Effects of Temperature Increase on Microbiome of Carnivorous Plant Utricularia vulgaris L. in Peat Bog Ecosystems
Simple Summary
Abstract
1. Introduction
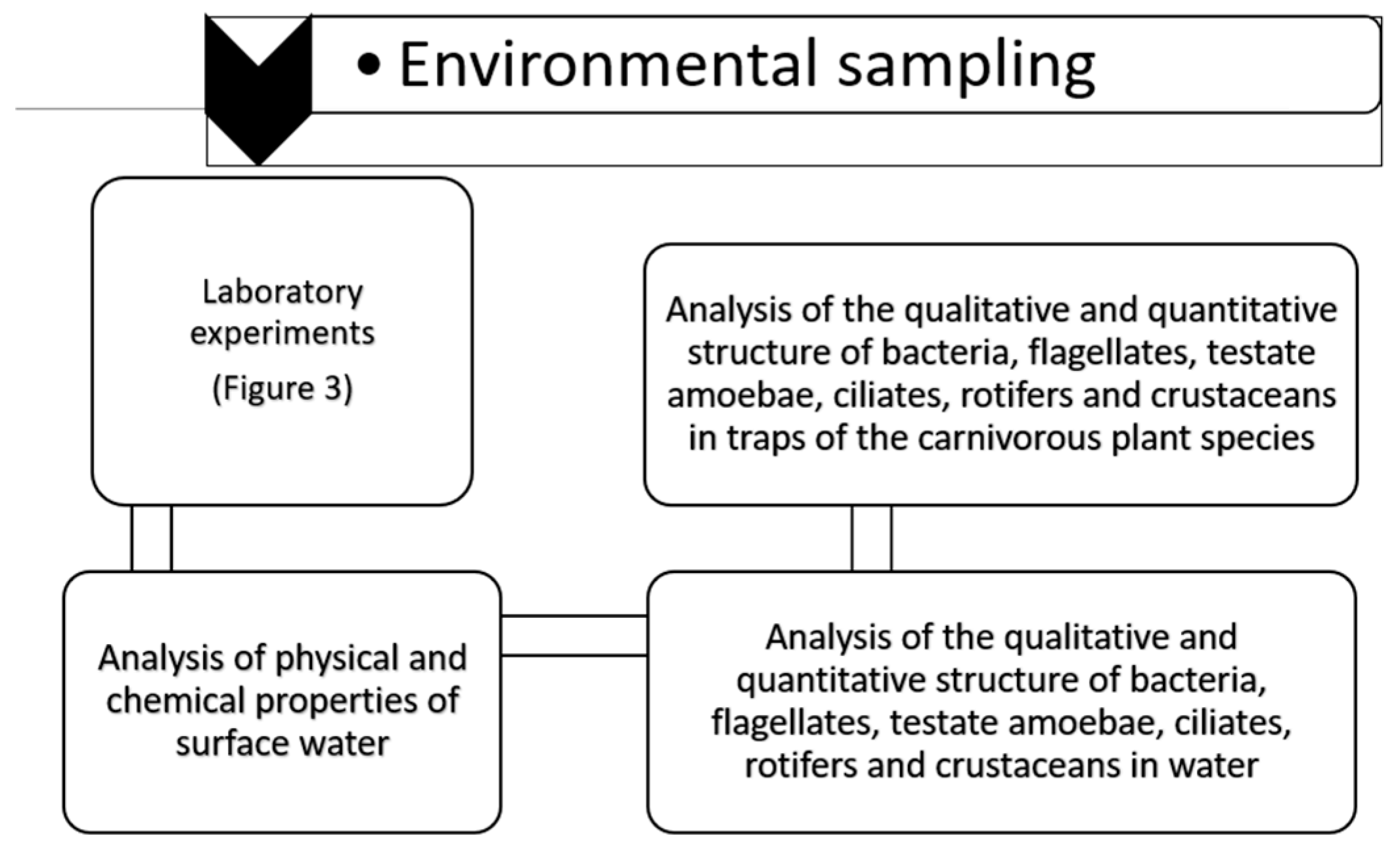
2. Materials and Methods
2.1. Experiment and Laboratory Analyses
2.2. Statistical Analysis
3. Results
3.1. Environmental Variables
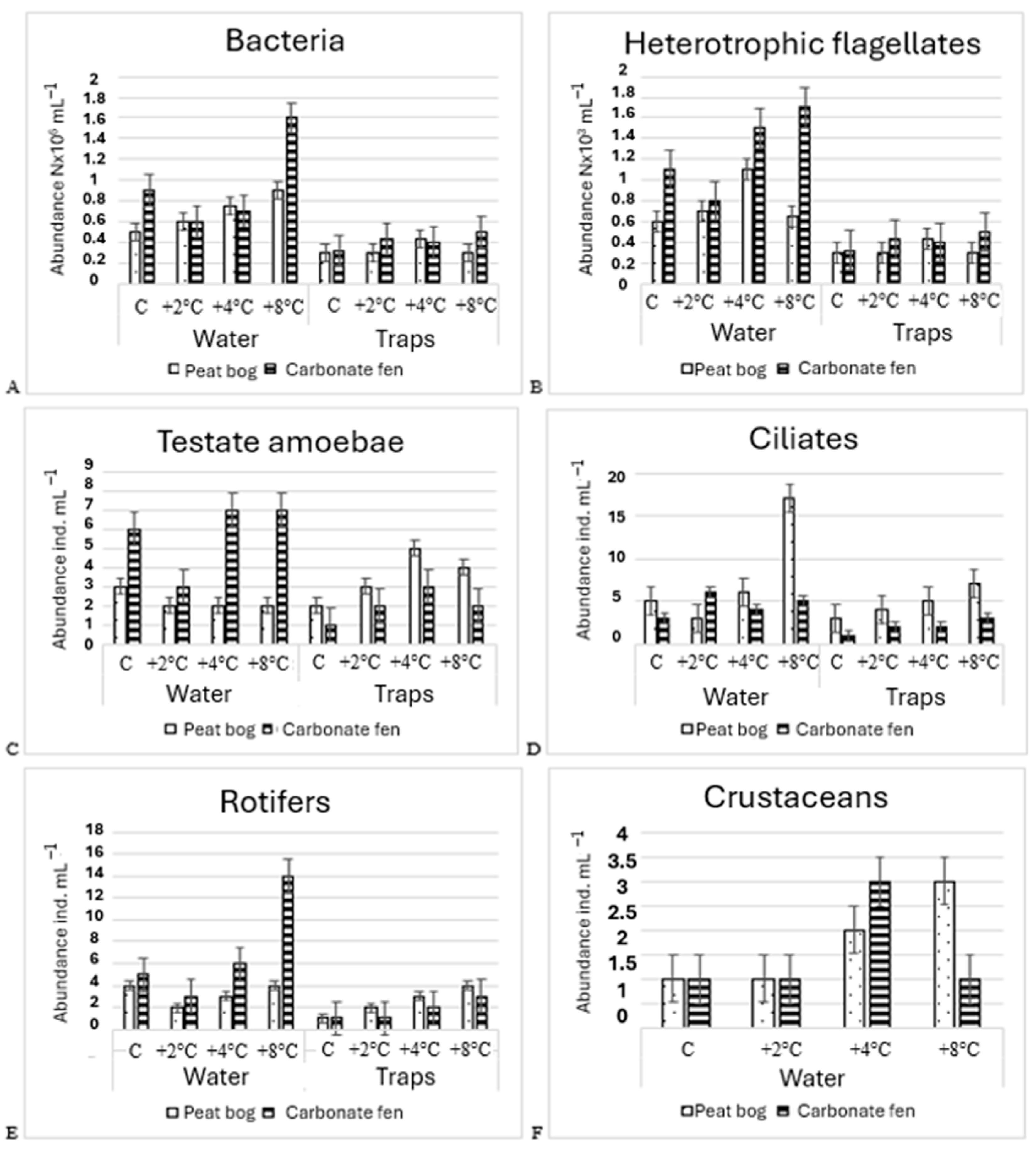
3.2. Species Diversity and Abundance of Microorganism and Small Metazoa
3.2.1. Abundance of Bacteria
3.2.2. Abundance of Heterotrophic Falgellates
3.2.3. Species Diversity and Abundance of Testate Amoebae
3.2.4. Species Diversity and Abundance of Ciliates
3.2.5. Species Diversity and Abundance of Small Metazoa
3.3. Biomass of Microbial Communities
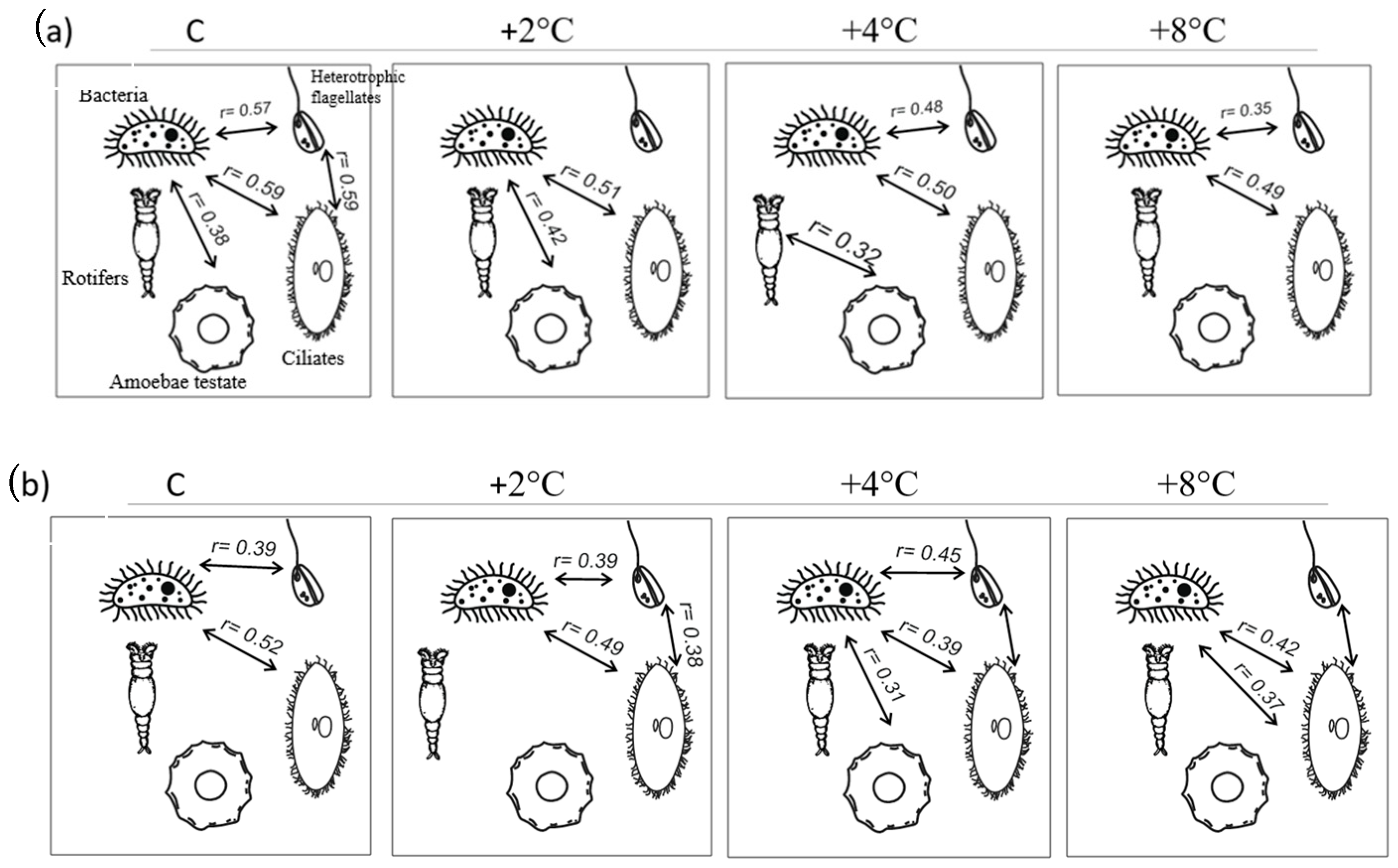
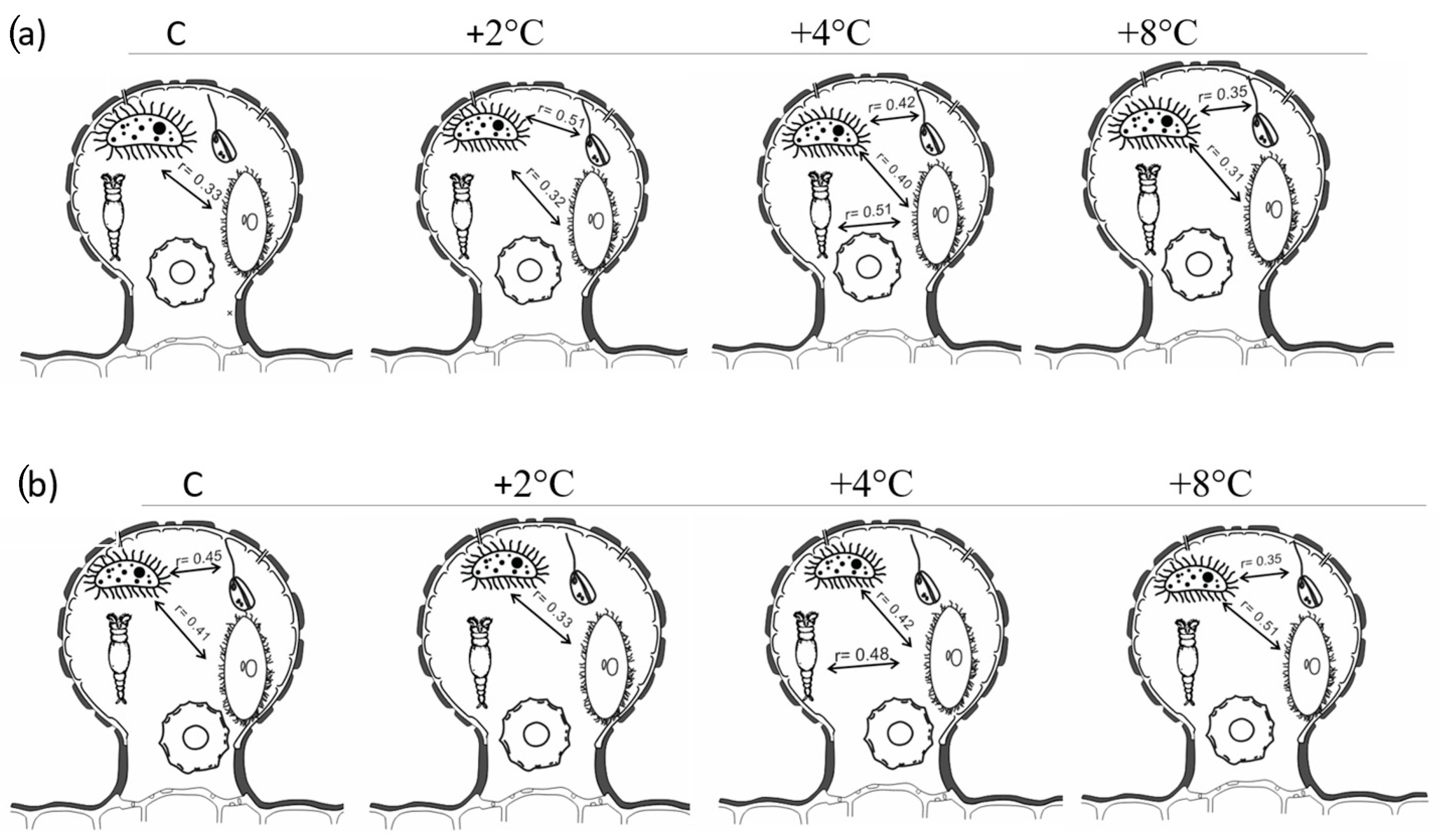
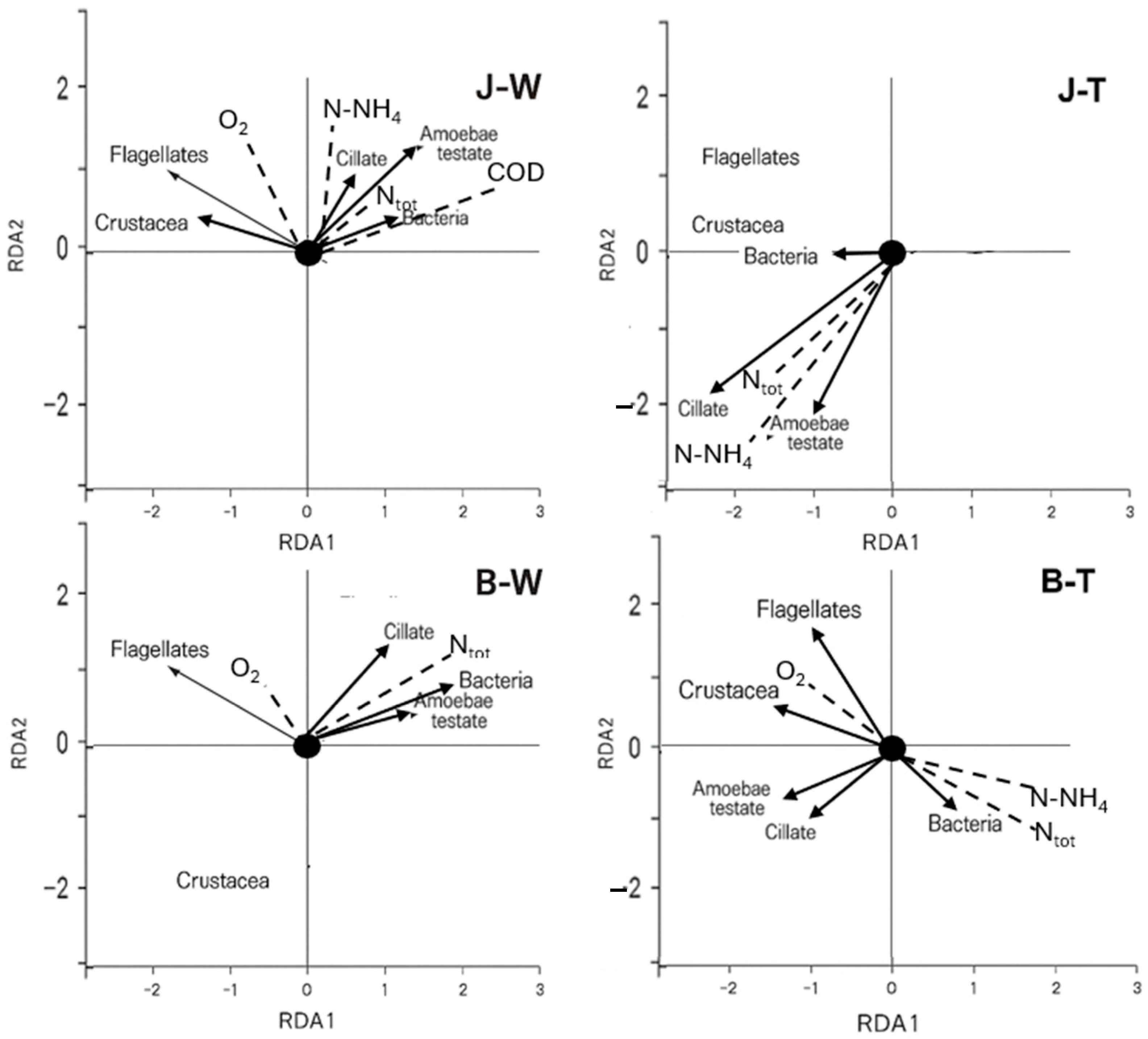
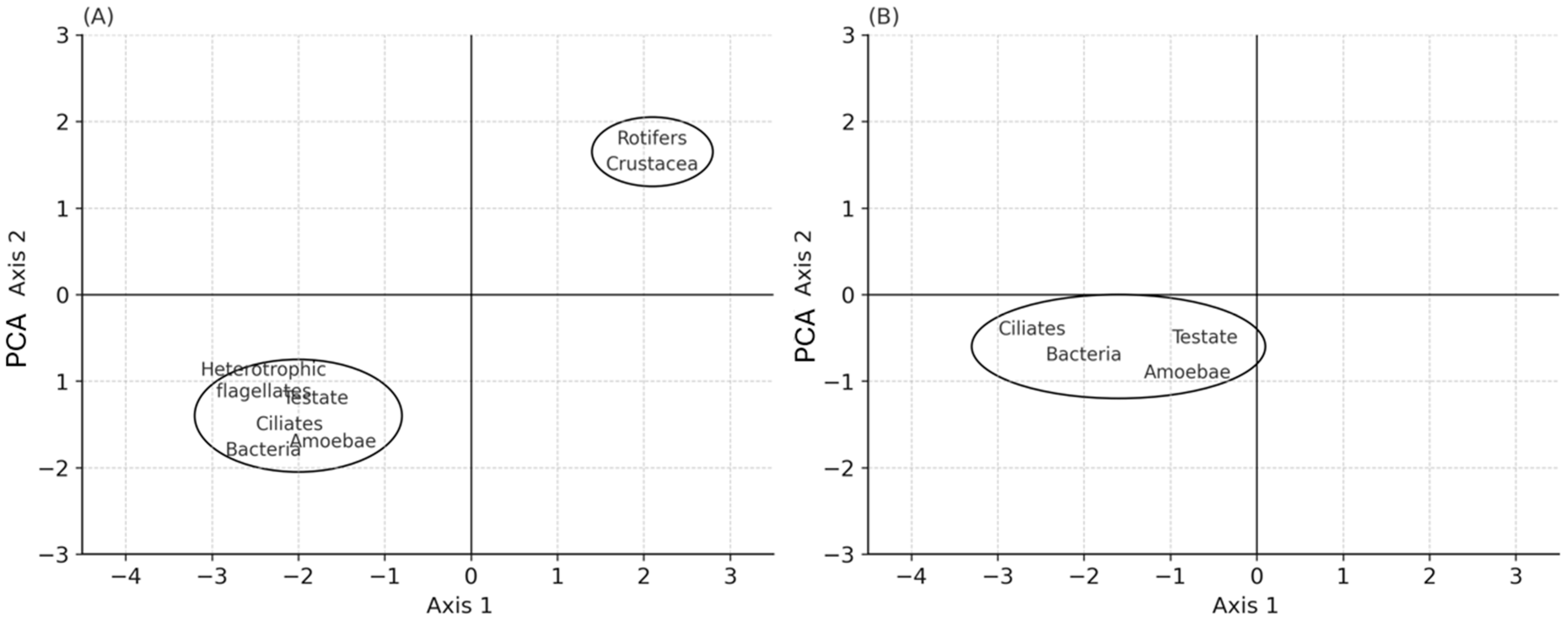
3.4. Size Structure, Correlations, and Ordination Analysis
Size Structure and Correlations Between Food Web Components
3.5. Ordination Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cross, A. Red List Assessment—Aldrovanda vesiculosa (Common Aldrovanda, Waterwheel); IUCN Red List: Gland, Switzerland, 2020. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.; Miller, H.L. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- IPCC. Climate Change 2021: The Physical Science Basis. Summary for Policymakers; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2021. [Google Scholar]
- Lee, H. IPCC Sixth Assessment Report—Synthesis Report; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2022. [Google Scholar]
- Bjorkman, A.D.; Myers-Smith, I.H.; Elmendorf, S.C.; Normand, S.; Rüger, N.; Beck, P.S.A.; Blach-Overgaard, A.; Blok, D.; Cornelissen, J.H.C.; Forbes, B.C.; et al. Plant functional trait change across a warming tundra biome. Nature 2018, 562, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, Y.; Boulanger, Y.; Girardin, M.; Bernier, P.Y.; Gauthier, S.; Beaudoin, A.; Guindon, L. Changes in mean forest age in Canada’s forests could limit future increases in area burned but compromise potential harvestable conifer volumes. Can. J. For. Res. 2017, 47, 755–764. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Mastepanov, M.; Sigsgaard, C.; Tagesson, T.; Ström, L. Large tundra methane burst during onset of freezing. Nature 2008, 456, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Dorrepaal, E.; Aerts, R.; Cornelissen, J.H.C.; van Logtestijn, R.S.P.; Callaghan, T.V. Carbon respiration from subsurface peat accelerated by climate warming in the subarctic. Nature 2009, 460, 616–619. [Google Scholar] [CrossRef]
- Basińska, A.M.; Pawlik-Skowrońska, B.; Mieczan, T. Experimental warming and precipitation reduction affect the biomass of microbial communities in a Sphagnum peatland. Ecol. Indic. 2020, 112, 106059. [Google Scholar] [CrossRef]
- FAO. Peatlands Mapping and Monitoring; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Loisel, J.; Gallego-Sala, A.V.; Yu, Z. Expert assessment of future vulnerability of the global peatland carbon sink. Nat. Clim. Change 2021, 11, 70–77. [Google Scholar] [CrossRef]
- Leifeld, J.; Wüst-Galley, C.; Page, S. Intact and managed peatland soils as a source and sink of GHGs from 1850 to 2100. Nat. Clim. Chang. 2019, 9, 945–947. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Abbott, B.W.; Jones, M.C.; Anthony, K.W.; Olefeldt, D.; Schuur, E.A.G.; Grosse, G.; Kuhry, P.; Hugelius, G.; Lawrence, D.M.; et al. Carbon release through abrupt permafrost thaw. Nat. Geosci. 2020, 13, 138–143. [Google Scholar] [CrossRef]
