrahari, G.; Seo, Y.; Yang, J.W.; An, E.J.; Bang, C.H.; Lee, M.J.; Kim, H.S.; et al. Superoxide Dismutase 3-Transduced Mesenchymal Stem Cells Preserve Epithelial Tight Junction Barrier in Murine Colitis and Attenuate Inflammatory Damage in Epithelial Organoids. Int. J. Mol. Sci. 2021, 22, 6431. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Suo, H.; Lv, Y.; Liu, S.; Gao, Z.; Chen, Y.; Zhang, M.; Meng, X.; Gao, S. Modulation of miR-466d-3p on Wnt signaling pathway in response to DEPs-induced blood-brain barrier disruption. Ecotoxicol. Environ. Saf. 2024, 284, 116869. [Google Scholar] [CrossRef]
- Singh, D.; Rai, V.; Agrawal, D.K. Regulation of collagen I and collagen III in tissue injury and regeneration. Cardiol. Cardiovasc. Med. 2023, 7, 5. [Google Scholar] [CrossRef]
- Herawati, E.; Akhsanitaqwim, Y.; Agnesia, P.; Listyawati, S.; Pangastuti, A.; Ratriyanto, A. In Vitro Antioxidant and Antiaging Activities of Collagen and Its Hydrolysate from Mackerel Scad Skin (Decapterus macarellus). Mar. Drugs 2022, 20, 516. [Google Scholar] [CrossRef]
- Cui, B.L.; Zhang, C.C.; Gan, B.; Liu, W.; Liang, J.Q.; Fan, Z.Q.; Wen, Y.Y.; Yang, Y.; Peng, X.S.; Zhou, Y.F. Collagen-tussah silk fibroin hybrid scaffolds loaded with bone mesenchymal stem cells promote skin wound repair in rats. Mater. Sci. Eng. C 2020, 109, 110611. [Google Scholar] [CrossRef]
- Luo, K. Signaling cross talk between TGF-β/Smad and other signaling pathways. Cold Spring Harb. Perspect. Biol. 2017, 9, a022137. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Zhong, W.; Zhu, M.; Hu, S.G.; Su, X.K. Nodal regulates bladder cancer cell migration and invasion via the ALK/Smad signaling pathway. OncoTargets Ther. 2018, 2018, 6589–6597. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.D. Pancreatic cancer is suppressed by fibroblast-derived collagen I. Cancer Cell 2021, 39, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Zhang, H.; Ning, L.; Wu, K.; Limbu, S.M.; Shi, Q.; Qin, C.; Wen, X. The transforming growth factor beta (TGF-β/Smads) pathway regulates collagen synthesis and deposition in swim bladder of Chu’s croaker (Nibea coibor) stimulated by proline. Aquaculture 2022, 558, 738360. [Google Scholar] [CrossRef]
- Xia, Y.; Yu, E.; Li, Z.; Zhang, K.; Tian, J.; Wang, G.; Xie, J.; Gong, W. Both TGF-β1 and Smad4 regulate type I collagen expression in the muscle of grass carp, Ctenopharyngodon idella. Fish Physiol. Biochem. 2021, 47, 907–917. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.Y.; Ji, Q.Q.; Song, J.X.; Wang, L.; Liu, B.K.; Wang, J.H.; Li, C. The function of Apostichopus japonicus catalase in sea cucumber intestinal immunity. Aquaculture 2020, 521, 735103. [Google Scholar] [CrossRef]
