Integrating Computational Analysis of In Vivo Investigation of Modulatory Effect of Fagonia cretica Plant Extract on Letrozole-Induced Polycystic Ovary Syndrome in Female Rats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Housing of Animals
2.3. Collection of Plant
- Extract preparation.
- Characterization of plant extract.
- Phytochemical analysis of extract.
- Designing the experimental protocol.
2.4. Preparation of Hydro-Alcoholic Extract
2.5. Characterization of Plant Extract
2.5.1. Qualitative Phytochemical Analysis Test
2.5.2. Total Phenolic Content (TPC)
2.5.3. Total Flavanoid Content (TFC)
2.6. Experimental Protocol
- (a)
- Control group (n = 3):
- (b)
- Disease group (n = 3):
- (c)
- Treatment groups (n = 9):
2.7. Ultrasound Examination of Polycystic Rat
2.8. Collection of Blood
2.9. Biochemical Analysis
2.9.1. Serum Hormone Levels (Testosterone and Insulin)
2.9.2. Fasting Blood Glucose
2.9.3. Insulin Resistance by HOMA-IR Index
2.9.4. Lipid Profile
2.10. Histopathological Evaluation
2.11. Statistical and Graphical Analysis
3. Results
3.1. Percentage Yield of Extract
3.2. Total Phenolic Content (TPC)
3.3. Total Flavonoid Content (TFC)
3.4. Qualitative Phytochemical Analysis
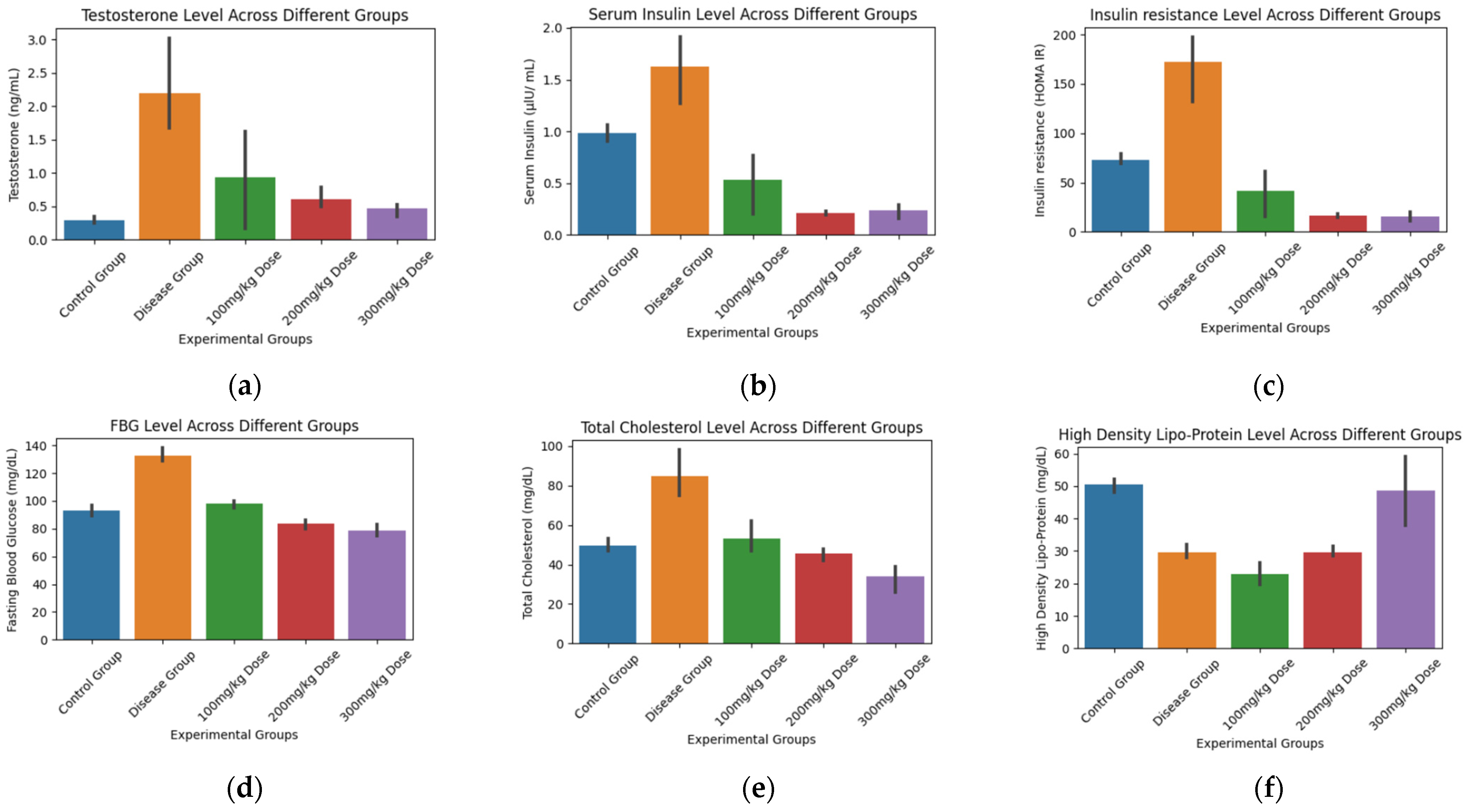
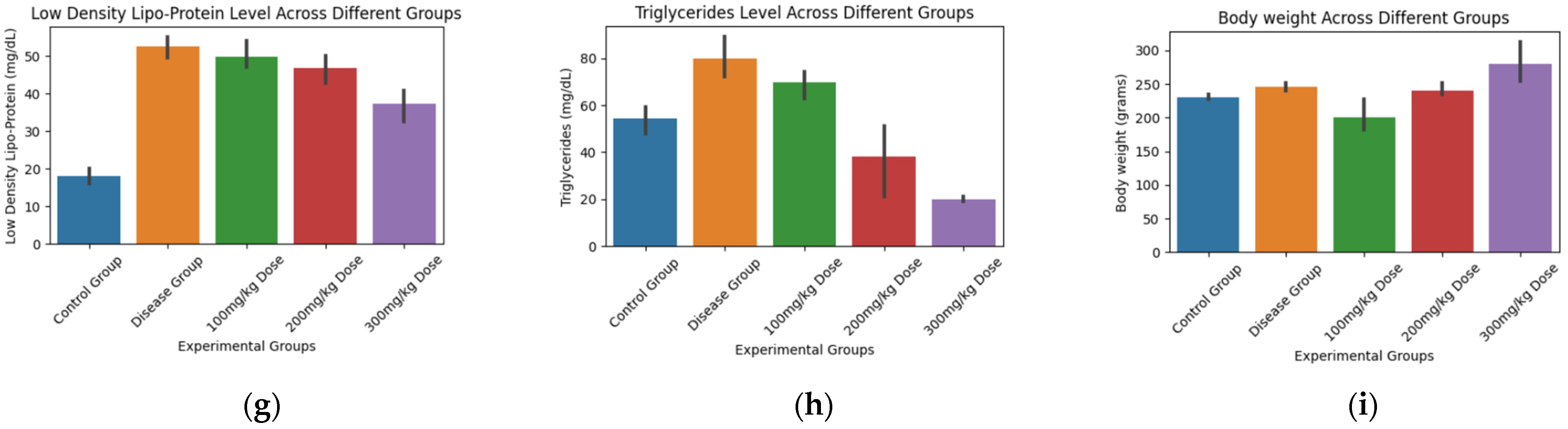
3.5. Biochemical and Statistical Analysis
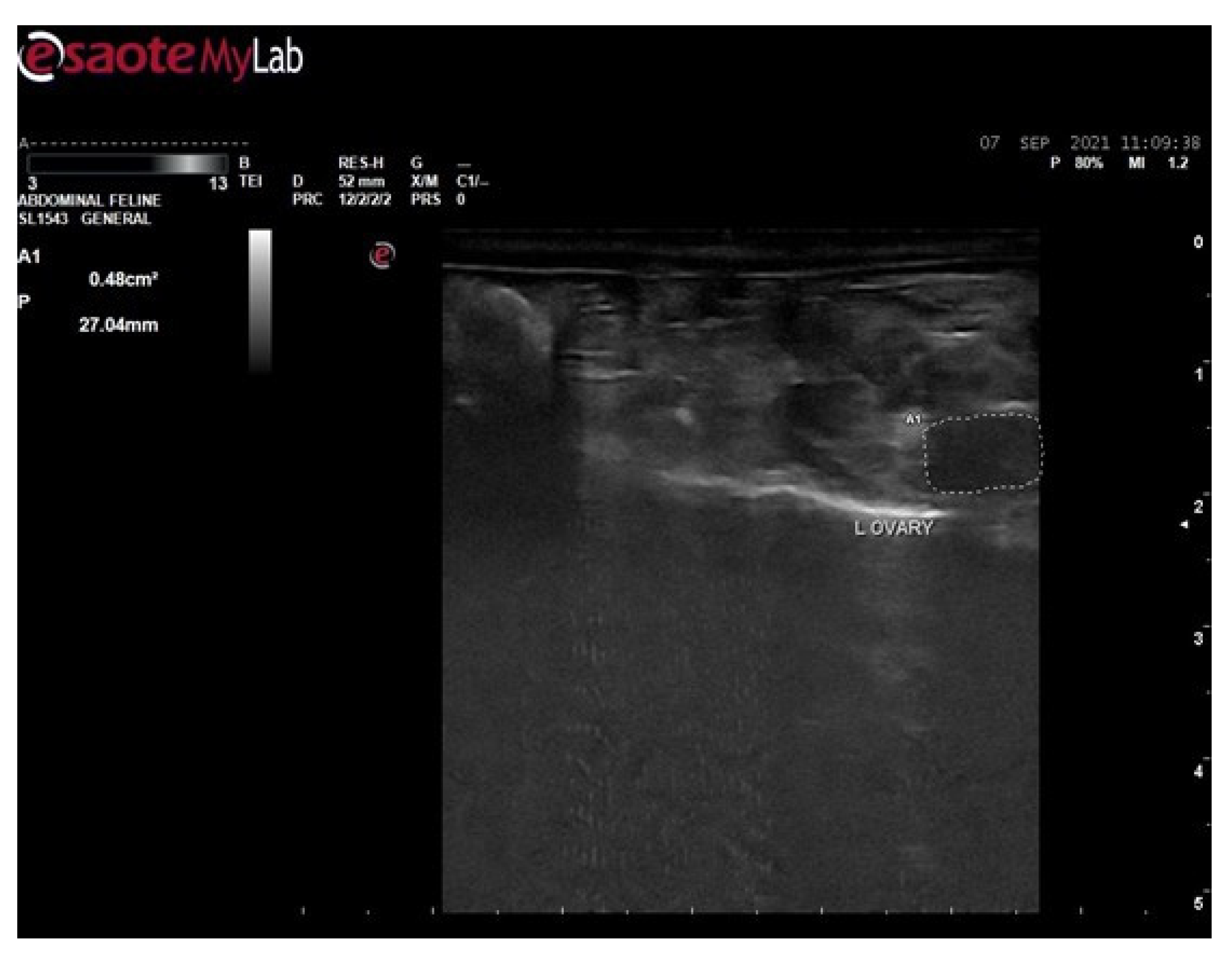
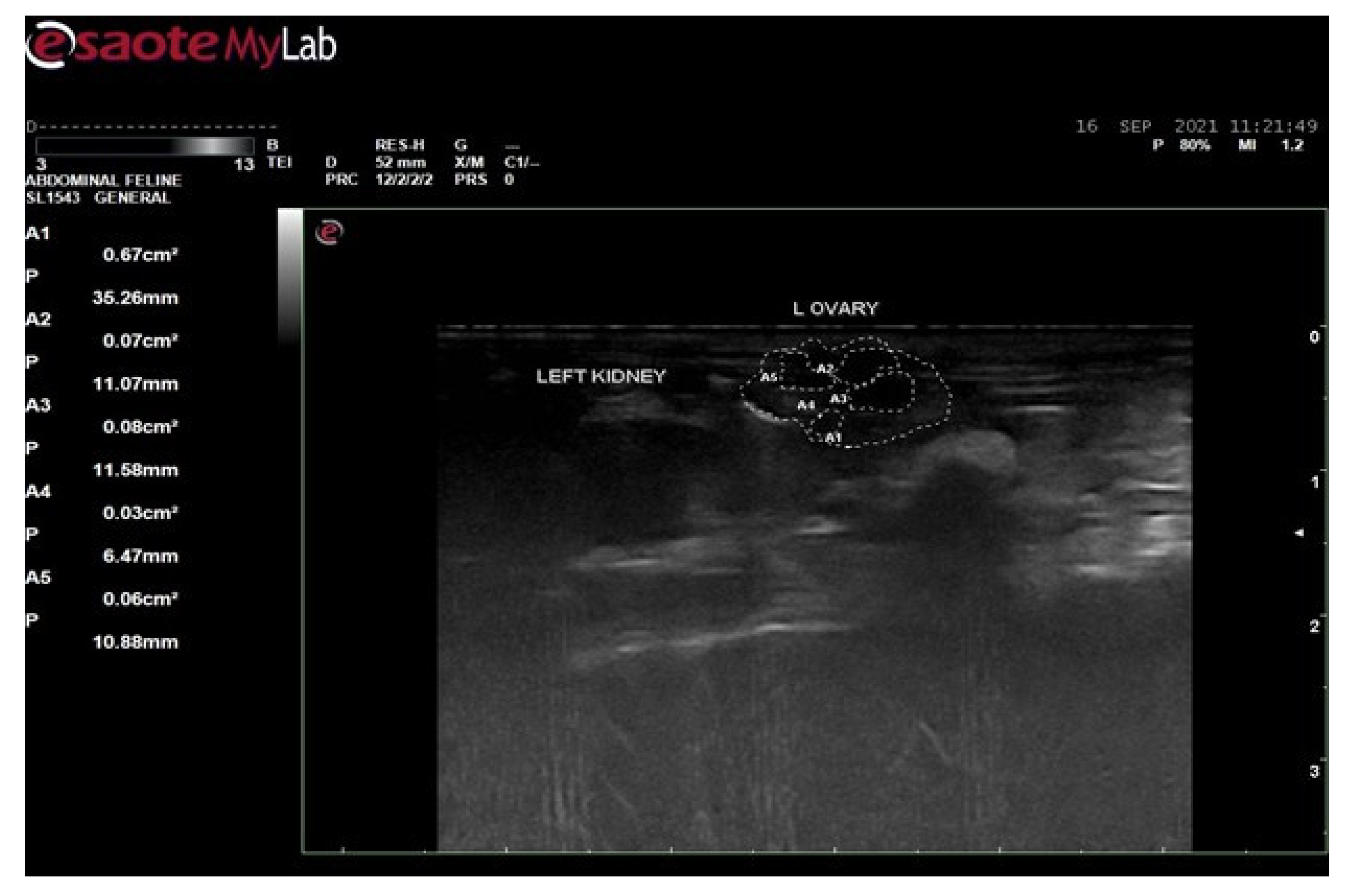
3.6. Ultrasound Examination for Diagnosis of PCOS
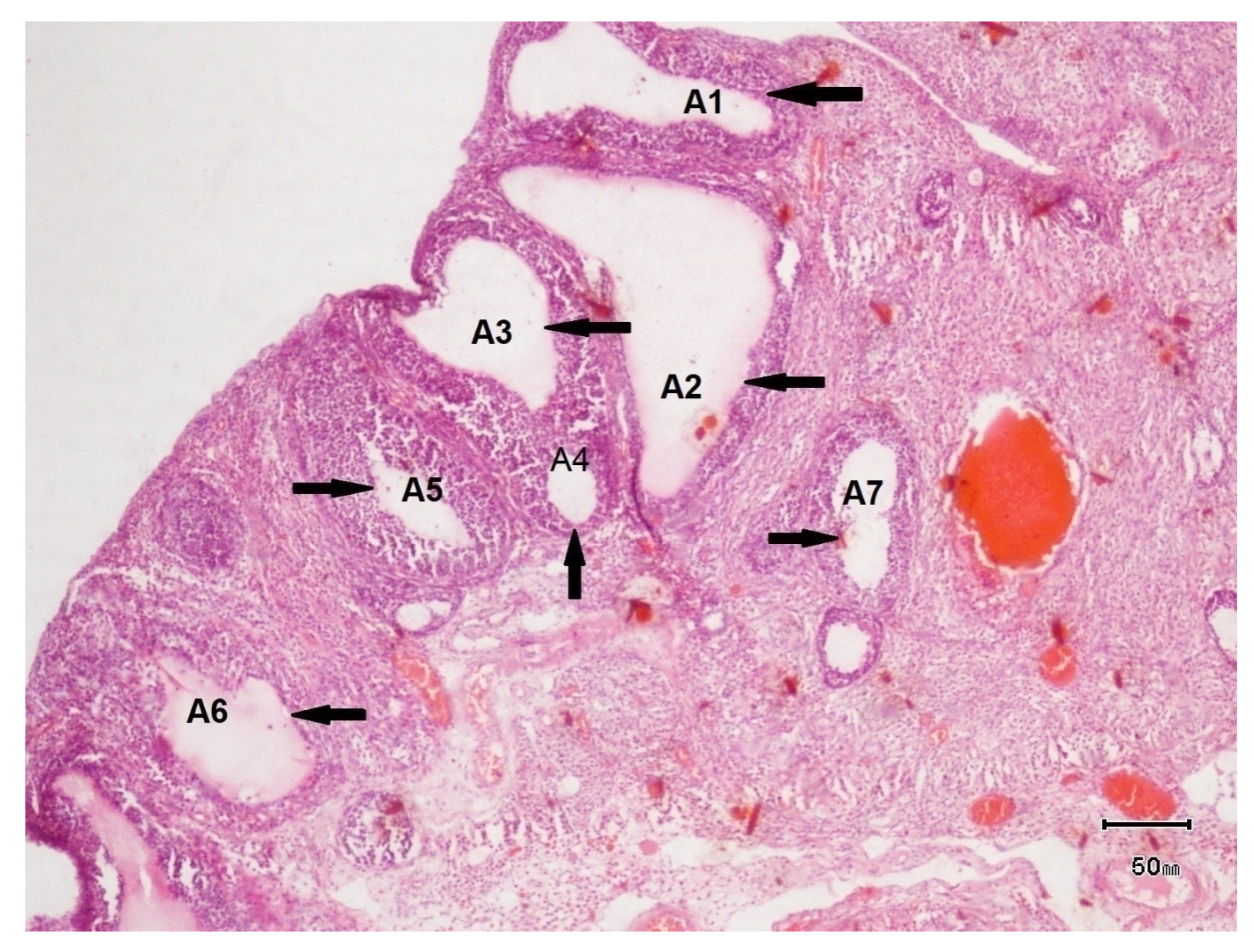
3.6.1. Ultrasound Examination of Female Rats of Control Group
3.6.2. Ultrasound Examination of Female Rats of Disease Group
3.7. Histopathological Evaluation
3.7.1. Histopathology of Ovary of Female Rats of Control Group
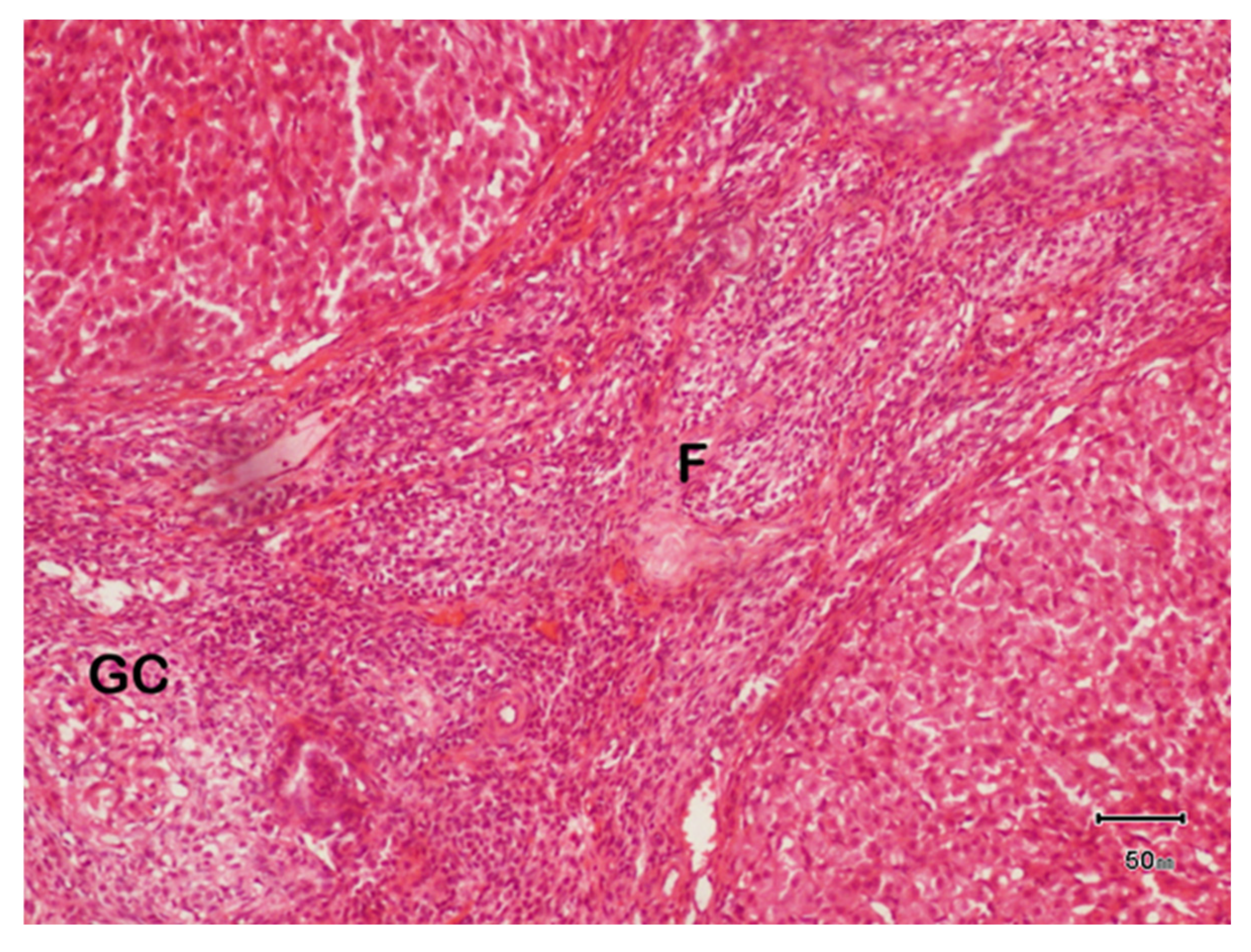
3.7.2. Histopathology of Ovary of Female Rat of Disease Group
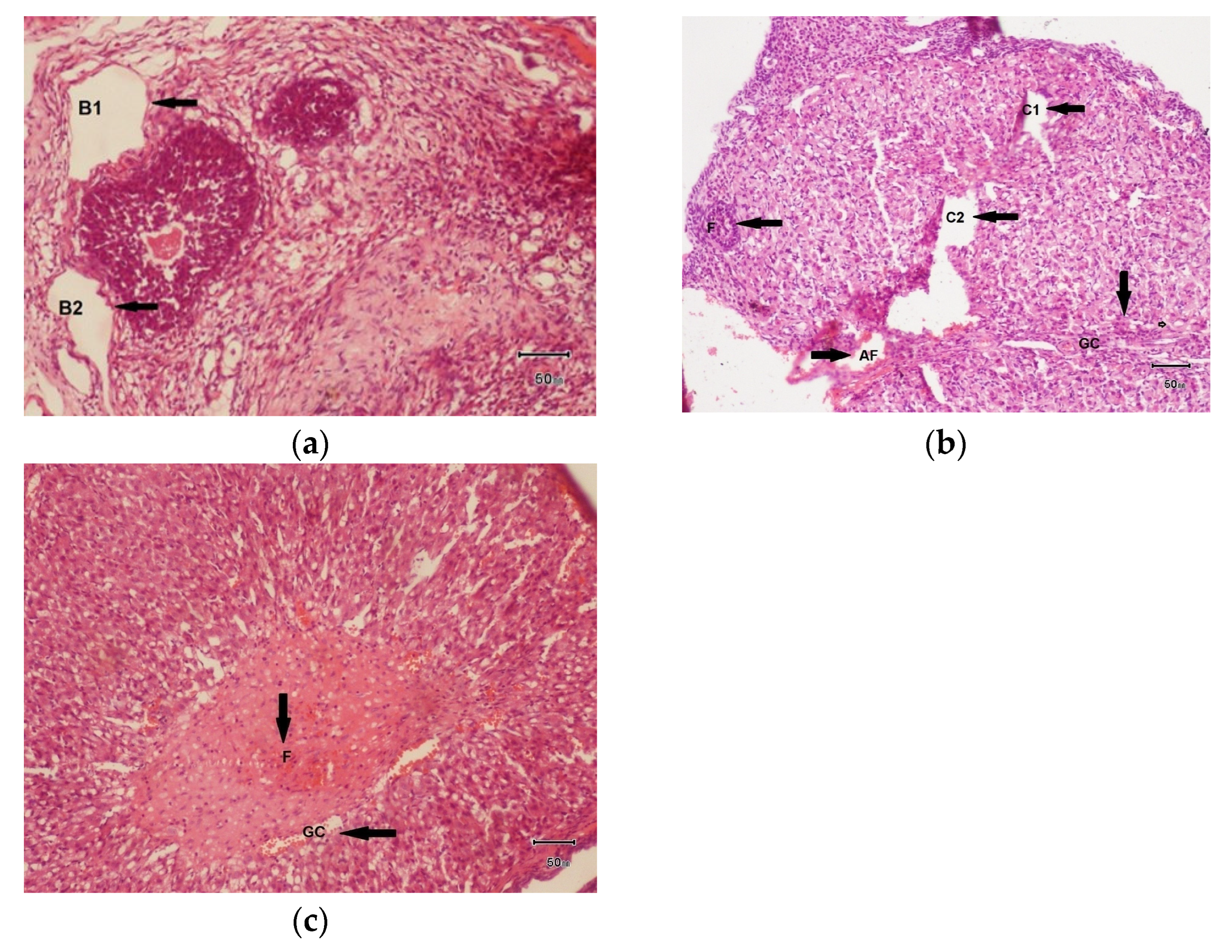
