Single-Cell Atlas of Spleen Remodeling Reveals Macrophage Subset-Driven ASFV Pathogenesis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. scRNA-Seq Data Acquisition and Quality Control
2.2. scRNA Data Processing and Annotation
2.3. Differential Expression and Functional Enrichment
2.4. Cell–Cell Communication Inference
2.5. Transcription Factor Network Reconstruction
2.6. Pseudotime Trajectory Analysis
2.7. Statistical Analysis for Differential Expression
3. Results
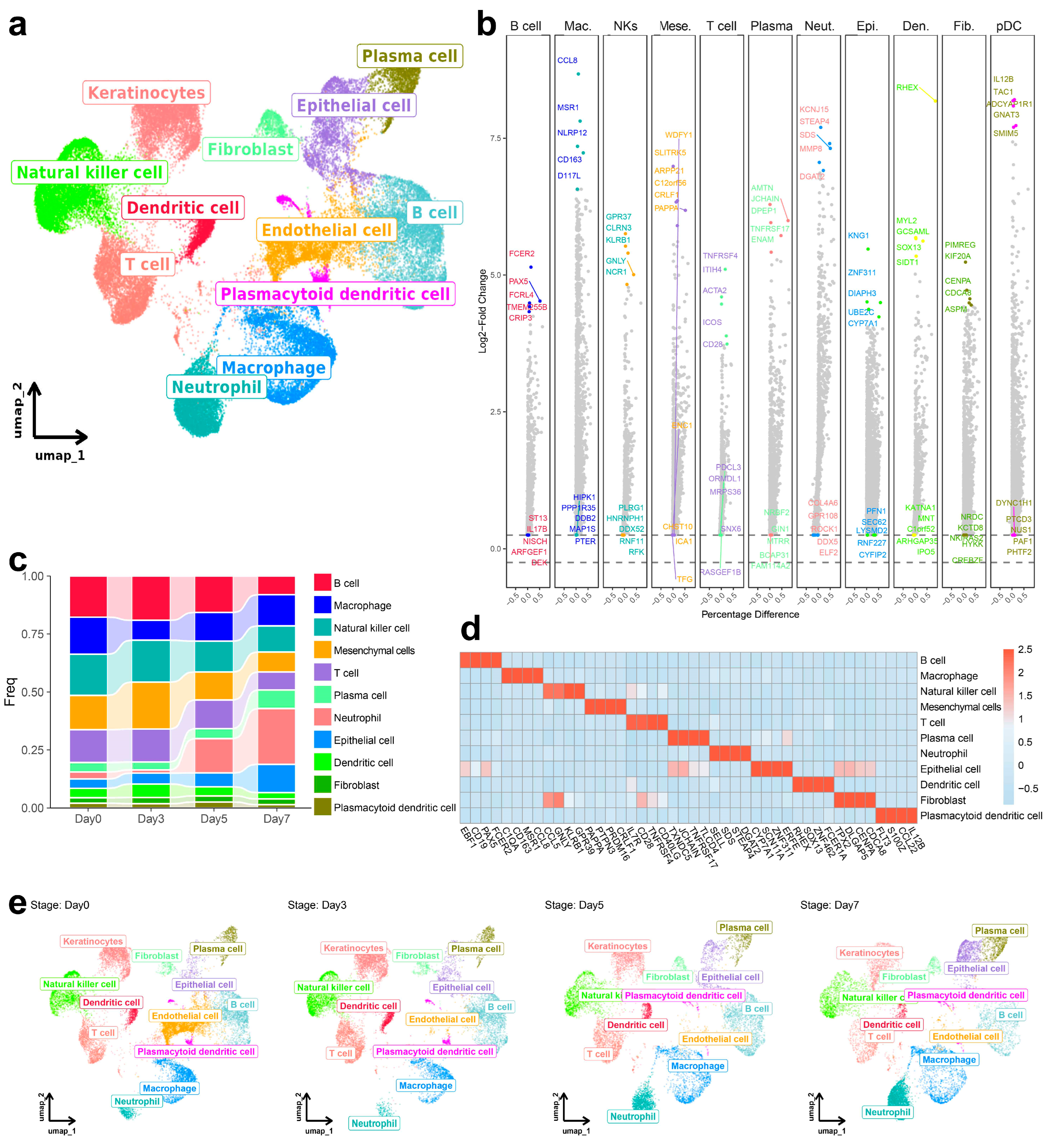
3.1. Dynamic Change in Immune Cell Composition in the Spleen Following ASFV Infection
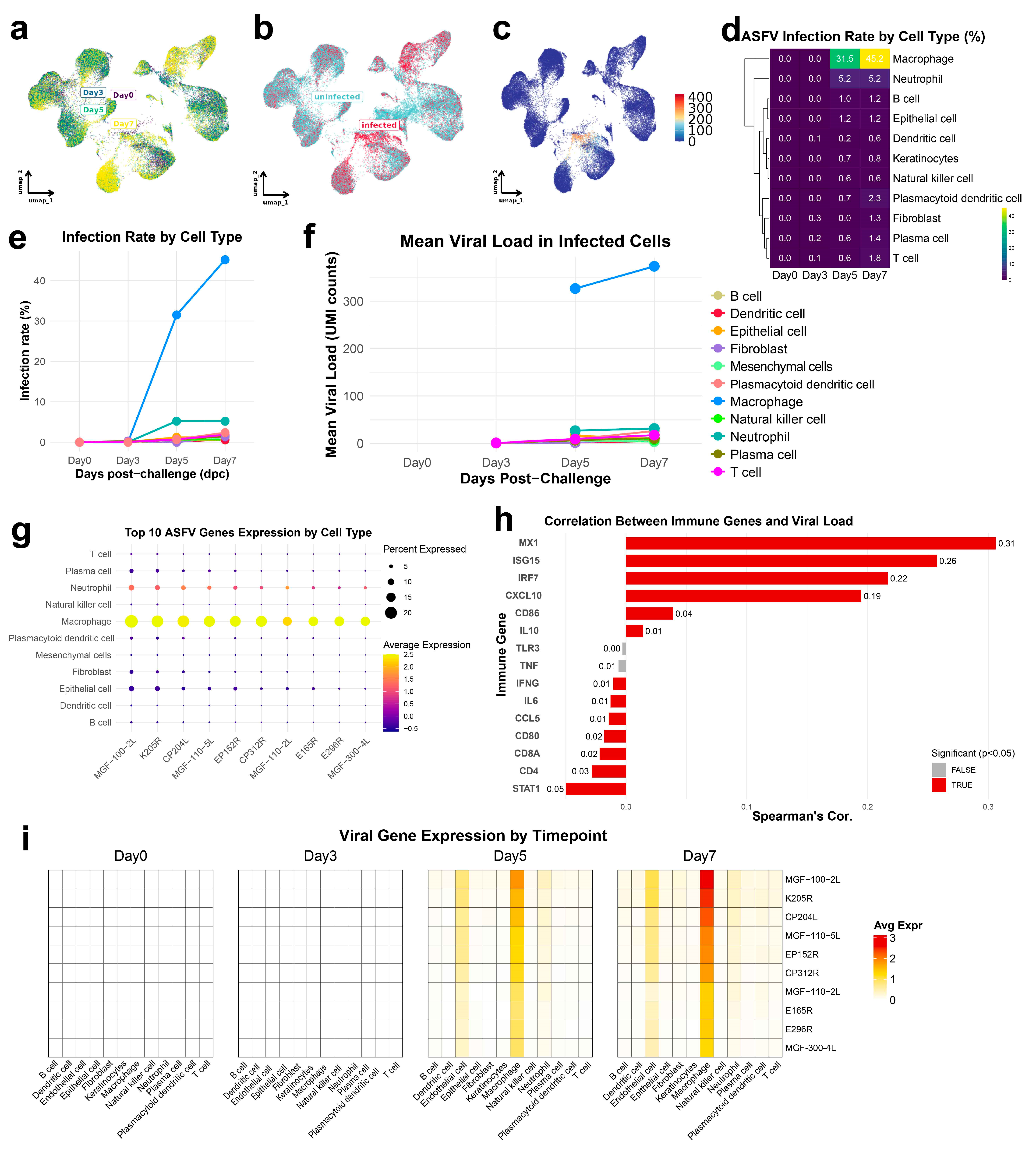
3.2. ASFV Preferentially Infects Macrophages with Increasing Viral Loads and Transcriptional Reprogramming over Time
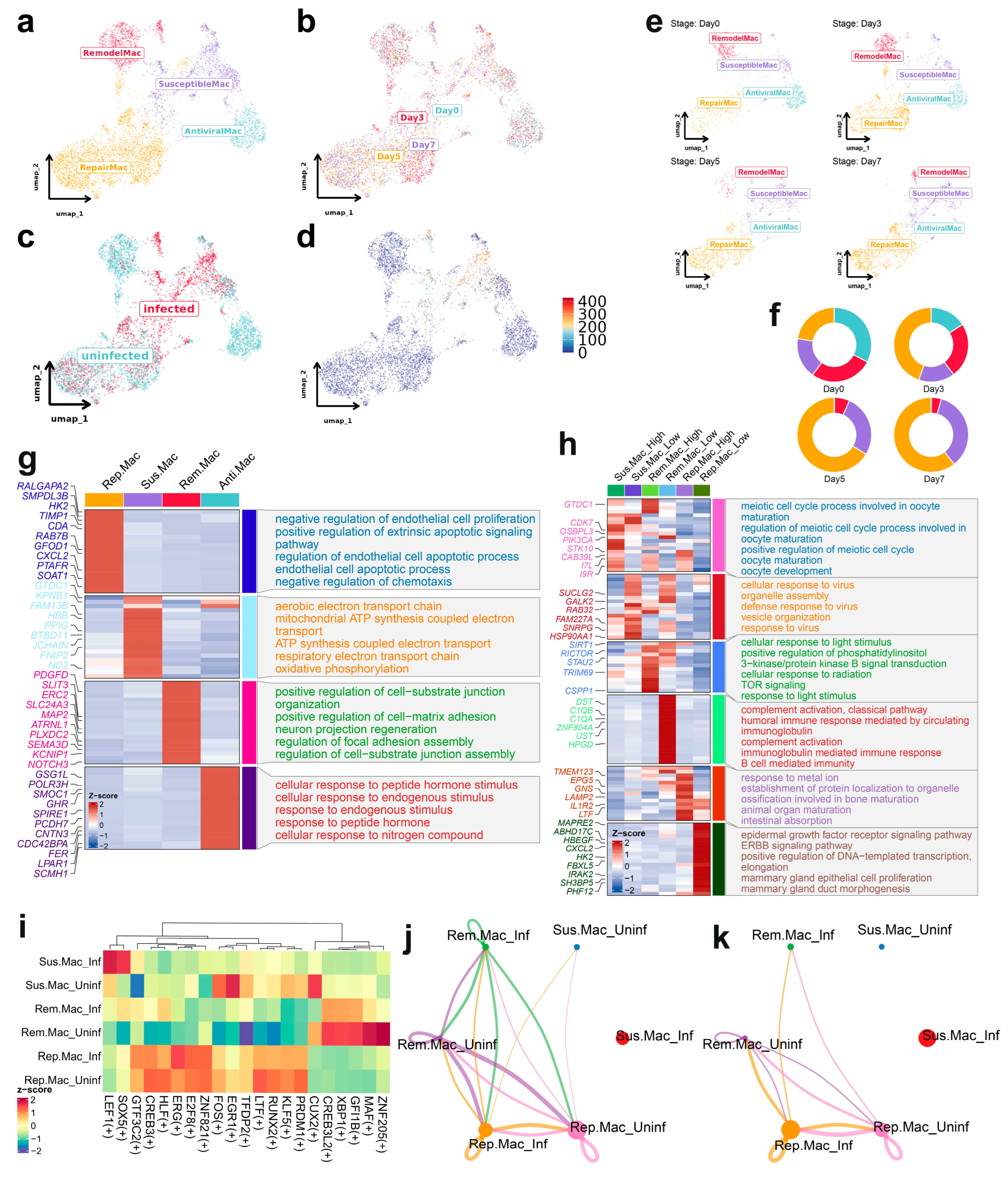
3.3. Subclustering Analysis of Macrophages Reveals Distinct Functional States and Susceptibility to ASFV Infection
3.4. Infection-Phase-Specific Transcriptional Programs in Macrophage Subpopulations
3.5. Pseudotemporal Analysis of Macrophage Subtypes During ASFV Infection
3.6. Transcriptional Regulation and Cellular Crosstalk in Macrophage Subsets During ASFV Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASF | African swine fever |
| ASFV | African swine fever virus |
| PCA | Principal component analysis |
| TF | Transcription factors |
| UMAP | Uniform manifold approximation and projection |
| GO | Gene ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| DEGs | Differentially expressed genes |
| PPR | Pattern recognition receptor |
References
- Niu, S.; Guo, Y.; Wang, X.; Wang, Z.; Sun, L.; Dai, H.; Peng, G. Innate immune escape and adaptive immune evasion of African swine fever virus: A review. Virology 2023, 587, 109878. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Chapman, D.A.; Netherton, C.L.; Upton, C. African swine fever virus replication and genomics. Virus. Res. 2013, 173, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Sirakanyan, S.; Arabyan, E.; Hakobyan, A.; Hakobyan, T.; Chilingaryan, G.; Sahakyan, H.; Sargsyan, A.; Arakelov, G.; Nazaryan, K.; Izmailyan, R.; et al. A new microtubule-stabilizing agent shows potent antiviral effects against African swine fever virus with no cytotoxicity. Emerg. Microbes Infec. 