Chnoospora minima Polysaccharide-Mediated Green Synthesis of Silver Nanoparticles: Potent Anticancer and Antimicrobial Activities
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Material and Polysaccharide Extraction
2.2. Green Synthesis of Silver Nanoparticles (PAgNPs)
2.3. Characterization of Polysaccharide and PAgNPs
2.3.1. Fourier Transform Infrared (FTIR) Spectroscopy
2.3.2. Biochemical Characterization of Seaweed Extract
2.3.3. UV–Visible (UV-Vis) Spectroscopy
2.3.4. Dynamic Light Scattering (DLS) and Zeta Potential
2.3.5. Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray (EDX) Analysis
2.3.6. Raman Spectroscopy
2.3.7. Total Phenolic Content (TPC)
2.4. Antioxidant Activity (DPPH Radical Scavenging Assay)
2.5. Antimicrobial Susceptibility Testing
2.5.1. Microbial Strains and Culture Conditions
2.5.2. Agar Well Diffusion Assay
2.6. Cell Lines and Cell Culture
2.7. Antiproliferative Activity (CellTiter-Glo Assay)
2.8. Statistical Analysis
3. Results
3.1. Characterization of Algal Polysaccharide
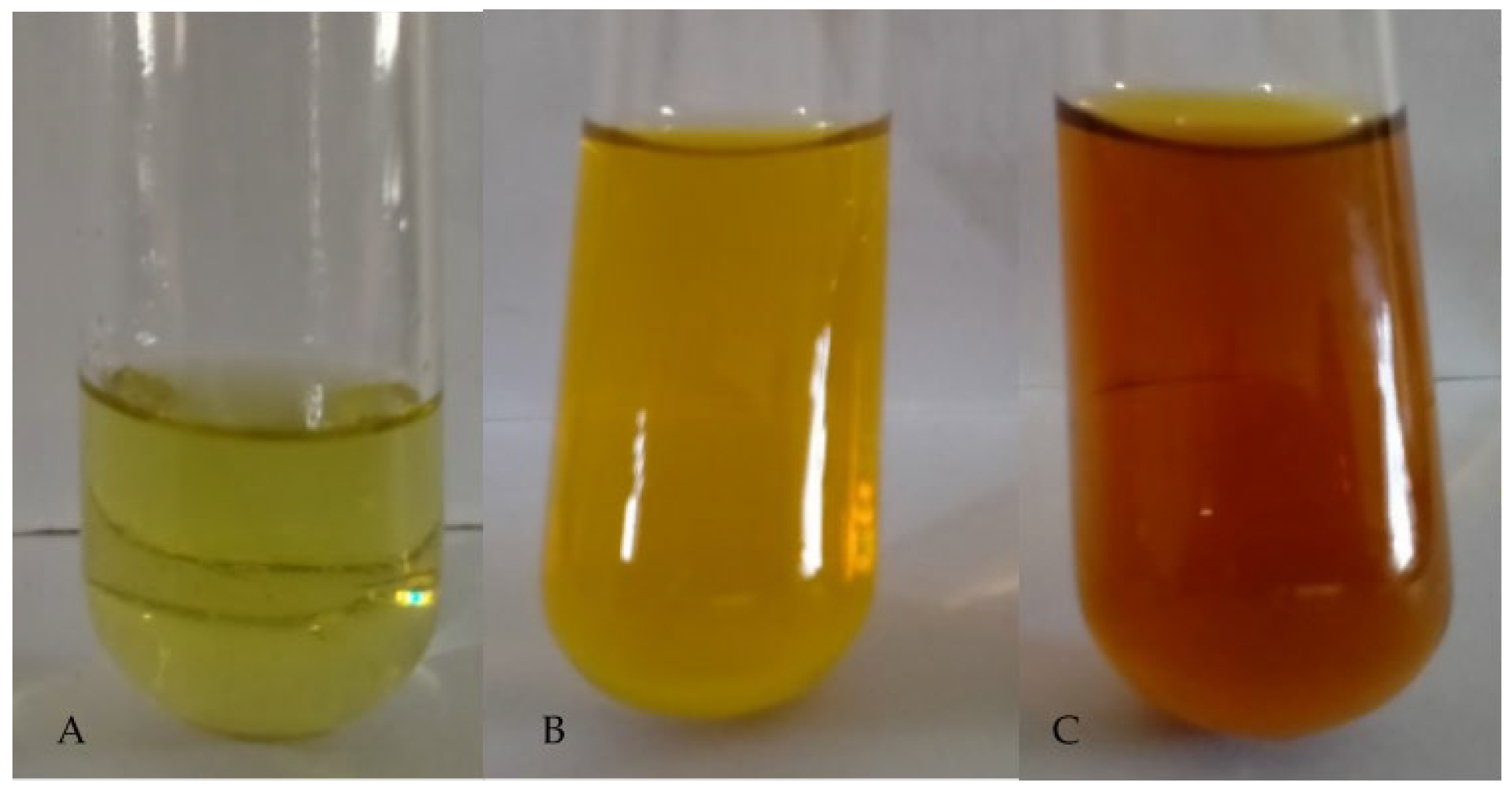