- Payton, L.; Perrigault, M.; Bourdineaud, J.P.; Marcel, A.; Massabuau, J.C.; Tran, D. Trojan horse strategy for non-invasive interference of clock gene in the oyster Crassostrea gigas. Mar. Biotechnol. 2017, 19, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Charoonnart, P.; Worakajit, N.; Zedler, J.A.Z.; Meetam, M.; Robinson, C.; Saksmerprome, V. Generation of microalga Chlamydomonas reinhardtii expressing shrimp antiviral dsRNA without supplementation of antibiotics. Sci. Rep. 2019, 9, 3164. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.R.; Dong, S.L.; Wang, F.; Tian, X.L.; Gao, Q.F. Effects of density on variation in individual growth and differentiation in endocrine response of Japanese sea cucumber (Apostichopus japonicus Selenka). Aquaculture 2012, 356, 398–403. [Google Scholar] [CrossRef]
- Olejniczak, M.; Galka, P.; Krzyzosiak, W.J. Sequence-non-specific effects of RNA interference triggers and microRNA regulators. Nucleic Acids Res. 2010, 38, 1–16. [Google Scholar] [CrossRef]
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, O.; Martins, I.M.; Hou, H.; Zhao, X.; Blumberg, J.B.; Li, B. Collagen peptides ameliorate intestinal epithelial barrier dysfunction in immunostimulatory Caco-2 cell monolayers via enhancing tight junctions. Food Funct. 2017, 8, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | Vitamin E Levels (mg/kg) | ||
|---|---|---|---|
| 0 mg/kg | 200 mg/kg | 400 mg/kg | |
| Fish meal | 40 | 40 | 40 |
| Fermented soybean meal | 50 | 50 | 50 |
| Sargassum thunbergii meal | 290 | 290 | 290 |
| Fish oil | 10 | 10 | 10 |
| Vitamin premix | 5 | 5 | 5 |
| DL-α-Tocopherol acetate (97% purity) | - | 0.2 | 0.4 |
| Mineral premix | 5 | 5 | 5 |
| Sea mud | 600 | 599.8 | 599.6 |
| Proximate composition | |||
| Crude protein (%) | 8.48 ± 0.29 | 8.39 ± 0.12 | 8.41 ± 0.32 |
| Crude lipid (%) | 0.53 ± 0.09 | 0.55 ± 0.01 | 0.58 ± 0.01 |
| Genes | Primer Sequences (5′-3′) | Gene IDs/References |
|---|---|---|
| Cytb-R 1 | F:5′-TGAGCCGCAACAGTAATC-3′ | KP170618.1 |
| R:5′-AAGGGAAAAGGAAGTGAAAG-3′ | ||
| Tenascin 2 | F:5′-CCCTGATGGTGCTCT-3′ | c64081.graph_c1 |
| R:5′-GGGACGAATCTTCATTTCTGTA-3′ | ||
| COL1A2 3 | F:5′-CGGACTTTTACTTTGGCGTTAT-3′ | c54738.graph_c0 |
| R:5′-TTTCTGGCGGTCTGCCTAT-3′ | ||
| COL3A1 4 | F:5′-TCTCTTTGGTCACGATTGGC-3′ | BSL78_04953 |
| R:5′-CACCACGGGCATCTGTTAG-3′ | ||
| SMAD2/3 5 | F:5′-CAGAACCACCACGAACTCAA-3′ | BSL78_11878 |
| R:5′-TGACTCACAGATACCACGGT-3′ | ||
| SMAD1 6 | F:5′-ACACATTTACTTGGCTCCCC-3′ | BSL78_15508 |
| R:5′-GTCCAGTTGTGAAGAGGCTT-3′ | ||