2021, 10, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Droesbeke, B.; Balmelle, N.; Cay, A.B.; Han, S.J.; Oh, D.; Nauwynck, H.J.; Tignon, M. Replication Kinetics and Infectivity of African Swine Fever Virus (ASFV) Variants with Different Genotypes or Levels of Virulence in Cell Culture Models of Primary Porcine Macrophages. Microbiol. Res. 2024, 15, 1690–1708. [Google Scholar] [CrossRef]
- Gómez-Villamandos, J.C.; Bautista, M.J.; Sánchez-Cordón, P.J.; Carrasco, L. Pathology of African swine fever: The role of monocyte-macrophage. Virus. Res. 2013, 173, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Ru, Y.; Yang, W.; Ren, J.; Hao, R.; Qin, X.; Li, D.; Zheng, H. Research progress on the proteins involved in African swine fever virus infection and replication. Front. Immunol. 2022, 13, 947180. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhu, Z.; Deng, J.; Tian, K.; Li, X. Antagonisms of ASFV towards Host Defense Mechanisms: Knowledge Gaps in Viral Immune Evasion and Pathogenesis. Viruses 2023, 15, 574. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Cao, Y.; Jiao, P.; Yu, P.; Zhang, H.; Chen, T.; Zhou, X.; Qi, Y.; Sun, L.; Liu, D.; et al. Synergistic effect of the responses of different tissues against African swine fever virus. Transbound. Emerg. Dis. 2022, 69, e204–e215. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Mao, R.; Liu, B.; Liu, H.; Shi, Z.; Zhang, K.; Liu, H.; Zhang, D.; Liu, J.; Zhao, Z.; et al. Single-cell profiling of African swine fever virus disease in the pig spleen reveals viral and host dynamics. Proc. Natl. Acad. Sci. USA 2024, 121, e2312150121. [Google Scholar] [CrossRef] [PubMed]
- Machuka, E.M.; Juma, J.; Muigai, A.W.T.; Amimo, J.O.; Pelle, R.; Abworo, E.O. Transcriptome profile of spleen tissues from locally-adapted Kenyan pigs (Sus scrofa) experimentally infected with three varying doses of a highly virulent African swine fever virus genotype IX isolate: Ken12/busia.1 (ken-1033). BMC Genom. 2022, 23, 522. [Google Scholar] [CrossRef] [PubMed]