3.2. Synthesis and Visual Observation of PAgNPs
3.3. Spectroscopic Characterization of PAgNPs
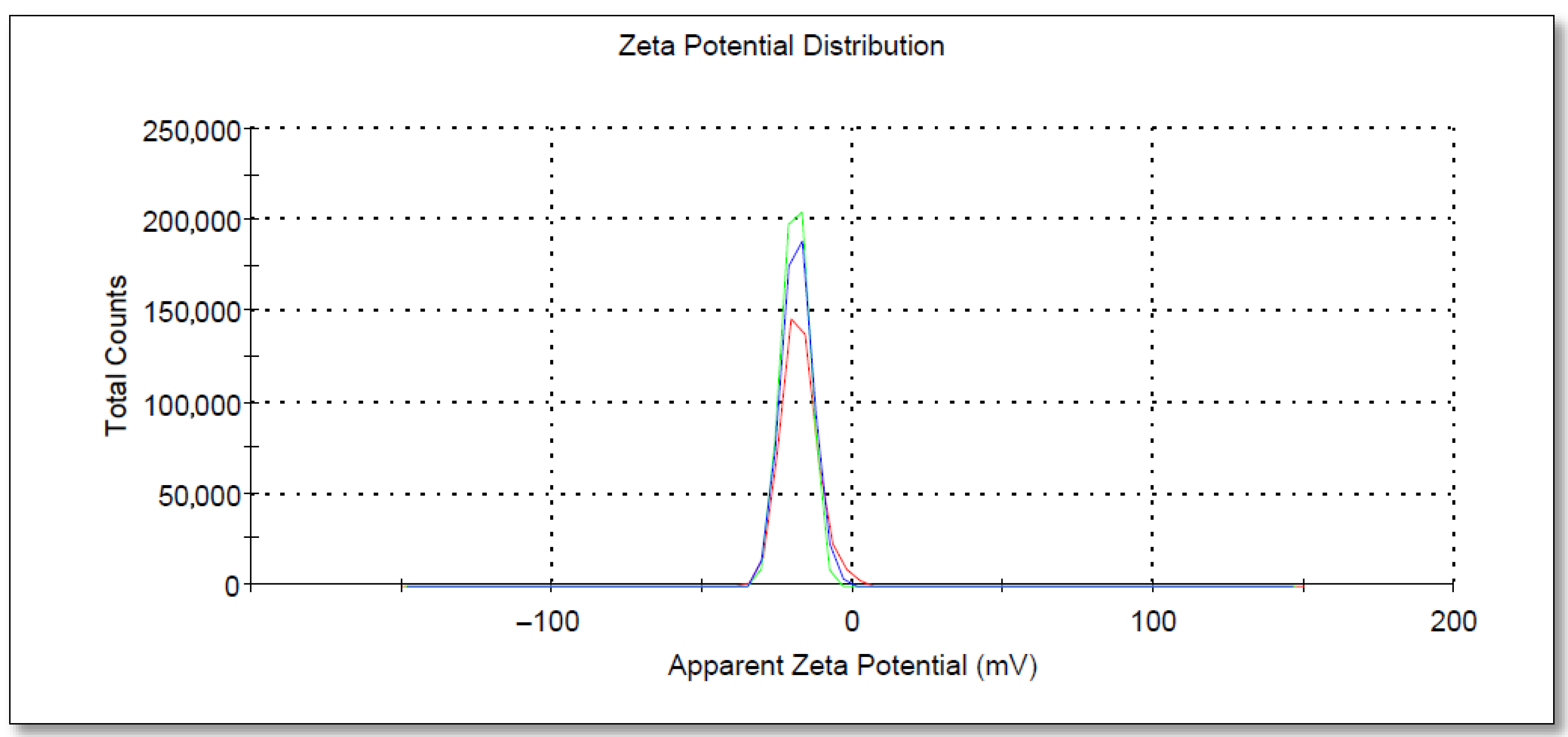
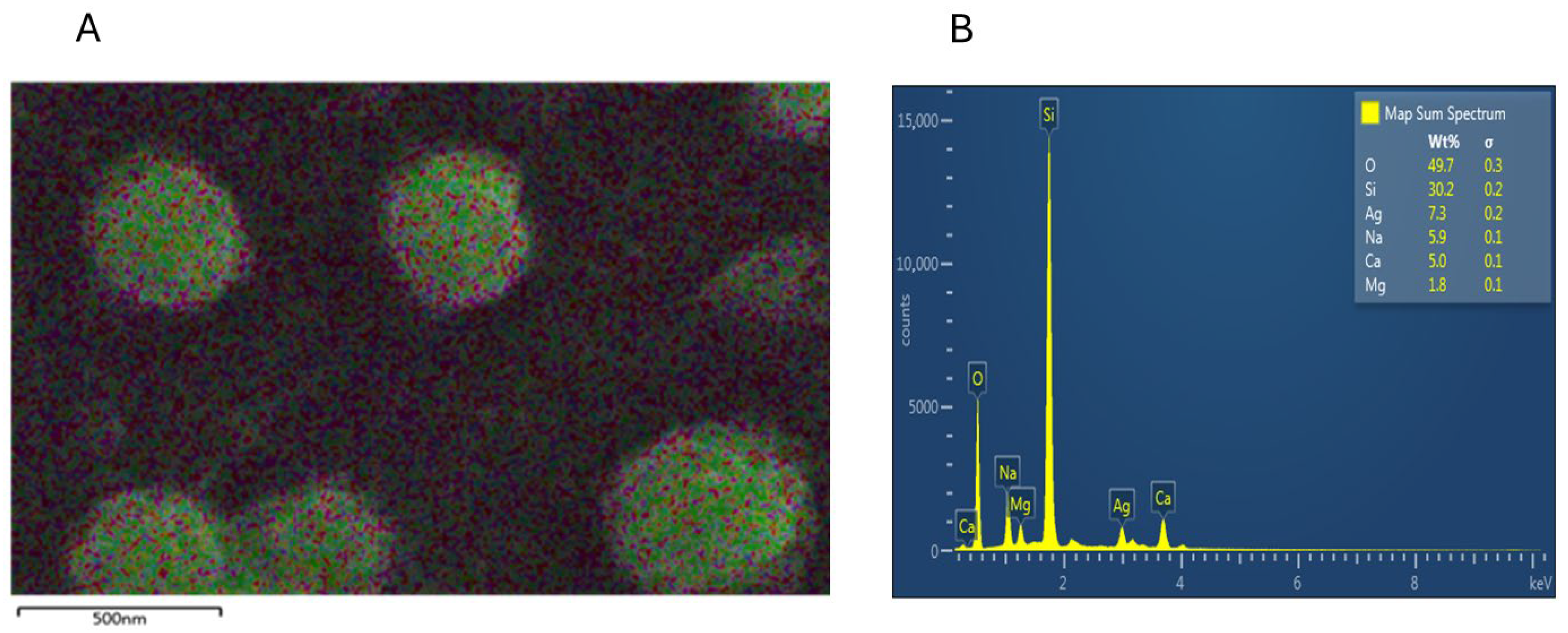
3.4. DLS, SEM, and EDX Analyses of PAgNPs
3.5. Raman Spectroscopy and Total Phenolic Content (TPC) of PAgNPs
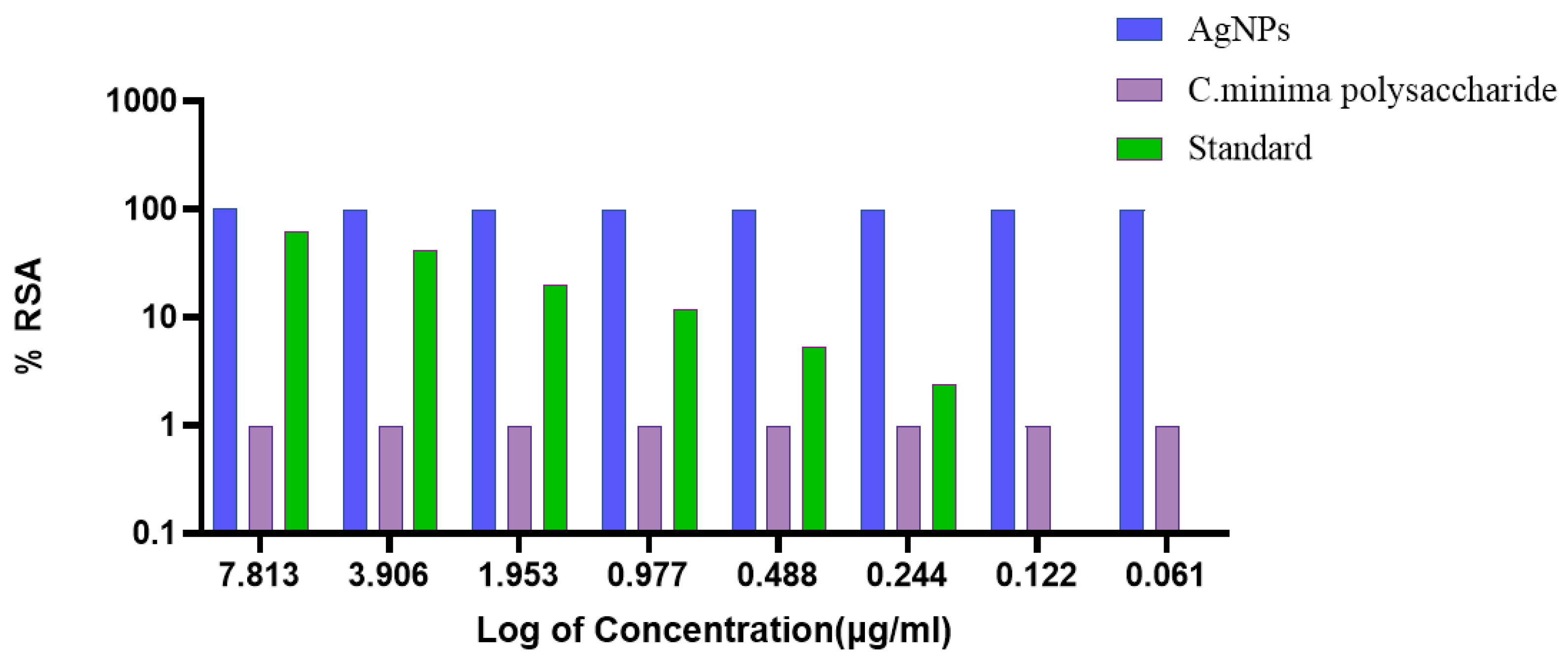
3.6. Antioxidant Activity of PAgNPs
3.7. Antimicrobial Susceptibility of PAgNPs
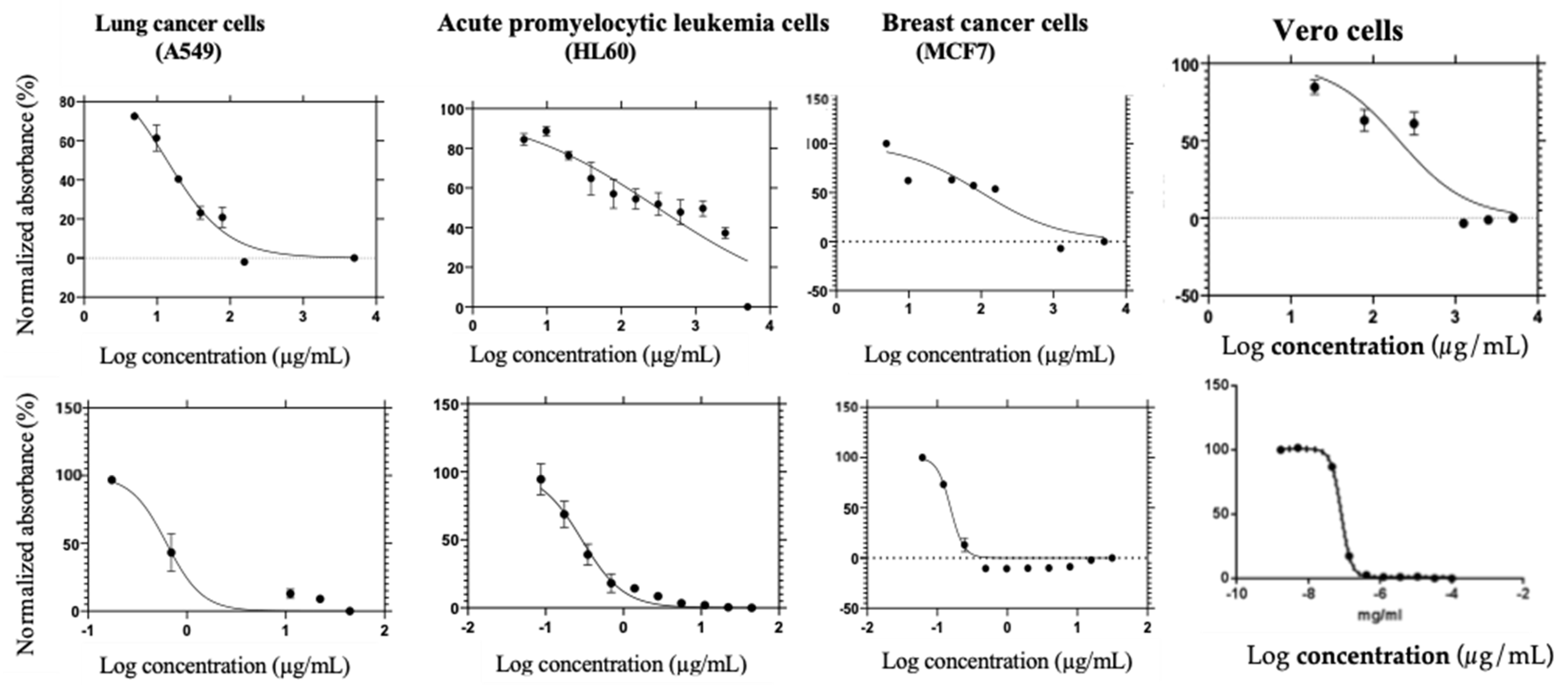
3.8. Antiproliferative Activity of PAgNPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAgNPs | Polysaccharide-based Silver Nanoparticles |
| AgNPs | Silver Nanoparticles |
| FTIR | Fourier Transform Infrared Spectroscopy |
| DLS | Dynamic Light Scattering |
| TPC | Total Phenolic Content |
| EDX | Energy Disperse X-ray |
| MIC | Minimum Inhibitory Concentration |
References
- Zulfiqar, Z.; Khan, R.R.M.; Summer, M.; Saeed, Z.; Pervaiz, M.; Rasheed, S.; Shehzad, B.; Kabir, F.; Ishaq, S. Plant-Mediated Green Synthesis of Silver Nanoparticles: Synthesis, Characterization, Biological Applications, and Toxicological Considerations: A Review. Biocatal. Agric. Biotechnol. 2024, 57, 103121. [Google Scholar] [CrossRef]