3.7.3. Histopathology of Ovaries of Female Rat Treated with F. cretica Plant Extract
- (a)
- 100 mg/kg dose of hydro-alcoholic plant extract of F. cretica
- (b)
- 200 mg/kg dose of hydro-alcoholic extract of Fagonia cretica
- (c)
- 300 mg/kg dose of hydro-alcoholic extract of Fagonia cretica
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leite, P.M.; Camargos, L.M.; Castilho, R.O. Recent progress in phytotherapy: A Brazilian perspective. Eur. J. Integr. Med. 2021, 41, 101270. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Ahmad, I. Herbal medicine: Current trends and future prospects. In New Look to Phytomedicine, 1st ed.; Khan, M.S.A., Ahmad, I., Chattopadhyay, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 3–13. [Google Scholar]
- Comhaire, F.; Decleer, W. Nutraceutical treatment of patients with polycystic ovary syndrome. Open J. Obstet. Gynecol. 2021, 11, 1005–1019. [Google Scholar] [CrossRef]
- Huseini, H.F.; Fakhrzadeh, H.; Larijani, B.; Shikh Samani, A.H. Review of anti-diabetic medicinal plants used in traditional medicine. J. Med. Plants 2006, 5, 1–8. [Google Scholar]
- Jazani, A.M.; Hamdi, K.; Tansaz, M.; Nazemiyeh, H.; Bazargani, H.S.; Fazljou, S.M.B.; Azgomi, R.N.D. Herbal medicine for oligomenorrhea and amenorrhea: A systematic review of ancient and conventional medicine. BioMed Res. Int. 2018, 2018, 3052768. [Google Scholar] [CrossRef] [PubMed]
- Vannuccini, S.; Bocchi, C.; Severi, F.M.; Challis, J.R.; Petraglia, F. Endocrinology of human parturition. Ann. Endocrinol. (Paris) 2016, 77, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Cheon, C.K. Evaluation and management of amenorrhea related to congenital sex hormonal disorders. Ann. Pediatr. Endocrinol. Metab. 