- Truong, A.D.; Kang, S.; Dang, H.V.; Hong, Y.; Vu, T.H.; Heo, J.; Chu, N.T.; Nguyen, H.T.; Tran, H.T.T.; Hong, Y.H. Small RNA sequencing and profiling of serum-derived exosomes from African swine fever virus-infected pigs. J. Anim. Sci. 2023, 101, skac400. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Li, S.H.; Yu, S.; Wang, Q.; Zhang, K.; Qu, L.; Sun, Y.; Bi, Y.; Tang, F.; et al. Transcriptome profiling in swine macrophages infected with African swine fever virus at single-cell resolution. Proc. Natl. Acad. Sci. USA 2022, 119, e2201288119. [Google Scholar] [CrossRef] [PubMed]
- Salinas, S.; Schiavo, G.; Kremer, E.J. A hitchhiker’s guide to the nervous system: The complex journey of viruses and toxins. Nat. Rev. Microbiol. 2010, 8, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Ziegon, L.; Schlegel, M. Netrin-1: A Modulator of Macrophage Driven Acute and Chronic Inflammation. Int. J. Mol. Sci. 2021, 23, 275. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Hu, Z.; Wang, S.; Yin, K. Netrin-1: An emerging player in inflammatory diseases. Cytokine Growth Factor Rev. 2022, 64, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Ducarouge, B.; Redavid, A.R.; Victoor, C.; Chira, R.; Fonseca, A.; Hervieu, M.; Berge, R.; Lengrand, J.; Vieugue, P.; Neves, D.; et al. Netrin-1 blockade inhibits tumor associated Myeloid-derived suppressor cells, cancer stemness and alleviates resistance to chemotherapy and immune checkpoint inhibitor. Cell Death Differ. 2023, 30, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.Y.; Zhang, X.X.; Xiong, J.; Yu, J.P.; Wei, H.P. Rapid genome-wide sequence typing of African swine fever virus based on alleles. Virus. Res. 2021, 297, 198357. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Stuart, T.; Kowalski, M.H.; Choudhary, S.; Hoffman, P.; Hartman, A.; Srivastava, A.; Molla, G.; Madad, S.; Fernandez-Granda, C.; et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 2024, 42, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Corbett, S.E.; Koga, Y.; Wang, Z.; Johnson, W.E.; Yajima, M.; Campbell, J.D. Decontamination of ambient RNA in single-cell RNA-seq with DecontX. Genome Biol. 2020, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.X.; Li, T.Y.; Xu, Y.Q.; Zhang, X.X.; Li, F.; Bai, J.; Chen, J.; Jiang, W.Q.; Yang, K.Y.; Ou, Q.; et al. CellMarker 2.0: An updated database of manually curated cell markers in human/mouse and web tools based on scRNA-seq data. Nucleic Acids Res. 2023, 51, D870–D876. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.Q.; Plikus, M.V.; Nie, Q. CellChat for systematic analysis of cell-cell communication from single-cell transcriptomics. Nat. Protoc. 2025, 20, 180–219. [Google Scholar] [CrossRef] [PubMed]
- van de Sande, B.; Flerin, C.; Davie, K.; De Waegeneer, M.; Hulselmans, G.; Aibar, S.; Seurinck, R.; Saelens, W.; Cannoodt, R.; Rouchon, Q.; et al. A scalable SCENIC workflow for single-cell gene regulatory network analysis. Nat. Protoc. 2020, 15, 2247–2276. [Google Scholar] [CrossRef] [PubMed]
- Tng, P.Y.L.; Al-Adwani, L.; Pauletto, E.; Hui, J.Y.K.; Netherton, C.L. Capsid-Specific Antibody Responses of Domestic Pigs Immunized with Low-Virulent African Swine Fever Virus. Vaccines 2023, 11, 1577. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, L.; Wang, J.; Su, D.; Li, D.; Du, Y.; Yang, G.; Zhang, G.; Chu, B. African Swine Fever Virus K205R Induces ER Stress and Consequently Activates Autophagy and the NF-kappaB Signaling Pathway. Viruses 2022, 14, 394. [Google Scholar] [CrossRef]
- Vuono, E.A.; Ramirez-Medina, E.; Pruitt, S.; Rai, A.; Espinoza, N.; Spinard, E.; Valladares, A.; Silva, E.; Velazquez-Salinas, L.; Borca, M.; et al. Deletion of the EP296R Gene from the Genome of Highly Virulent African Swine Fever Virus Georgia 2010 Does Not Affect Virus Replication or Virulence in Domestic Pigs. Viruses 2022, 14, 1682. [Google Scholar] [CrossRef] [PubMed]