- Cross, A.T.; Krueger, T.A.; Gonella, P.M.; Robinson, A.S.; Fleischmann, A.S. Conservation of carnivorous plants in the age of extinction. Glob. Ecol. Conserv. 2020, 24, e01272. [Google Scholar] [CrossRef]
- Wardle, D. Aboveground-Belowground Linkages, Ecosystem Development, and Ecosystem Restoration; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Leifeld, J.; Menichetti, L. The underappreciated potential of peatlands in global climate change mitigation strategies. Nat. Commun. 2018, 9, 1071. [Google Scholar] [CrossRef] [PubMed]
- Tarnocai, C. The effect of climate change on carbon in Canadian peatlands. Glob. Planet Chang. 2006, 53, 4. [Google Scholar] [CrossRef]
- Seward, J.; Bräuer, S.; Beckett, P.; Roy-Léveillée, P.; Emilson, E.; Watmough, S.; Basiliko, N. Recovery of Smelter-Impacted Peat and Sphagnum Moss: A Microbial Perspective. Microb. Ecol. 2023, 86, 2894–2903. [Google Scholar] [CrossRef] [PubMed]
- Pociecha, A.; Buczek, K.; Margielewski, W.; Kupryjanowicz, M.; Fiłoc, M.; Korzeń, K.; Krąpiec, M.; Sala, D.; Obidowicz, A.; Michczyńska, D.J.; et al. Appearance of the rotifer community as a potential indicator of stable paleohydrological conditions in peatlands since the Late Glacial: A case study of three wetlands in Poland. Hydrobiologia 2024, 851, 2965–2981. [Google Scholar] [CrossRef]
- Fitzpatrick, M.C.; Ellison, A.M. Estimating the exposure of carnivorous plants to rapid climatic change. In Carnivorous Plants: Physiology, Ecology, and Evolution; Ellison, A.M., Adamec, L., Eds.; Oxford University Press: Oxford, UK, 2018; pp. 389–407. [Google Scholar] [CrossRef]
- Cross, A.T.; Skates, L.M.; Adamec, L.; Hammond, C.M.; Sheridan, P.M.; Dixon, K.W. Population ecology of the endangered aquatic carnivorous macrophyte Aldrovanda vesiculosa at a naturalised site in North America. Freshw. Biol. 2015, 60, 1772–1783. [Google Scholar] [CrossRef]
- Cross, A.T.; Adamec, L.; Turner, S.R.; Dixon, K.W.; Merritt, D.J. Seed reproductive biology of the rare aquatic carnivorous plant Aldrovanda vesiculosa (Droseraceae). Bot. J. Linn. Soc. 2016, 180, 515–529. [Google Scholar] [CrossRef]
- Adamec, L. Biological flora of Central Europe: Aldrovanda vesiculosa L. Perspect. Plant Ecol. Evol. Syst. 2018, 35, 8–21. [Google Scholar] [CrossRef]
- Jennings, D.E.; Rohr, J.R. A review of the conservation threats to carnivorous plants. Biol. Cons. 2011, 144, 1356–1363. [Google Scholar] [CrossRef]
- Robroek, B.J.; Albrecht, R.J.; Hamard, S.; Pulgarin, A.; Bragazza, L.; Buttler, A.; Jassey, V.E. Peatland vascular plant functional types affect dissolved organic matter chemistry. Plant Soil 2016, 407, 135–143. [Google Scholar] [CrossRef]
- Laine, A.M.; Lindholm, T.; Nilsson, M.; Kuznetsov, O.; Jassey, V.E.; Tuittila, E.-S. Functional diversity and trait composition of vascular plant and Sphagnum moss communities during peatland succession across land uplift regions. J. Ecol. 2021, 109, 1774–1789. [Google Scholar] [CrossRef]
- Bengtsson, F.; Rydin, H.; Baltzer, J.L.; Bragazza, L.; Bu, Z.J.; Caporn, S.J.M. Environmental drivers of Sphagnum growth in peatlands across the Holarctic region. J. Ecol. 2021, 109, 417–431. [Google Scholar] [CrossRef]
- Płachno, B.J.; Adamec, L.; Kaińska, I. Relationship between trap anatomy and function in Australian carnivorous bladderworts (Utricularia) of the subgenus Polypompholyx. Aquat. Bot. 2015, 120, 290–296. [Google Scholar] [CrossRef]