2019, 24, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Ghare Naz, M.S.; Tehrani, F.R.; Majd, H.A.; Ahmadi, F.; Ozgoli, G.; Fakari, F.R.; Ghasemi, V. The prevalence of polycystic ovary syndrome in adolescents: A systematic review and meta-analysis. Int. J. Reprod. Biomed. 2019, 17, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Y.R.; Abdullah, M.A.; Gul, R.; Bhutta, H.R.; Imran, M.; Mazhar, S.B.; Tariq, N. Prevalence of anxiety and depression among women with polycystic ovarian syndrome: A cross-sectional study from a tertiary care hospital of Islamabad, Pakistan. Cureus 2024, 16, e52540. [Google Scholar] [CrossRef] [PubMed]
- Harada, M. Pathophysiology of polycystic ovary syndrome revisited: Current understanding and perspectives regarding future research. Reprod. Med. Biol. 2022, 21, e12487. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.; Dawalbhakta, M.; Nampoothiri, L. GnRH dysregulation in polycystic ovarian syndrome (PCOS) is a manifestation of an altered neurotransmitter profile. Reprod. Biol. Endocrinol. 2018, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Zafar, U.; Memon, Z.; Moin, K.; Agha, S.; Hassan, J.A.; Zehra, D. Prevalence of PCOS with associated symptoms and complications at tertiary care hospital of Karachi. J. Adv. Med. Med. Res. 2019, 30, 1–9. [Google Scholar] [CrossRef]
- Miazgowski, T.; Martopullo, I.; Widecka, J.; Miazgowski, B.; Brodowska, A. National and regional trends in the prevalence of polycystic ovary syndrome since 1990 within Europe: The modeled estimates from the Global Burden of Disease Study 2016. Arch. Med. Sci. 2019, 17, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Kiani, B.H.; Ikram, F.; Fatima, H.; Alhodaib, A.; Haq, I.U.; Ur-Rehman, T.; Naz, I. Comparative evaluation of biomedical and phytochemical applications of zinc nanoparticles by using Fagonia cretica extracts. Sci. Rep. 2022, 12, 10024. [Google Scholar] [CrossRef] [PubMed]