- Vuono, E.A.; Ramirez-Medina, E.; Pruitt, S.; Rai, A.; Espinoza, N.; Silva, E.; Velazquez-Salinas, L.; Gladue, D.P.; Borca, M. Deletion of the ASFV dUTPase Gene from the Genome of Highly Virulent African Swine Fever Virus Georgia 2010 Does Not Affect Virus Replication or Virulence in Domestic Pigs. Viruses 2022, 14, 1409. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.V.; Jayakumar, C.; Ramesh, G. Netrin-1-treated macrophages protect the kidney against ischemia-reperfusion injury and suppress inflammation by inducing M2 polarization. Am. J. Physiol.-Ren. Physiol. 2013, 304, F948–F957. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Duan, Y.; Li, X.; Zhang, Y.; Cao, L.; Feng, T.; Ge, J.; Wang, Q.; Zheng, H. Transcriptional and ultrastructural changes of macrophages after african swine fever virus infection. Vet. Microbiol. 2024, 293, 110074. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, W.; Li, X.; Wu, K.; Wang, X.; Wang, W.; Li, Y.; Yi, L.; Zhao, M.; Ding, H.; et al. Transcriptome Profiling in Swine Macrophages Infected with African Swine Fever Virus (ASFV) Uncovers the Complex and Close Relationship with Host. Pathogens 2022, 11, 1411. [Google Scholar] [CrossRef] [PubMed]
- Hubner, A.; Petersen, B.; Keil, G.M.; Niemann, H.; Mettenleiter, T.C.; Fuchs, W. Efficient inhibition of African swine fever virus replication by CRISPR/Cas9 targeting of the viral p30 gene (CP204L). Sci. Rep. 2018, 8, 1449. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ge, H.L.; Li, Y.H.; Zhang, K.H.; Yu, S.X.; Cao, H.W.; Wang, Y.J.; Deng, H.; Li, J.Q.; Dai, J.W.; et al. The E301R protein of African swine fever virus functions as a sliding clamp involved in viral genome replication. Mbio 2023, 14, e01645-23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.L.; Xi, F.; Zeng, W.X.; Zhao, Y.F.; Cao, W.J.; Liu, C.; Yang, F.; Ru, Y.; Xiao, S.Q.; Zhang, S.L.; et al. Structural basis of RNA polymerase complexes in African swine fever virus. Nat. Commun. 2025, 16, 501. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Chai, Y.; Song, H.; Weng, C.J.; Qi, J.X.; Sun, Y.P.; Gao, G.F. Crystal Structure of African Swine Fever Virus dUTPase Reveals a Potential Drug Target. Mbio 2019, 10, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Fu, Y.N.; Li, G.S.; Shen, Z.; Guo, F.L.; Shi, J.L.; Guo, Y.L.; Zhang, D.; Wang, Z.X.; Chen, C.E.; et al. EP152R-mediated endoplasmic reticulum stress contributes to African swine fever virus infection via the PERK-eIF2α pathway. FASEB J. 2024, 38, e70187. [Google Scholar] [CrossRef] [PubMed]