- Cao, H.X.; Yin, J.; Wang, C.; Li, W.; Jin, Y.; Li, J.; Wang, Y. Metatranscriptome analysis reveals host-microbiome interactions in traps of carnivorous Genlisea species. Front. Microbiol. 2015, 6, 526. [Google Scholar] [CrossRef] [PubMed]
- Peroutka, M.; Adamec, L.; Sirová, D.; Vrba, J. Utricularia: A vegetarian carnivorous plant? Algae as prey of bladderwort in oligotrophic bogs. Plant Ecol. 2008, 199, 153–162. [Google Scholar] [CrossRef]
- Adamec, L. Functional characteristics of traps of aquatic carnivorous Utricularia species. Aquat. Bot. 2011, 95, 226–233. [Google Scholar] [CrossRef]
- Płachno, B.J.; Łukaszek, M.; Wołowski, K.; Adamec, L.; Stolarczyk, P. Aging of Utricularia traps and variability of microorganisms associated with that microhabitat. Aquat. Bot. 2012, 97, 44–48. [Google Scholar] [CrossRef]
- Sirová, D.; Bárta, J.; Šimek, K.; Posch, T.; Pech, J.; Stone, J.; Borovec, J.; Adamec, L.; Vrba, J. Hunters or farmers? Microbiome characteristics help elucidate the diet composition in an aquatic carnivorous plant. Microbiome 2018, 6, 225, Corrected in Microbiome 2019, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Fenner, N.; Freeman, C.; Lock, M.A.; Harmens, H.; Reynolds, B.; Sparks, T. Interactions between elevated CO2 and warming could amplify DOC exports from peatland catchments. Environ. Sci. Technol. 2007, 41, 3146–3152. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Rochefort, L.; Hogue-Hugron, S.; Alqulaiti, Z.; Dunn, C.; Pouliot, R.; Jones, T.G.; Freeman, C.; Kang, H. Water table fluctuation in peatlands facilitates fungal proliferation, impedes Sphagnum growth and accelerates decomposition. Front. Earth Sci. 2021, 8, 579329. [Google Scholar] [CrossRef]
- Delarue, F.; Laggoun-Défarge, F.; Buttler, A.; Gogo, S.; Jassey, V.E.J.; Disnar, J.R. Experimental warming differentially affects microbial structure and activity in two contrasted moisture sites in a Sphagnum-dominated peatland. Sci. Total Environ. 2015, 511, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Song, Y.; Sun, L.; Song, C.; Wang, X.; Ma, X.; Liu, C.; Gao, J. Effects of warming on carbon emission and microbial abundances across different soil depths of a peatland in the permafrost region under anaerobic condition. Appl. Soil Ecol. 2020, 156, 103712. [Google Scholar] [CrossRef]
- Song, Y.; Liu, C.; Song, C.; Wang, X.; Ma, X.; Gao, J.; Gao, S.; Wang, L. Linking soil organic carbon mineralization with soil microbial and substrate properties under warming in permafrost peatlands of Northeastern China. CATENA 2021, 203, 105348. [Google Scholar] [CrossRef]
- Carrell, A.A.; Kolton, M.; Glassman, S.I.; Booth, M.G.; Carey, C.J.; Turetsky, M.R. Habitat-adapted microbial communities mediate Sphagnum peatmoss resilience to warming. New Phytol. 2022, 234, 2111–2125. [Google Scholar] [CrossRef] [PubMed]
- Mieczan, T.; Bartkowska, A. The effect of experimentally simulated climate warming on the microbiome of carnivorous plants—A microcosm experiment. Glob. Ecol. Conserv. 2022, 34, e02040. [Google Scholar] [CrossRef]
- Jassey, V.E.J.; Chiapusio, G.; Gilbert, D.; Binet, P.; Toussaint, M.L.; Delarue, F. An unexpected role for mixotrophs in the response of peatland carbon cycling to climate warming. Sci. Rep. 2015, 5, 16931. [Google Scholar] [CrossRef] [PubMed]