- Soni, M.; Mehta, P.; Soni, A.; Goswami, G.K. Green Nanoparticles: Synthesis and Applications. IOSR J. Biotechnol. Biochem. 2018, 4, 78–83. [Google Scholar]
- Priyadarshni, K.C.; Mahalingam, P.U. Biofunctionalization of Mycosynthesized Silver Nanoparticles on Selected Drug Resistant Human Pathogens. Mater. Res. Express 2019, 6, 85056. [Google Scholar] [CrossRef]
- Elsaesser, A.; Howard, C.V. Toxicology of Nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Fahimirad, S.; Ajalloueian, F.; Ghorbanpour, M. Synthesis and Therapeutic Potential of Silver Nanomaterials Derived from Plant Extracts. Ecotoxicol. Environ. Saf. 2019, 168, 260–278. [Google Scholar] [CrossRef]
- Hu, R.; Yong, K.-T.; Roy, I.; Ding, H.; He, S.; Prasad, P.N. Metallic Nanostructures as Localized Plasmon Resonance Enhanced Scattering Probes for Multiplex Dark-Field Targeted Imaging of Cancer Cells. J. Phys. Chem. C 2009, 113, 2676–2684. [Google Scholar] [CrossRef]
- Javaid, A.; Oloketuyi, S.F.; Khan, M.M.; Khan, F. Diversity of Bacterial Synthesis of Silver Nanoparticles. Bionanoscience 2018, 8, 43–59. [Google Scholar] [CrossRef]
- Amini, N.; Amin, G.; Azar, Z.J. Green Synthesis of Silver Nanoparticles Using Avena sativa L. Extract. Nanomed. Res. J. 2017, 2, 57–63. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; de Andrade Tomaz, A.C.; Vanderlei de Souza, M.d.F.; Leitão da Cunha, E.V.; Kiss, R.; Mathieu, V.; Ioannou, E.; Roussis, V. Halogenated Diterpenes with In Vitro Antitumor Activity from the Red Alga Sphaerococcus coronopifolius. Mar. Drugs 2019, 18, 29. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sarkar, D.; Sasmal, S. A Review of Green Synthesis of Metal Nanoparticles Using Algae. Front. Microbiol. 2021, 12, 693899. [Google Scholar] [CrossRef]
- Gunathilaka, T.L.; Samarakoon, K.W.; Ranasinghe, P.; Peiris, L.D.C. In-Vitro Antioxidant, Hypoglycemic Activity, and Identification of Bioactive Compounds in Phenol-Rich Extract from the Marine Red Algae Gracilaria edulis (Gmelin) Silva. Molecules 2019, 24, 3708. [Google Scholar] [CrossRef]
- National Cancer Institute. SEER Cancer Statistics Review. 1975–2014. Available online: https://seer.cancer.gov/archive/csr/1975_2014/ (accessed on 29 November 2019).
- National Cancer Institute. SEER Cancer Statistics Review. 1975–2018. Available online: https://seer.cancer.gov/archive/csr/1975_2018/ (accessed on 29 November 2019).
- Martínez Andrade, K.; Lauritano, C.; Romano, G.; Ianora, A. Marine Microalgae with Anti-Cancer Properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.; Iqbal, J.; Ali, M.; Khalil, A.T.; Ahsan Abbasi, B.; Numan, M.; Shinwari, Z.K.; Abbasi, B.A.; Numan, M.; Shinwari, Z.K. Green Synthesis of Zinc Nanoparticles through Plant Extracts: Establishing a Novel Era in Cancer Theranostics. Mater. Res. Express 2019, 6, 102005. [Google Scholar] [CrossRef]
- National Breast Cancer Foundations INC Breast Cancer Facts. Available online: https://www.nationalbreastcancer.org/breast-cancer-facts (accessed on 20 August 2020).