| Nodal 7 | F:5′-GTTGTCTACACGAAGGGGTC-3′ | BSL78_20284 |
| R:5′-CTGCATTTCAACCACACGAC-3′ | ||
| AjCTL2-p | F:5′-ACCGCTCCTCACCCTTTAACAC-3′ | [47] |
| R:5′-CCACTAGCACTAAGCAGCATATCAG-3′ | ||
| AjNox | F:5′-TAGCCCAAGAAAATCAAGGAGAA-3′ | [48] |
| R:5′-CAGCATTTGTGAAGGATGTGTGA-3′ | ||
| IL-17 | F:5′-ACCGCTCCTCACCCTTTAACAC-3′ | |
| R:5′-ACACAAACCTGCCCTACATCA-3′ | ||
| AjGAPDH | F:5′-GGCATTCCGTGTACCTGTCCC-3′ | |
| R:5′-TACTGCTGGCTGCTTTTTTGA-3′ | ||
| Laccase 8 | F:5′-TGTAGGGCATAATCAACCGG-3′ | BSL78_13504 |
| R:5′-CAAACCTCTCCCCTGCATTG-3′ | ||
| LZM 9 | F:5′-TCCTCTTCCCTAGCTCTACA-3′ | BSL78_07135 |
| R:5′-AGTGAATGGCGATGTTGGTC-3′ | ||
| Toll 10 | F:5′-CCGGGTTACATGTCACTGTT-3′ | BSL78_01296 |
| R:5′-ACAACCCATTCAACTGCACT-3′ | ||
| Toll-R3 11 | F:5′-TTATCGAGAACATCACCGGC-3′ | BSL78_17684 |
| R:5′-GCCTCCTTCAACTTTTCCCA-3′ | ||
| TRAF6 12 | F:5′-CTTCCGTTTCAAGCAGTCCT-3′ | BSL78_15285 |
| R:5′-ATAACATTGTCGAGTGGCCC-3′ |
| VE 0 | VE 200 | VE 400 | ||||
|---|---|---|---|---|---|---|
| C 1 | Ds 2 | C | ds | C | ds | |
| Survival rate (%) | 100 | 100 | 100 | 100 | 100 | 100 |
| Weight growth rate (%) | 55.22 ± 0.04 Aa | −12.28 ± 0.02 Ba | 88.93 ± 0.02 Ab | 32.26 ± 0.05 Bb | 52.47 ± 0.03 Ac | 15.55 ± 0.01 Bc |
| Specific growth rate (%/d) | 2.09 ± 0.01 Aa | −0.63 ± 0.01 Ba | 3.03 ± 0.01 Ab | 1.32 ± 0.02 Bb | 2.01 ± 0.01 Aa | 0.69 ± 0.01 Bc |
| Body wall index (%) | 63.11 ± 1.22 Aa | 52.71 ± 3.81 Ba | 64.36 ± 1.56 Aa | 61.29 ± 1.83 Ba | 59.68 ± 1.12 Aa | 59.19 ± 2.33 Aa |
| VE 0 | VE 200 | VE 400 | ||||
|---|---|---|---|---|---|---|
| C 1 | Ds 2 | C | ds | C | ds | |
| SOD (U/mL) | 202.37 ± 5.94 Aa | 195.75 ± 1.09 Ba | 266.10 ± 0.70 Ab | 219.78 ± 3.94 Bb | 253.78 ± 2.74 Ac | 231.04 ± 2.63 Bc |
| POD (U/mL) | 0.65 ± 0.01 Aa | 0.44 ± 0.19 Ba | 2.19 ± 0.22 Ab | 1.39 ± 0.08 Bb | 1.92 ± 0.18 Ab | 1.96 ± 0.10 Ac |
| GSH-PX (U/mL) | 6.73 ± 0.24 Aa | 6.36 ± 0.28 Ba | 15.70 ± 0.16 Ab | 8.88 ± 0.57 Bb | 11.52 ± 1.09 Ac | 10.91 ± 0.01 Bc |
| CAT (U/mL) | 0.38 ± 0.07 Aa | 0.32 ± 0.09 Aa | 0.72 ± 0.05 Ab | 0.32 ± 0.08 Ba | 0.17 ± 0.02 Bc | 0.20 ± 0.06 Aa |
| MDA (nmol/mL) | 0.77 ± 0.03 Aa | 0.59 ± 0.05 Ba | 0.24 ± 0.02 Bb | 0.33 ± 0.01 Ab | 0.97 ± 0.02 Bc | 1.19 ± 0.02 Ac |
| LZM (U/mL) | 56.15 ± 2.70 Aab | 39.74 ± 1.36 Ba | 102.56 ± 3.98 Ac | 51.79 ± 0.68 Bb | 58.72 ± 0.26 Aa | 33.59 ± 2.45 Bc |
| ACP (King’s units/100 mL) | 0.55 ± 0.02 Aab | 0.50 ± 0.03 Ba | 1.08 ± 0.04 Ac | 0.59 ± 0.01 Bab | 0.96 ± 0.08 Ab | 0.68 ± 0.06 Bc |
| ALP (King’s units/100 mL) | 0.96 ± 0.03 Aa | 0.44 ± 0.08 Ba | 2.71 ± 0.21 Ab | 1.40 ± 0.12 Bb | 1.21 ± 0.14 Ac | 1.06 ± 0.04 Bc |
| VE 0 | VE 200 | VE 400 | ||||
|---|---|---|---|---|---|---|