- Mieczan, T.; Adamczuk, M.; Pawlik-Skowrońska, B.; Toporowska, M. Eutrophication of peatbogs: Consequences of P and N enrichment for microbial and metazoan communities in mesocosm experiments. Aquat. Microb. Ecol. 2015, 74, 121–141. [Google Scholar] [CrossRef]
- Özen, A.; Adrian, R.; Maberly, S.C.; Andersen, T.; Jeppesen, E.; Mooij, W.M. Long-term effects of warming and nutrients on microbes and other plankton in mesocosms. Freshw. Biol. 2013, 58, 483–493. [Google Scholar] [CrossRef]
- Bartkowska, A.; Mieczan, T. The microbiome of peatland plants—Literature review. J. Water Land Dev. 2023, 59, 25–34. [Google Scholar] [CrossRef]
- Adamec, L. Soil fertilization enhances growth of the carnivorous plant Genlisea violacea. Biologia 2008, 63, 201–203. [Google Scholar] [CrossRef]
- Porter, K.G.; Feig, Y.S. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 1980, 25, 943–948. [Google Scholar] [CrossRef]
- Caron, D.A. Technique for enumeration of heterotrophic and phototrophic nanoplankton, using epifluorescence microscopy, and comparison with other procedures. Appl. Environ. Microbiol. 1983, 46, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Int. Ver. Theor. Angew. Limnol. Mitt. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Gilbert, D.; Amblard, C.; Bourdier, G.; Francez, A.J. The microbial loop at the surface of a peatland: Structure, function, and impact of nutrient input. Microb. Ecol. 1998, 35, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Bottrell, H.H. A review of some problems in zooplankton production studies. Nor. J. Zool. 1976, 24, 419–456. [Google Scholar]
- Amezaga, J.; Santamaria, L.; Green, A. Biotic wetland connectivity—Supporting a new approach for wetland policy. Acta Oecologia. 2002, 23, 213–222. [Google Scholar] [CrossRef]
- Nolan, C.; Overpeck, J.T.; Allen, J.R.M.; Anderson, P.M.; Betancourt, J.L.; Binney, H.A.; Brewer, S.; Bush, M.B.; Chase, B.M.; Cheddadi, R.; et al. Past and future global transformation of terrestrial ecosystems under climate change. Science 2018, 361, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Steffen, W.; Rockström, J.; Richardson, K.; Lenton, T.M.; Folke, C.; Liverman, D.; Summerhayes, C.P.; Barnosky, A.D.; Cornell, S.E.; Crucifix, M.; et al. Trajectories of the Earth System in the Anthropocene. Proc. Natl. Acad. Sci. USA 2018, 115, 8252–8259. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Bragazza, L.; Buttler, A.; Siegenthaler, A.; Yli-Petäys, M.; Mitchell, E.A.D.; Rydin, H.; Gerdol, R. Persistent high temperature and low precipitation reduce peat carbon accumulation. Glob. Chang. Biol. 2016, 22, 4114–4123. [Google Scholar] [CrossRef] [PubMed]
- Santschi, F.; Gounand, I.; Harvey, E.; Altermatt, F. Leaf litter diversity and structure of microbial decomposer communities modulate litter decomposition in aquatic systems. Funct. Ecol. 2018, 32, 522–532. [Google Scholar] [CrossRef]
- Geisen, S.; Mitchell, E.A.D.; Adl, S.; Bonkowski, M.; Dunthorn, M.; Ekelund, F.; Fernández, L.D.; Jousset, A.; Krashevska, V.; Singer, D.; et al. Soil protistology rebooted: 30 fundamental questions to start with. Soil Biol. Biochem. 2017, 111, 94–103. [Google Scholar] [CrossRef]
- Peltoniemi, K.; Fritze, H.; Laiho, R. Response of fungal and actinobacterial communities to water-level drawdown in boreal peatland sites. Soil Biol. Biochem. 2009, 41, 1902–1914. [Google Scholar] [CrossRef]
- Le Geay, M.; Lauga, B.; Walcker, R.; Jassey, V.E.J. A meta-analysis of peatland microbial diversity and function responses to climate change. Soil Biol. Biochem. 2024, 189, 109245. [Google Scholar] [CrossRef]