- Puri, D.; Bhandari, A. Fagonia: A potential medicinal desert plant. J. Nepal Pharm. Assoc. 2015, 27, 28–33. [Google Scholar] [CrossRef]
- Hussain, M.; Zia, A.; Mirza, B. Cytotoxic and antitumor potential of Fagonia cretica L. Turk. J. Biol. 2007, 31, 143–150. [Google Scholar]
- Qureshi, H.A.H.; Asif, S.; Hayat, K. Chemical composition and medicinal significance of Fagonia cretica: A review. Nat. Prod. Res. 2015, 30, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.L.; Kumbi, Y.; Wu, W.; Lee, H.-S.; Reed, R.L.; Stevens, J.F. Phytochemical characterization and bioactivity toward breast cancer cells of unhydrolyzed and acid-hydrolyzed extracts of Fagonia indica. Nat. Prod. Commun. 2022, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abirami, V.; Khosa, R.L.; Dhar, S.K.; Sahai, M. Investigation on Fagonia cretica—Its effect on hormonal profile and immunomodulation in rats. Anc. Sci. Life 1996, 15, 259–263. [Google Scholar] [PubMed]
- Rashid, U.; Khan, M.R.; Jan, S.; Bokhari, J.; Shah, N.A. Assessment of phytochemicals, antimicrobial and cytotoxic activities of extract and fractions from Fagonia olivieri (Zygophyllaceae). BMC Complement. Altern. Med. 2013, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, R.H.K.; Roberts, J.; Docherty, P.D.; Lunt, H.; Campbell, R.E.; Möller, K. A review of the hormones involved in the endocrine dysfunctions of polycystic ovary syndrome and their interactions. Front. Endocrinol. 2022, 13, 1017468. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Yuan, X.; Ye, R.; Zhou, H.; Lin, J.; Zhang, C.; Zhang, H.; Wei, G.; Dong, M.; Huang, Y.; et al. Brown adipose tissue activation by rutin ameliorates polycystic ovary syndrome in rat. J. Nutr. Biochem. 