| C 1 | Ds 2 | C | ds | C | ds | |
| SOD (U/mgprot) | 348.90 ± 0.44 Aa | 285.33 ± 3.84 Ba | 451.33 ± 4.69 Ab | 364.36 ± 2.07 Bb | 340.73 ± 3.18 Aa | 277.80 ± 1.48 Ba |
| POD (U/mgprot) | 5.52 ± 0.54 Aa | 4.70 ± 0.21 Ba | 10.34 ± 0.54 Ab | 6.52 ± 0.33 Bb | 4.44 ± 0.66 Aa | 4.94 ± 0.67 Aab |
| GSH-PX (U/mgprot) | 15.23 ± 0.69 Aab | 12.20 ± 0.28 Ba | 26.30 ± 1.69 Ac | 16.04 ± 1.30 Bb | 16.18 ± 1.67 Ab | 9.68 ± 0.85 Bc |
| CAT (U/mgprot) | 66.69 ± 4.52 Aab | 46.36 ± 8.86 Ba | 113.99 ± 1.20 Ac | 34.08 ± 5.39 Bc | 72.35 ± 4.28 Ab | 40.84 ± 1.43 Bab |
| MDA (nmol/mgprot) | 1.06 ± 0.15 Ba | 2.84 ± 0.30 Aa | 0.46 ± 0.22 Bb | 1.75 ± 0.34 Ab | 2.00 ± 0.06 Bc | 2.30 ± 0.07 Aab |
| LZM (U/mgprot) | 59.00 ± 2.10 Aa | 33.77 ± 3.83 Ba | 98.95 ± 0.95 Ab | 39.59 ± 1.38 Bb | 33.23 ± 1.65 Ac | 29.43 ± 1.03 Bc |
| ACP (King’s units/gprot) | 113.49 ± 2.16 Aa | 107.30 ± 1.03 Ba | 219.69 ± 15.20 Ac | 150.49 ± 14.25 Bb | 130.52 ± 2.90 Aab | 114.13 ± 9.44 Ba |
| ALP (King’s units/gprot) | 1078.08 ± 17.06 Aa | 1028.65 ± 15.64 Aa | 1372.85 ± 85.32 Ab | 499.07 ± 87.84 Bb | 1307.32 ± 80.93 Aab | 1133.30 ± 82.36 Bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, Y.; Wang, X.; Zhao, G.; Zeng, H.; Xiao, H.; Han, L.; Ding, J.; Chang, Y.; Zuo, R. Vitamin E Enhances Immune Function and the Intestinal Histological Structure by Regulating the Nodal-Mediated Signaling Pathway: A Case Study on the Sea Cucumber Apostichopus japonicus. Biology 2025, 14, 1008. https://doi.org/10.3390/biology14081008
Wang Z, Wang Y, Wang X, Zhao G, Zeng H, Xiao H, Han L, Ding J, Chang Y, Zuo R. Vitamin E Enhances Immune Function and the Intestinal Histological Structure by Regulating the Nodal-Mediated Signaling Pathway: A Case Study on the Sea Cucumber Apostichopus japonicus. Biology. 2025; 14(8):1008. https://doi.org/10.3390/biology14081008
Chicago/Turabian StyleWang, Zitong, Yan Wang, Xianyu Wang, Guangyao Zhao, Haiqing Zeng, Haoran Xiao, Lingshu Han, Jun Ding, Yaqing Chang, and Rantao Zuo. 2025. "Vitamin E Enhances Immune Function and the Intestinal Histological Structure by Regulating the Nodal-Mediated Signaling Pathway: A Case Study on the Sea Cucumber Apostichopus japonicus" Biology 14, no. 8: 1008. https://doi.org/10.3390/biology14081008
APA StyleWang, Z., Wang, Y., Wang, X., Zhao, G., Zeng, H., Xiao, H., Han, L., Ding, J., Chang, Y., & Zuo, R. (2025). Vitamin E Enhances Immune Function and the Intestinal Histological Structure by Regulating the Nodal-Mediated Signaling Pathway: A Case Study on the Sea Cucumber Apostichopus japonicus. Biology, 14(8), 1008. https://doi.org/10.3390/biology14081008