- Meistrich, M.L. Effects of Chemotherapy and Radiotherapy on Spermatogenesis in Humans. Fertil. Steril. 2013, 100, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.R.; Mukherjee, S.; Kotcherlakota, R. Biosynthesized Silver Nanoparticles: A Step Forward for Cancer Theranostics? Nanomedicine 2014, 9, 1445–1448. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.-S.; Kim, E.-A.; Gunasekara, U.K.D.S.S.; Abeytunga, D.T.U.; Nanayakkara, C.; de Silva, E.D.; et al. FTIR Characterization and Antioxidant Activity of Water Soluble Crude Polysaccharides of Sri Lankan Marine Algae. Algae 2017, 32, 75–86. [Google Scholar] [CrossRef]
- Udayangani, R.M.A.C.; Somasiri, G.D.P.; Wickramasinghe, I.; Kim, S. Potential Health Benefits of Sulfated Polysaccharides from Marine Algae. In Encyclopedia of Marine Biotechnology; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 629–635. [Google Scholar] [CrossRef]
- Gómez, I.; Huovinen, P. Brown Algal Phlorotannins: An Overview of Their Functional Roles. In Antarctic Seaweeds; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 365–388. [Google Scholar]
- Lakmal, H.C.; Samarakoon, K.W.; Lee, W.; Lee, J.-H.; Abeytunga, D.; Lee, H.-S.; Jeon, Y.-J. Anticancer and Antioxidant Effects of Selected Sri Lankan Marine Algae. J. Natl. Sci. Found. 2014, 42, 315. [Google Scholar] [CrossRef]
- El-Rafie, H.M.; El-Rafie, M.H.; Zahran, M.K. Green Synthesis of Silver Nanoparticles Using Polysaccharides Extracted from Marine Macro Algae. Carbohydr. Polym. 2013, 96, 403–410. [Google Scholar] [CrossRef]
- Kang, M.-C.; Kim, S.-Y.; Kim, E.-A.; Lee, J.-H.; Kim, Y.-S.; Yu, S.-K.; Chae, J.B.; Choe, I.-H.; Cho, J.H.; Jeon, Y.-J. Antioxidant Activity of Polysaccharide Purified from Acanthopanax koreanum Nakai Stems in Vitro and in Vivo Zebrafish Model. Carbohydr. Polym. 2015, 127, 38–46. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Deshavath, N.N.; Mukherjee, G.; Goud, V.V.; Veeranki, V.D.; Sastri, C.V. Pitfalls in the 3, 5-Dinitrosalicylic Acid (DNS) Assay for the Reducing Sugars: Interference of Furfural and 5-Hydroxymethylfurfural. Int. J. Biol. Macromol. 2020, 156, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, F.A.; Abdala-Díaz, R.T.; Pérez, C.; Casas-Arrojo, V.; Nesic, A.; Tapia, C.; Durán, C.; Valdes, O.; Parra, C.; Bravo-Arrepol, G.; et al. Sulfated Polysaccharide Extracted from the Green Algae Codium Bernabei: Physicochemical Characterization and Antioxidant, Anticoagulant and Antitumor Activity. Mar. Drugs 2022, 20, 458. [Google Scholar] [CrossRef] [PubMed]
- Siddhanta, S.; Kuzmin, A.N.; Pliss, A.; Baev, A.S.; Khare, S.K.; Chowdhury, P.K.; Ganguli, A.K.; Prasad, P.N. Advances in Raman Spectroscopy and Imaging for Biomedical Research. Adv. Opt. Photonics 2023, 15, 318–384. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Nasir, N.A.H.A.; Roslly, N.A.L.; Rosli, N.M.; Razali, Z. Phytochemical Screening and Potential DPPH Radical Scavenging Activity of Banana Peel Extract. In Charting the Sustainable Future of ASEAN in Science and Technology; Springer: Singapore, 2020; pp. 249–258. [Google Scholar]
- Salayová, A.; Bedlovičová, Z.; Daneu, N.; Baláž, M.; Lukáčová Bujňáková, Z.; Balážová, Ľ.; Tkáčiková, Ľ. Green Synthesis of Silver Nanoparticles with Antibacterial Activity Using Various Medicinal Plant Extracts: Morphology and Antibacterial Efficacy. Nanomaterials 2021, 11, 1005. [Google Scholar] [CrossRef]