2017, 47, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Requena, A.; Herrero, J.; Landeras, J.; Navarro, E.; Neyro, J.L.; Salvador, C.; Tur, R.; Callejo, J.; Checa, M.A.; Farré, M.; et al. Use of letrozole in assisted reproduction: A systematic review and meta-analysis. Hum. Reprod. Update 2008, 14, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.; Mir, S.A.; Kareem, O.; Ali, T.; Ara, R.; Malik, A.; Amin, F.; Bader, G.N. Polycystic ovarian syndrome—Current pharmacotherapy and clinical implications. Taiwan J. Obstet. Gynecol. 2022, 61, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, P.; Ahmed, D.; Asghar, M.N. A comparative in vitro antioxidant potential profile of extracts from different parts of Fagonia cretica. Asian Pac. J. Trop. Med. 2014, 7, S473–S480. [Google Scholar] [CrossRef] [PubMed]
- Mihanfar, A.; Nouri, M.; Roshangar, L.; Khadem-Ansari, M.H. Therapeutic potential of quercetin in an animal model of PCOS: Possible involvement of AMPK/SIRT-1 axis. Eur. J. Pharmacol. 2021, 900, 174062. [Google Scholar] [CrossRef] [PubMed]
- Ndefo, U.A.; Eaton, A.; Green, M.R. Polycystic ovary syndrome: A review of treatment options with a focus on pharmacological approaches. P T 2013, 38, 336–355. [Google Scholar] [PubMed]
- Sadeghi, H.M.; Adeli, I.; Calina, D.; Docea, A.O.; Mousavi, T.; Daniali, M.; Nikfar, S.; Tsatsakis, A.; Abdollahi, M. Polycystic ovary syndrome: A comprehensive review of pathogenesis, management, and drug repurposing. Int. J. Mol. Sci. 2022, 23, 583. [Google Scholar] [CrossRef] [PubMed]
- Bayona, A.; Martínez-Vaello, V.; Zamora, J.; Nattero-Chávez, L.; Luque-Ramírez, M.; Escobar-Morreale, H.F. Prevalence of PCOS and related hyperandrogenic traits in premenopausal women with type 1 diabetes: A systematic review and meta-analysis. Hum. Reprod. Update 2022, 28, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Ahmad-Rukhsana, M.A.; Hafeez, S.; Kamran, S.; Shoaib, R.M. Antidiabetic and renoprotective effect of Fagonia cretica L. methanolic extract and Citrus paradisi Macfad. juice in alloxan induced diabetic rabbits. J. Pharm. Pharmacogn. Res. 2017, 5, 161–169. [Google Scholar]