- Tamás, V.; Righi, C.; Mészáros, I.; D’Errico, F.; Olasz, F.; Casciari, C.; Zádori, Z.; Magyar, T.; Petrini, S.; Feliziani, F. Involvement of the MGF 110-11L Gene in the African Swine Fever Replication and Virulence. Vaccines 2023, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Chen, L.Q.; Chen, H.; Zhang, H.; Dong, P.P.; Sun, L.F.; Huang, X.J.; Lin, P.D.; Wu, L.J.; Jing, D.D.; et al. Structural insights into the CP312R protein of the African swine fever virus. Biochem. Biophys. Res. Commun. 2022, 624, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, H.H.; Denyer, M.S.; Lacasta, A.; Stirling, C.M.; Argilaguet, J.M.; Netherton, C.L.; Oura, C.A.; Martins, C.; Rodriguez, F. Cellular immunity in ASFV responses. Virus. Res. 2013, 173, 110–121. [Google Scholar] [CrossRef] [PubMed]
- He, W.R.; Yuan, J.; Ma, Y.H.; Zhao, C.Y.; Yang, Z.Y.; Zhang, Y.; Han, S.; Wan, B.; Zhang, G.P. Modulation of Host Antiviral Innate Immunity by African Swine Fever Virus: A Review. Animals 2022, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Bouhlel, M.A.; Derudas, B.; Rigamonti, E.; Díèvart, R.; Brozek, J.; Haulon, S.; Zawadzki, C.; Jude, B.; Torpier, G.; Marx, N.; et al. PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007, 6, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.F.; Zheng, W.Y.; Xu, Z.Y.; Wang, S.H.; Wang, Y.; Cheng, J.S.; Li, Z.W.; Cheng, K.A.; Zhang, S.S.; Chen, X.M.; et al. RBM4 regulates M1 macrophages polarization through targeting STAT1-mediated glycolysis. Int. Immunopharmacol. 2020, 83, 106432. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Miller, L.C.; Blecha, F. Macrophage polarization in virus-host interactions. J. Clin. Cell. Immunol. 2015, 6, 311. [Google Scholar] [PubMed]