- Alexandrov, G.A.; Brovkin, V.; Kleinen, T.; Zicheng, Y. The capacity of northern peatlands for long-term carbon sequestration. Biogeosciences 2020, 17, 47–54. [Google Scholar] [CrossRef]
- Straková, P.; Niemi, R.M.; Freeman, C.; Peltoniemi, K.; Toberman, H.; Heiskanen, I.; Fritze, H.; Laiho, R. Litter type affects the activity of aerobic decomposers in a boreal peatland more than site nutrient and water table regimes. Biogeosciences 2011, 8, 2741–2755. [Google Scholar] [CrossRef]
- Urbanová, Z.; Bárta, J. Effects of long-term drainage on microbial community composition vary between peatland types. Soil Biol. Biochem. 2016, 92, 16–26. [Google Scholar] [CrossRef]
- Mpamah, P.A.; Taipale, S.; Rissanen, A.J.; Biasis, C.; Nykänen, H.N. The impact of long-term water level draw-down on microbial biomass: A comparative study from two peatland sites with different nutrient status. Eur. J. Soil Biol. 2017, 80, 59–68. [Google Scholar] [CrossRef]
- Potter, C.; Freeman, C.; Fenner, N.; Kipling, R.P. Subtle shifts in microbial communities occur alongside the release of carbon induced by drought and rewetting in contrasting peatland ecosystems. Sci. Rep. 2017, 7, 11314. [Google Scholar] [CrossRef] [PubMed]
- Adlassnig, W.; Peroutka, M.; Lendl, T. Traps of carnivorous pitcher plants as a habitat: Composition of the fluid, biodiversity and mutualistic activities. Ann. Bot. 2011, 107, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Auer, B.; Arndt, H. Taxonomic composition and biomass of heterotrophic flagellates in relation to lake trophy and season. Freshw. Biol. 2001, 46, 959–972. [Google Scholar] [CrossRef]
- Carballeira, R.; Pontevedra-Pombal, X. Diversity of testate amoebae as an indicator of the conservation status of peatlands in southwest Europe. Diversity 2021, 13, 122. [Google Scholar] [CrossRef]
- Koenig, I.; Schwendener, F.; Mulot, M.; Mitchell, E.A.D. Response of Sphagnum testate amoebae to drainage, subsequent re-wetting and associated changes in the moss carpet—Results from a three year mesocosm experiment. Acta Protozool. 2017, 56, 191–210. [Google Scholar] [CrossRef]
- Jassey, V.E.J.; Gilbert, D.; Binet, P.; Toussaint, M.L.; Chiapusio, G. Effect of a temperature gradient on Sphagnum fallax and its associated living microbial communities: A study under controlled conditions. Can. J. Microbiol. 2011, 57, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Magnan, G.; van Bellen, S.; Davies, L.; Froese, D.; Garneau, M.; Mullan-Boudreau, G.; Zaccone, C.; Shotyk, W. Impact of the Little Ice Age cooling and 20th century climate change on peatland vegetation dynamics in central and northern Alberta using a multi-proxy approach and high-resolution peat chronologies. Quat. Sci. Rev. 2018, 185, 230–243. [Google Scholar] [CrossRef]
- Payne, R.J.; Babeshko, K.V.; Van Bellen, S.; Blackford, J.J.; Booth, R.K.; Charman, D.J.; Ellershaw, M.R.; Gilbert, D.; Hughes, P.D.; Jassey, V.E.; et al. Significance testing testate amoeba water table reconstructions. Quat. Sci. Rev. 2016, 15, 131–135. [Google Scholar] [CrossRef]
- Gałka, M.; Tobolski, K.; Lamentowicz, Ł.; Ersek, V.; Jassey, V.; van der Knaap, W.O.; Lamentowicz, M. Unveiling exceptional Baltic bog ecohydrology, autogenic succession and climate change during the last 2000 years in CE Europe using replicate cores, multi-proxy data and functional traits of testate amoebae. Quat. Sci. Rev. 2017, 156, 90–106. [Google Scholar] [CrossRef]