- El-Tantawy, W.H.; Temraz, A. Natural products for controlling hyperlipidemia: Review. Arch. Physiol. Biochem. 2019, 125, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Jarald, E.E.; Joshi, S.B.; Jain, D.C.; Edwin, S. Biochemical evaluation of the hypoglycemic effects of extract and fraction of Cassia fistula Linn. in alloxan-induced diabetic rats. Indian J. Pharm. Sci. 2013, 75, 427–434. [Google Scholar] [CrossRef] [PubMed]
| Sr. No | Constituents Expected | Test Name |
|---|---|---|
| 1 | Carbohydrate | Molisch Test |
| 2 | Flavonoid | Lead Acetate Test |
| 3 | Glycosides | Legal’s Test |
| 4 | Phenolic Compounds | Iodine Test |
| 5 | Phytosterols | Salkowski’s Test |
| 6 | Proteins and Amino Acids | Ninhydrin Test |
| 7 | Reducing Sugar | Fehling’s Test |
| 8 | Saponins | Foam Test |
| 9 | Tannins | Braymer’s Test |
| 10 | Terpenoids | Salkowski’s Test |
| Sr. No. | Test Name | Outcome |
|---|---|---|
| 1. | Molisch test | Positive |
| 2. | Lead acetate test | Positive |
| 3. | Legal’s test | Positive |
| 4. | Iodine test | Positive |
| 5. | Salkowski’s test | Negative |
| 6. | Ninhydrin test | Positive |
| 7. | Fehling’s test | Positive |
| 8. | Foam test | Positive |
| 9. | Braymer’s test | Positive |
| 10. | Test for Terpenoids | Negative |
| Sr. No. | Biochemical Tests | F-Value | p-Value | Control vs. Disease | Control vs. 100 mg/kg | Control vs. 200 mg/kg | Control vs. 300 mg/kg |
|---|---|---|---|---|---|---|---|
| 1. | Testosterone | 5.09 | 0.01 | −3.79 | −0.84 | −2.61 | −2.28 |
| 2. | SI | 26.31 | 2.73 × 10−5 | −2.73 | 2.12 | 14.04 | 10.34 |
| 3. | HOMA IR | 34.39 | 8.08 × 10−6 | −3.95 | 1.82 | 14.37 | 11.72 |
| 4. | FBG | 89.58 | 8.65 × 10−8 | −8.75 | −1.47 | 2.57 | 3.59 |