- Razzuoli, E.; Franzoni, G.; Carta, T.; Zinellu, S.; Amadori, M.; Modesto, P.; Oggiano, A. Modulation of Type I Interferon System by African Swine Fever Virus. Pathogens 2020, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Jiao, P.; Zhang, H.; Chen, T.; Zhou, X.; Qi, Y.; Sun, L.; Shang, Y.; Zhu, H.; Hu, R.; et al. Inhibition of African Swine Fever Virus Replication by Porcine Type I and Type II Interferons. Front. Microbiol. 2020, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Wimmers, F.; Donato, M.; Kuo, A.; Ashuach, T.; Gupta, S.; Li, C.; Dvorak, M.; Foecke, M.H.; Chang, S.E.; Hagan, T.; et al. The single-cell epigenomic and transcriptional landscape of immunity to influenza vaccination. Cell 2021, 184, 3915–3935. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Kotliar, D.; Lin, A.E.; Logue, J.; Hughes, T.K.; Khoury, N.M.; Raju, S.S.; Wadsworth, M.H., 2nd; Chen, H.; Kurtz, J.R.; Dighero-Kemp, B.; et al. Single-Cell Profiling of Ebola Virus Disease In Vivo Reveals Viral and Host Dynamics. Cell 2020, 183, 1383–1401.e19. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yang, B.; Yuan, X.; Hong, J.; Peng, M.; Chen, J.L.; Song, Z. Regulation and Evasion of Host Immune Response by African Swine Fever Virus. Front. Microbiol. 2021, 12, 698001. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudabadi, G.; Milo, R.; Phillips, R. Energetic cost of building a virus. Proc. Natl. Acad. Sci. USA 2017, 114, E4324–E4333. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, S.; Borrajo, A.; Pellegrino, M.; Svicher, V. Mechanisms underlying of antiretroviral drugs in different cellular reservoirs with a focus on macrophages. Virulence 2020, 11, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Sanchez-Cordon, P.J.; Galindo, I.; Alonso, C. Investigations of Pro- and Anti-Apoptotic Factors Affecting African Swine Fever Virus Replication and Pathogenesis. Viruses 2017, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lu, Z.; Liu, J.; Tao, Y.; Si, Y.; Ye, J.; Cao, S.; Zhu, B. Host Innate and Adaptive Immunity Against African Swine Fever Virus Infection. Vaccines 2024, 12, 1278. [Google Scholar] [CrossRef] [PubMed]