- Herbert, R.P.; Peters, S.C.; Nelson, D.M.; Booth, R.K. Light variability and mixotrophy: Responses of testate amoeba communities and shell δ13C values to a peatland shading experiment. Eur. J. Protistol. 2019, 67, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Lamentowicz, M.; Gałka, M.; Miotk-Szpiganowicz, G.; Tobolski, K.; Mitchell, E.A.D. Testate amoebae taxonomy and trait diversity are coupled along an openness and wetness gradient in pine-dominated Baltic bogs. Eur. J. Protistol. 2020, 73, 125674. [Google Scholar] [CrossRef] [PubMed]
- Collins, E.S.; McCarthy, F.M.G.; Medioli, F.S.; Scott, D.B.; Honig, C.A. Biogeographic distribution of modern thecamoebians in a transect along the Eastern North American coast. In Paleoecology, Biostratigraphy, Paleoceanography and Taxonomy of Agglutinated Foraminifera; Hemleben, C., Kaminski, M.A., Kuhnt, W., Scott, D.B., Eds.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1990; pp. 783–792. [Google Scholar]
- Lara, E.; Gomaa, F. Symbiosis between Testate Amoebae and Photosynthetic Organisms. In Algal and Cyanobacteria Symbioses; Seckbach, J., Grube, M., Eds.; World Scientific Publishing: Singapore, 2017; pp. 399–419. [Google Scholar] [CrossRef]
- Zhang, H.; Mitchell, E.A.D.; Liu, W.; Xiong, S.; Peng, C. Recent Changes in Peatland Testate Amoeba Functional Traits and Hydrology Within a Replicated Site Network in Northwestern Québec, Canada. Front. Ecol. Evol. 2020, 8, 228. [Google Scholar] [CrossRef]
- Koenig, I.; Mulot, M.; Mitchell, E.A.D. Taxonomic and functional traits responses of Sphagnum peatland testate amoebae to experimentally manipulated water table. Ecol. Indic. 2018, 85, 342–351. [Google Scholar] [CrossRef]
- Reczuga, M.K.; Lamentowicz, M.; Mulot, M.; Mitchell, E.A.D.; Buttler, A.; Chojnicki, B.; Słowiński, M.; Binet, P.; Chiapusio, G.; Gilbert, D.; et al. Predator–prey mass ratio drives microbial activity under dry conditions in Sphagnum peatlands. Ecol. Evol. 2018, 8, 5752–5764. [Google Scholar] [CrossRef] [PubMed]
- Singer, D.; Seppey, C.V.W.; Lentendu, G.; Dunthorn, M.; Bass, D.; Belbahri, L.; Blandenier, Q.; Debroas, D.; Izzo, A.; Kaczmarek, Ł.; et al. Contrasted micro-eukaryotic diversity associated with Sphagnum mosses in tropical, subtropical and temperate climatic zones. Microb. Ecol. 2019, 78, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Lamentowicz, M.; Gałka, M.; Kołaczek, P.; Pawlyta, J.; Piotrowska, N.; Stefaniak, K.; Tobolski, K.; van der Knaap, W.O. Unveiling tipping points in long-term ecological records from Sphagnum-dominated peatlands. Biol. Lett. 2019, 15, 20190043. [Google Scholar] [CrossRef]
- Mitchell, E.A.D.; Gilbert, D.; Buttler, A.; Amblard, C.; Grosvernier, P.; Gobat, J.M. Structure of microbial communities in Sphagnum peatlands and effect of atmospheric carbon dioxide enrichment. Microb. Ecol. 2003, 46, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Xu, M.; Liu, B.; Cao, H. Characteristics of microfauna and their relationships with the performance of an activated sludge plant in China. J. Environ. Sci. 2007, 20, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Viet, H.; Gilbert, D.; Mitchell, E.A.D.; Badot, P.M.; Bernard, N. Effects of experimental lead pollution on the microbial communities associated with Sphagnum fallax (Bryophyta). Microb. Ecol. 2007, 54, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.M.; Mitchell, E.A.D. Testate amoebae and nutrient cycling with particular reference to soils. Geomicrobiol. J. 2010, 27, 520–533. [Google Scholar] [CrossRef]