| 5. | CHOL | 20.34 | 8.56 × 10−5 | −4.10 | −0.62 | 1.33 | 2.90 |
| 6. | TGR | 22.43 | 5.57 × 10−5 | −3.46 | −2.65 | 1.36 | 8.40 |
| 7. | LDLP | 51.66 | 1.20 × 10−6 | −14.06 | 10.62 | −9.81 | −5.85 |
| 8. | HDLP | 17.43 | 0.0001 | 9.775 | 9.62 | 11.06 | 0.24 |
| 9. | BW | 7.48 | 0.004 | −3.13 | 1.73 | −1.36 | −2.30 |
| Sr. No. | Biochemical Tests | Group 1 | Group 2 | Mean Diff | p-adj | Reject |
|---|---|---|---|---|---|---|
| 1. | Testosterone | Control | Disease | 1.91 | 0.017 | True |
| Disease | 100 mg/kg | 1.26 | 0.13 | False | ||
| Disease | 200 mg/kg | 1.59 | 0.04 | True | ||
| Disease | 300 mg/kg | 1.73 | 0.03 | True | ||
| 2. | Serum Insulin | Control | Disease | 0.64 | 0.01 | True |
| Disease | 100 mg/kg | 1.09 | 0.0004 | True | ||
| Disease | 200 mg/kg | 1.41 | 0.0 | True | ||
| Disease | 300 mg/kg | 1.39 | 0.0001 | True | ||
| 3. | HOMA-IR Index | Control | Disease | 99.45 | 0.0006 | True |
| Disease | 100 mg/kg | 130.50 | 0.0001 | True | ||
| Disease | 200 mg/kg | 155.49 | 0.0 | True | ||
| Disease | 300 mg/kg | 156.50 | 0.0 | True | ||
| 4. | FBG | Control | Disease | 39.33 | 0.0 | True |
| Disease | 100 mg/kg | 34.33 | 0.0 | True | ||
| Disease | 200 mg/kg | 48.66 | 0.0 | True | ||
| Disease | 300 mg/kg | 54.0 | 0.0 | True | ||
| 5. | CHOL (Cholesterol) | Control | Disease | 35.33 | 0.001 | True |
| Disease | 100 mg/kg | 31.66 | 0.0025 | True | ||
| Disease | 200 mg/kg | 39.43 | 0.0004 | True | ||
| Disease | 300 mg/kg | 51.00 | 0.0001 | True | ||
| 6. | TGR (Triglycerides) | Control | Disease | 25.33 | 0.0338 | True |
| Disease | 100 mg/kg | 10.0 | 0.6418 | False | ||
| Disease | 200 mg/kg | 41.33 | 0.0013 | True | ||
| Disease | 300 mg/kg | 59.66 | 0.0001 | True | ||
| 7. | LDLP (Low-Density Lipoprotein) | Control | Disease | 34.7 | 0.0 | True |
| Disease | 100 mg/kg | 2.93 | 0.8216 | False | ||