- Elkhateeb, M.I.; El-Bitar, A.M.H.; Saleh, S.R.; Abdelreheem, A.M.A. Evaluation of Bioactive Phytochemical Characterization, Antioxidant, Antimicrobial, and Antihemolytic Properties of Some Seaweeds Collected from Red Sea Coast, Egypt. Egypt. J. Aquat. Biol. Fish. 2021, 25, 417–436. [Google Scholar] [CrossRef]
- Heendeniya, S.N.; Keerthirathna, L.R.; Manawadu, C.K.; Dissanayake, I.H.; Ali, R.; Mashhour, A.; Alzahrani, H.; Godakumbura, P.; Boudjelal, M.; Peiris, D.C. Therapeutic Efficacy of Nyctanthes Arbor-tristis Flowers to Inhibit Proliferation of Acute and Chronic Primary Human Leukemia Cells, with Adipocyte Differentiation and in Silico Analysis of Interactions between Survivin Protein and Selected Secondary Meta. Biomolecules 2020, 10, 165. [Google Scholar] [CrossRef]
- Yudha, S.S.; Notriawan, D.; Angasa, E.; Eka Suharto, T.; Hendri, J.; Nishina, Y. Green Synthesis of Silver Nanoparticles Using Aqueous Rinds Extract of Brucea javanica (L.) Merr at Ambient Temperature. Mater. Lett. 2013, 97, 181–183. [Google Scholar] [CrossRef]
- Selvi, B.C.G.; Madhavan, J.; Santhanam, A. Cytotoxic Effect of Silver Nanoparticles Synthesized from Padina Tetrastromatica on Breast Cancer Cell Line. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035015. [Google Scholar] [CrossRef]
- He, R.; Qian, X.; Yin, J.; Zhu, Z. Preparation of Polychrome Silver Nanoparticles in Different Solvents. J. Mater. Chem. 2002, 12, 3783–3786. [Google Scholar] [CrossRef]
- Grigoras, A.G.; Grigoras, V.C. Eco-Friendly Silver Nanoparticles Obtained by Green Synthesis from Salvia officinalis. Sustain. Chem. 2024, 5, 215–228. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-Based Nanoparticles as Drug Delivery Systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef]
- Aboelfetoh, E.F.; El-Shenody, R.A.; Ghobara, M.M. Eco-Friendly Synthesis of Silver Nanoparticles Using Green Algae (Caulerpa serrulata): Reaction Optimization, Catalytic and Antibacterial Activities. Environ. Monit. Assess. 2017, 189, 349. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological Activities and Potential Health Benefits of Sulfated Polysaccharides Derived from Marine Algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Gibson, E.G.; Bax, B.; Chan, P.F.; Osheroff, N. Mechanistic and Structural Basis for the Actions of the Antibacterial Gepotidacin against Staphylococcus aureus Gyrase. ACS Infect. Dis. 2019, 5, 570–581. [Google Scholar] [CrossRef]
- Hongal, A.M.; Shettar, A.K.; Hoskeri, J.H.; Vedamurthy, A.B. Silver Nanoparticles Mediated Apoptosis and Cell Cycle Arrest in Lung Cancer A549. 3 Biotech. 2024, 14, 238. [Google Scholar] [CrossRef]
- Devasvaran, K.; Alallam, B.; Yunus, M.A.; Dewi, F.R.P.; Kamal, N.N.S.N.; Lim, V. Microwave-Assisted Green Synthesis of Silver Nanoparticles Using Alkaline Extracted Crude Polysaccharide of C. nutans: Optimisation, Characterisation, Toxicity, Anticancer Potential and Antibacterial Studies. J. Drug Deliv. Sci. Technol. 2023, 86, 104688. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the Abilities of Ambient and Manufactured Nanoparticles To Induce Cellular Toxicity According to an Oxidative Stress Paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef]
- Netala, V.; Bethu, M.; Pushpalatha, B.; Baki, V.; Aishwarya, S.; Rao, J.; Tartte, V. Biogenesis of Silver Nanoparticles Using Endophytic Fungus Pestalotiopsis Microspora and Evaluation of Their Antioxidant and Anticancer Activities. Int. J. Nanomed. 2016, 11, 5683–5696. [Google Scholar] [CrossRef]