- Lukić, D.; Limberger, R.; Agatha, S.; Montagnes, D.J.S.; Weisse, T. Thermal performance of planktonic ciliates differs between marine and freshwaters: A case study providing guidance for climate change studies. Limnol. Oceanogr. Lett. 2022, 7, 520–526. [Google Scholar] [CrossRef]
- Andersen, R.; Chapman, S.J.; Artz, R.R.E. Microbial communities in natural and disturbed peatlands: A review. Soil Biol. Biochem. 2013, 57, 979–994. [Google Scholar] [CrossRef]
- Wojewódka, M.; Hruševar, D. The role of paleolimnology in climate and environment reconstruction and lake restoration in light of research on selected bioindicators. Holist. Approach Environ. 2020, 10, 16–28. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, J.C.; Joo, G.J.; Choi, J.Y. Response of the rotifer community to human-induced changes in the trophic state of a reservoir. Oceanol. Hydrobiol. Stud. 2020, 49, 329–344. [Google Scholar] [CrossRef]
- Muller, S.D.; Richard, P.; Talon, B. Impact of disturbance on the Holocene development of a temperate peatland (Southern Quebe). Veg. Hist. Archaeobotany 2008, 17, 713–721. [Google Scholar] [CrossRef]
- Błędzki, L.A.; Bubier, J.L.; Ellison, A.M.; Moore, T.R. Ecology of rotifers and their unappreciated source of nitrogen and phosphorus in temperate northeastern American bogs. Fundam. Appl. Limnol. 2018, 191, 277–287. [Google Scholar] [CrossRef]
- Buosi, P.R.B.; Pauleto, G.M.; Lansac-Tôha, F.A.; Velho, L.F.M. Ciliate community associated with aquatic macrophyte roots: Effects of nutrient enrichment on the community composition and species richness. Eur. J. Protistol. 2011, 47, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, A.J.; Swenson, J.E.; Casamatta, D.A. Culturable bacteria present in the fluid of the hooded-pitcher plant Sarracenia minor based on 16S rDNA gene sequence data. Microb. Ecol. 2007, 54, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Sirová, D.; Adamec, L.; Vrba, J. Enzymatic activities in traps of four aquatic species of the carnivorous genus Utricularia. New Phytol. 2003, 159, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.; Pacheco, S. Prey composition in the carnivorous plant Utricularia inflata and U. gibba (Lentibulariaceae) from Paria Peninsula, Venezuela. Rev. Biol. Trop. 2007, 55, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Posch, T.; Eugster, B.; Pomati, F.; Pernthaler, J.; Psenner, R.; Kainz, M.J. Network of interactions between ciliates and phytoplankton during spring. Front. Microbiol. 2015, 6, 1289. [Google Scholar] [CrossRef] [PubMed]
- Weisse, T.; Anderson, R.; Arndt, H.; Calbet, A.; Hansen, P.J.; Montagnes, D.J.S. Functional ecology of aquatic phagotrophic protists—Concepts, limitations, and perspectives. Eur. J. Protistol. 2016, 55, 50–74. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a Metabolic Theory of Ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Price, C.A.; Gilooly, J.F.; Allen, A.P.; Weitz, J.S.; Niklas, K.J. The metabolic theory of ecology: Prospects and challenges for plant biology. New Phytol. 2010, 188, 696–710. [Google Scholar] [CrossRef] [PubMed]
- DeLong, J.P.; Gibert, J.P.; Luhring, T.M.; Bachman, G.; Reed, B.; Neyer, A.; Vasseur, D.A. The combined effects of reactant kinetics and enzyme stability explain the temperature dependence of metabolic rates. Ecol. Evol. 2017, 7, 3940–3950. [Google Scholar] [CrossRef] [PubMed]