| Disease | 200 mg/kg | 5.9 | 0.2779 | False | ||
| Disease | 300 mg/kg | 15.26 | 0.0018 | True | ||
| 8. | HDLP (High-Density Lipoprotein) | Control | Disease | −20.83 | 0.0041 | True |
| Disease | 100 mg/kg | 6.66 | 0.5394 | False | ||
| Disease | 200 mg/kg | 0.03 | 1.0 | False | ||
| Disease | 300 mg/kg | −19.0 | 0.0078 | True | ||
| 9. | Body Weight | Control | Disease | 15.0 | 0.839 | False |
| Disease | 100 mg/kg | 45.0 | 0.0702 | False | ||
| Disease | 200 mg/kg | 5.0 | 0.9966 | False | ||
| Disease | 300 mg/kg | −33.66 | 0.0 | True |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qasim, A.; Calvo, H.; Escobar, J.J.M.; Akhtar, Z.-u.-d. Integrating Computational Analysis of In Vivo Investigation of Modulatory Effect of Fagonia cretica Plant Extract on Letrozole-Induced Polycystic Ovary Syndrome in Female Rats. Biology 2025, 14, 903. https://doi.org/10.3390/biology14070903
Qasim A, Calvo H, Escobar JJM, Akhtar Z-u-d. Integrating Computational Analysis of In Vivo Investigation of Modulatory Effect of Fagonia cretica Plant Extract on Letrozole-Induced Polycystic Ovary Syndrome in Female Rats. Biology. 2025; 14(7):903. https://doi.org/10.3390/biology14070903
Chicago/Turabian StyleQasim, Ayesha, Hiram Calvo, Jesús Jaime Moreno Escobar, and Zia-ud-din Akhtar. 2025. "Integrating Computational Analysis of In Vivo Investigation of Modulatory Effect of Fagonia cretica Plant Extract on Letrozole-Induced Polycystic Ovary Syndrome in Female Rats" Biology 14, no. 7: 903. https://doi.org/10.3390/biology14070903
APA StyleQasim, A., Calvo, H., Escobar, J. J. M., & Akhtar, Z.-u.-d. (2025). Integrating Computational Analysis of In Vivo Investigation of Modulatory Effect of Fagonia cretica Plant Extract on Letrozole-Induced Polycystic Ovary Syndrome in Female Rats. Biology, 14(7), 903. https://doi.org/10.3390/biology14070903