- Venugopal, K.; Ahmad, H.; Manikandan, E.; Thanigai Arul, K.; Kavitha, K.; Moodley, M.K.; Rajagopal, K.; Balabhaskar, R.; Bhaskar, M. The Impact of Anticancer Activity upon Beta Vulgaris Extract Mediated Biosynthesized Silver Nanoparticles (Ag-NPs) against Human Breast (MCF-7), Lung (A549) and Pharynx (Hep-2) Cancer Cell Lines. J. Photochem. Photobiol. B Biol. 2017, 173, 99–107. [Google Scholar] [CrossRef]
- Vaikundamoorthy, R.; Krishnamoorthy, V.; Vilwanathan, R.; Rajendran, R. Structural Characterization and Anticancer Activity (MCF7 and MDA-MB-231) of Polysaccharides Fractionated from Brown Seaweed Sargassum wightii. Int. J. Biol. Macromol. 2018, 111, 1229–1237. [Google Scholar] [CrossRef]
- Hemlata; Meena, P.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of Silver Nanoparticles Using Cucumis Prophetarum Aqueous Leaf Extract and Their Antibacterial and Antiproliferative Activity Against Cancer Cell Lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef]







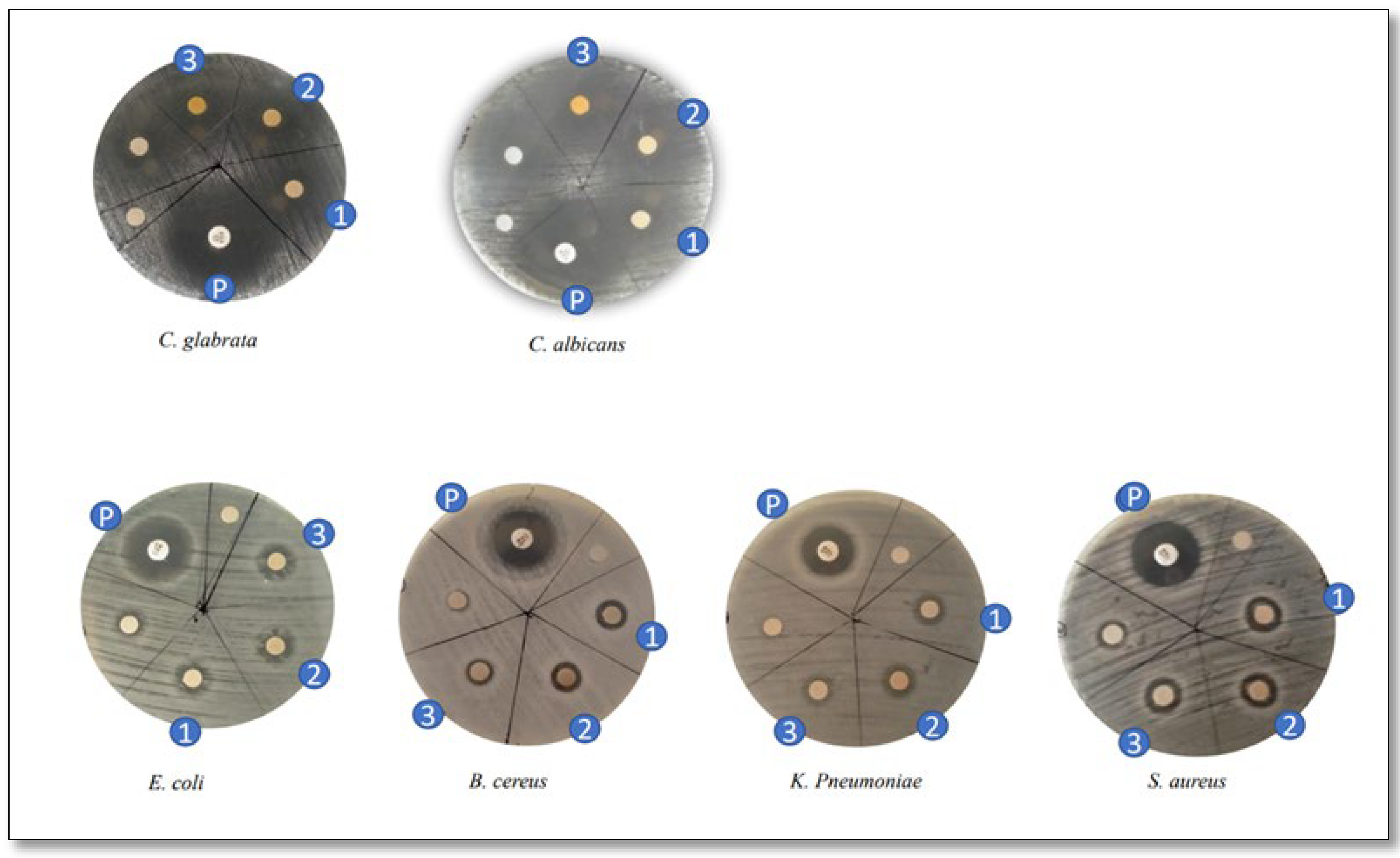
| Microbial Strains | Inhibition Zone (mm) | |
|---|---|---|
| Fluconazole | Ag-NPs | |
| C. albicans | 34 ± 0.04 | 15 ± 0.02 |
| C. glabrata | 35 ± 0.05 | 12 ± 0.1 |
| Gentamicin | Ag-NPs | |
| E. coli | 22 ± 0.04 | 8 ± 0.00 |
| P. aeruginosa | 20 ± 0.03 | 8 ± 0.00 |
| K. Pneumoniae | 15 ± 0.01 | 8 ± 0.00 |
| S. aureus | 18 ± 0.02 | 12 ± 0.015 |
| B. cereus | 18 ± 0.02 | 8 ± 0.000 |
| Mitoxantrone (Standard Drug) | Polysaccharide-AgNPs | |
|---|---|---|
| Cells | IC50 Value (µg/mL) | |
| A459 | 0.627 | 13.59 |
| HL60 | 0.291 | 306.1 |
| MCF7 | 0.153 | 100.7 |
| Vero | 8.54 | 300.2 |
| Extract Type | IC50 (μg/mL) |
|---|---|
| Polysaccharide-based silver nanoparticles | 3.921 |
| Crude polysaccharide | 200 |
| Vero cell line | 0.604 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keerthirathna, L.; Sigera, S.; Rathnayake, M.; Senarathne, A.; Udeshika, H.; Kodikara, C.; Sirimuthu, N.M.; Samarakoon, K.W.; Boudjelal, M.; Ali, R.; et al. Chnoospora minima Polysaccharide-Mediated Green Synthesis of Silver Nanoparticles: Potent Anticancer and Antimicrobial Activities. Biology 2025, 14, 904. https://doi.org/10.3390/biology14070904
Keerthirathna L, Sigera S, Rathnayake M, Senarathne A, Udeshika H, Kodikara C, Sirimuthu NM, Samarakoon KW, Boudjelal M, Ali R, et al. Chnoospora minima Polysaccharide-Mediated Green Synthesis of Silver Nanoparticles: Potent Anticancer and Antimicrobial Activities. Biology. 2025; 14(7):904. https://doi.org/10.3390/biology14070904
Chicago/Turabian StyleKeerthirathna, Lakshika, Sachini Sigera, Milan Rathnayake, Arunoda Senarathne, Hiruni Udeshika, Chamali Kodikara, Narayana M. Sirimuthu, Kalpa W. Samarakoon, Mohamad Boudjelal, Rizwan Ali, and et al. 2025. "Chnoospora minima Polysaccharide-Mediated Green Synthesis of Silver Nanoparticles: Potent Anticancer and Antimicrobial Activities" Biology 14, no. 7: 904. https://doi.org/10.3390/biology14070904
APA StyleKeerthirathna, L., Sigera, S., Rathnayake, M., Senarathne, A., Udeshika, H., Kodikara, C., Sirimuthu, N. M., Samarakoon, K. W., Boudjelal, M., Ali, R., & Peiris, D. C. (2025). Chnoospora minima Polysaccharide-Mediated Green Synthesis of Silver Nanoparticles: Potent Anticancer and Antimicrobial Activities. Biology, 14(7), 904. https://doi.org/10.3390/biology14070904