- Schafer, A.; Franzoni, G.; Netherton, C.L.; Hartmann, L.; Blome, S.; Blohm, U. Adaptive Cellular Immunity against African Swine Fever Virus Infections. Pathogens 2022, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Rajasekharan, S.; Kennedy, T.E. The netrin protein family. Genome Biol. 2009, 10, 239. [Google Scholar] [CrossRef]
- Schlegel, M.; Kohler, D.; Korner, A.; Granja, T.; Straub, A.; Giera, M.; Mirakaj, V. The neuroimmune guidance cue netrin-1 controls resolution programs and promotes liver regeneration. Hepatology 2016, 63, 1689–1705. [Google Scholar] [CrossRef] [PubMed]
- Ramkhelawon, B.; Hennessy, E.J.; Menager, M.; Ray, T.D.; Sheedy, F.J.; Hutchison, S.; Wanschel, A.; Oldebeken, S.; Geoffrion, M.; Spiro, W.; et al. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat. Med. 2014, 20, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Plissonnier, M.L.; Lahlali, T.; Michelet, M.; Lebossé, F.; Cottarel, J.; Beer, M.; Neveu, G.; Durantel, D.; Bartosch, B.; Accardi, R.; et al. Epidermal Growth Factor Receptor-Dependent Mutual Amplification between Netrin-1 and the Hepatitis C Virus. PLoS Biol. 2016, 14, e1002421. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Sun, S.; Liu, L.; Chen, Y.; Zheng, H.; Tang, Z. Single-Cell Atlas of Spleen Remodeling Reveals Macrophage Subset-Driven ASFV Pathogenesis. Biology 2025, 14, 882. https://doi.org/10.3390/biology14070882
Wang L, Sun S, Liu L, Chen Y, Zheng H, Tang Z. Single-Cell Atlas of Spleen Remodeling Reveals Macrophage Subset-Driven ASFV Pathogenesis. Biology. 2025; 14(7):882. https://doi.org/10.3390/biology14070882
Chicago/Turabian StyleWang, Liyuan, Shouzhang Sun, Lei Liu, Yun Chen, Haixue Zheng, and Zhonglin Tang. 2025. "Single-Cell Atlas of Spleen Remodeling Reveals Macrophage Subset-Driven ASFV Pathogenesis" Biology 14, no. 7: 882. https://doi.org/10.3390/biology14070882
APA StyleWang, L., Sun, S., Liu, L., Chen, Y., Zheng, H., & Tang, Z. (2025). Single-Cell Atlas of Spleen Remodeling Reveals Macrophage Subset-Driven ASFV Pathogenesis. Biology, 14(7), 882. https://doi.org/10.3390/biology14070882