- Dewitt, T.D.; Nordlund, D.A.; Evans, T.A.; Van Valkenburgh, B.; Crumpler, L.S.; Weiss, L.L.; Szunyogh, L.; Adams, P.; Stone, P.; Herkenhoff, K.E. Climatologically invariant scale invariance seen in distributions of cloud horizontal sizes. Atmos. Chem. Phys. 2024, 24, 109–122. [Google Scholar] [CrossRef]


| Peatland | Carbonate Fen | |||||||
|---|---|---|---|---|---|---|---|---|
| Variant I C = 22° | Variant II +2 °C | Variant III +4 °C | Variant IV +8 °C | Variant I C = 22° | Variant II +2 °C | Variant III +4 °C | Variant IV +8 °C | |
| T (°C) | 22.5 | 24.6 | 25.6 | 30.1 | 22.3 | 24.6 | 25.6 | 30.4 |
| pH | 4.98 | 6.96 | 6.9 | 6.91 | 6.51 | 5.9 | 6.4 | 6.44 |
| Cond. (μS cm−1) | 27.1 | 31 | 29.2 | 43.4 | 147.4 | 120.9 | 118.5 | 176.1 |
| O2 (mg L−1) | 7.83 | 8.09 | 7.77 | 7.06 | 6.12 | 7.72 | 7.98 | 4.06 |
| O2% | 94.2 | 97.6 | 95.3 | 89.4 | 73.7 | 93.6 | 98.8 | 52.4 |
| Ntot (mg L−1) | 1.843 | 1.203 | 1.397 | 1.418 | 2.987 | 3.05 | 3.270 | 4.444 |
| Ptot (mg L−1) | 0.124 | 0.071 | 0.01 | 0.046 | 0.131 | 0.06 | 0.02 | 0.209 |
| Chla (µg L−1) | 24 | 20 | 31 | 83 | 25.32 | 26 | 6.32 | 23 |
| SUR (mg L−1) | 23 | 22.4 | 22 | 22.8 | 34.5 | 35.5 | 36 | 35 |
| ChZT (mg L−1) | 68.5 | 65.6 | 65.5 | 68.8 | 85 | 85 | 86 | 85 |
| BZT (mg L−1) | 41.5 | 39.5 | 39.5 | 41 | 53 | 54 | 54.5 | 53.5 |
| TSS (mg L−1) | 156 | 176 | 168 | 180 | 294 | 315 | 300 | 305 |
| TOC (mg L−1) | 31.5 | 30 | 30 | 31 | 41 | 41.5 | 42 | 41.5 |
| . | Average Level of Biomass (µgC mg L−1) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peatland | Carbonate Fen | |||||||||||||||
Environment | Water | Traps | Water | Traps | ||||||||||||
Taxonomic group | ||||||||||||||||
| C | +2 °C | +4 °C | +8 °C | C | +2 °C | +4 °C | +8 °C | C | +2 °C | +4 °C | +8 °C | C | +2 °C | +4 °C | +8 °C | |
| Bacteria | 0.52 | 0.72 | 0.83 | 0.91 | 0.42 | 0.47 | 0.49 | 0.53 | 0.6 | 0.82 | 0.97 | 1.1 | 0.5 | 0.55 | 0.62 | 0.65 |
| Heterotrophic falgellates | 0.2 | 0.45 | 0.62 | 0.75 | 0.19 | 0.42 | 0.47 | 0.51 | 0.32 | 0.55 | 0.71 | 0.82 | 0.2 | 0.4 | 0.52 | 0.5 |
| Testate amoebae | 0.5 | 0.6 | 0.7 | 0.4 | 0.1 | 0.1 | 0.2 | 0.1 | 0.6 | 0.6 | 0.8 | 0.5 | 0.1 | 0.2 | 0.3 | 0.2 |
| Ciliates | 0.3 | 0.3 | 0.8 | 0.6 | 0.2 | 0.2 | 0.6 | 0.4 | 0.4 | 0.5 | 0.7 | 0.6 | 0.3 | 0.2 | 0.7 | 0.5 |
| Rotifers | 0.7 | 0.8 | 0.4 | 0.3 | 0.6 | 0.5 | 0.2 | 0.1 | 0.8 | 0.8 | 0.9 | 0.4 | 0.5 | 0.5 | 0.6 | 0.2 |
| Crustacea | 0.6 | 0.45 | 0.38 | 0.3 | - | - | - | - | 0.70 | 0.52 | 0.48 | 0.39 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartkowska-Bekasiewicz, A.; Mieczan, T. Effects of Temperature Increase on Microbiome of Carnivorous Plant Utricularia vulgaris L. in Peat Bog Ecosystems. Biology 2025, 14, 884. https://doi.org/10.3390/biology14070884
Bartkowska-Bekasiewicz A, Mieczan T. Effects of Temperature Increase on Microbiome of Carnivorous Plant Utricularia vulgaris L. in Peat Bog Ecosystems. Biology. 2025; 14(7):884. https://doi.org/10.3390/biology14070884
Chicago/Turabian StyleBartkowska-Bekasiewicz, Aleksandra, and Tomasz Mieczan. 2025. "Effects of Temperature Increase on Microbiome of Carnivorous Plant Utricularia vulgaris L. in Peat Bog Ecosystems" Biology 14, no. 7: 884. https://doi.org/10.3390/biology14070884
APA StyleBartkowska-Bekasiewicz, A., & Mieczan, T. (2025). Effects of Temperature Increase on Microbiome of Carnivorous Plant Utricularia vulgaris L. in Peat Bog Ecosystems. Biology, 14(7), 884. https://doi.org/10.3390/biology14070884





