Sex Differences in Human Myogenesis Following Testosterone Exposure
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Treatments
2.2. RNA Extraction, Reverse Transcription, and Real-Time Quantitative PCR
2.3. Protein Expression Analysis
2.4. Cytokines Assay
2.5. Statistical Analysis
3. Results
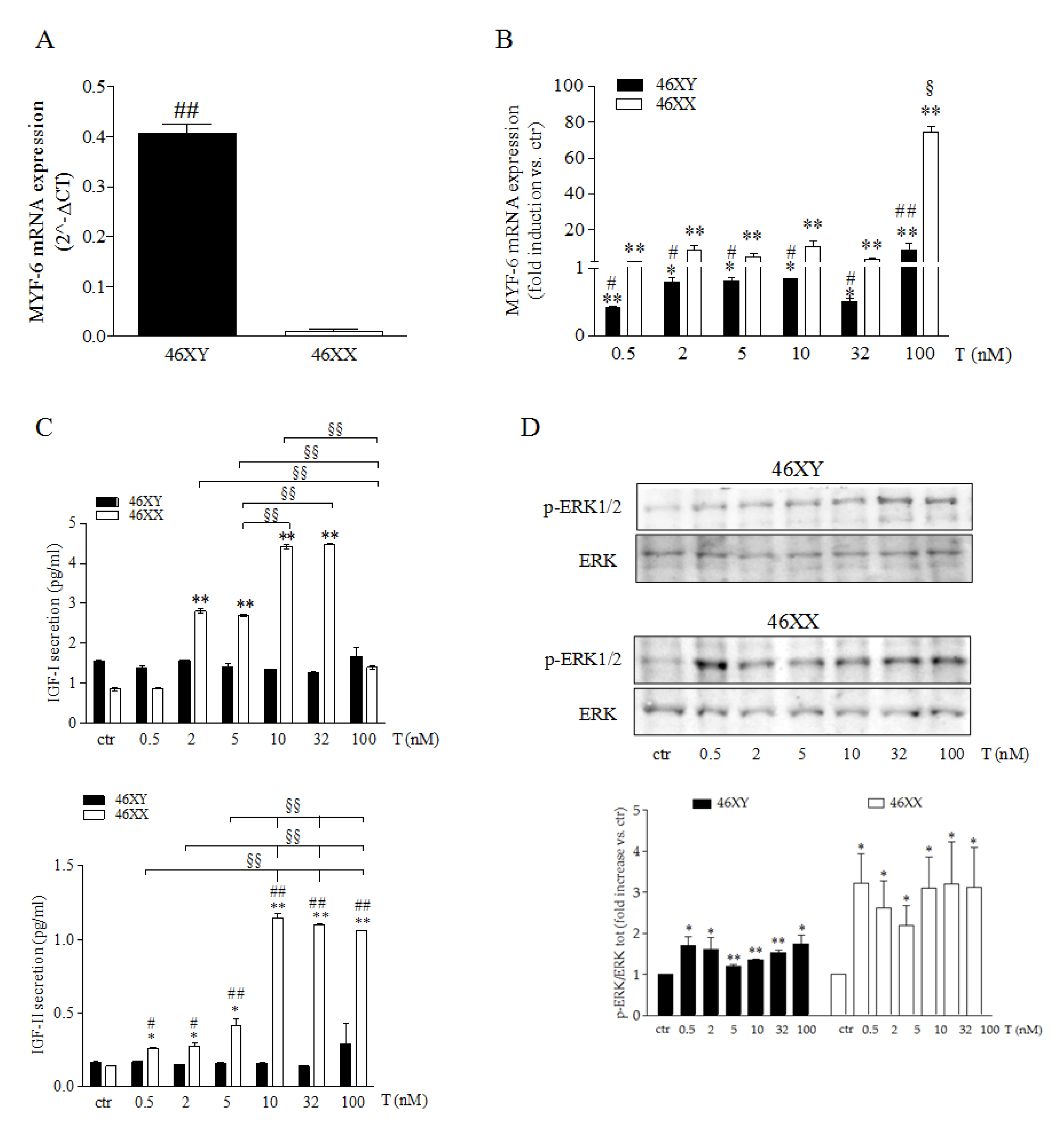
3.1. Testosterone Differently Modulates Myogenesis in Male and Female Human Skeletal Muscle Cells
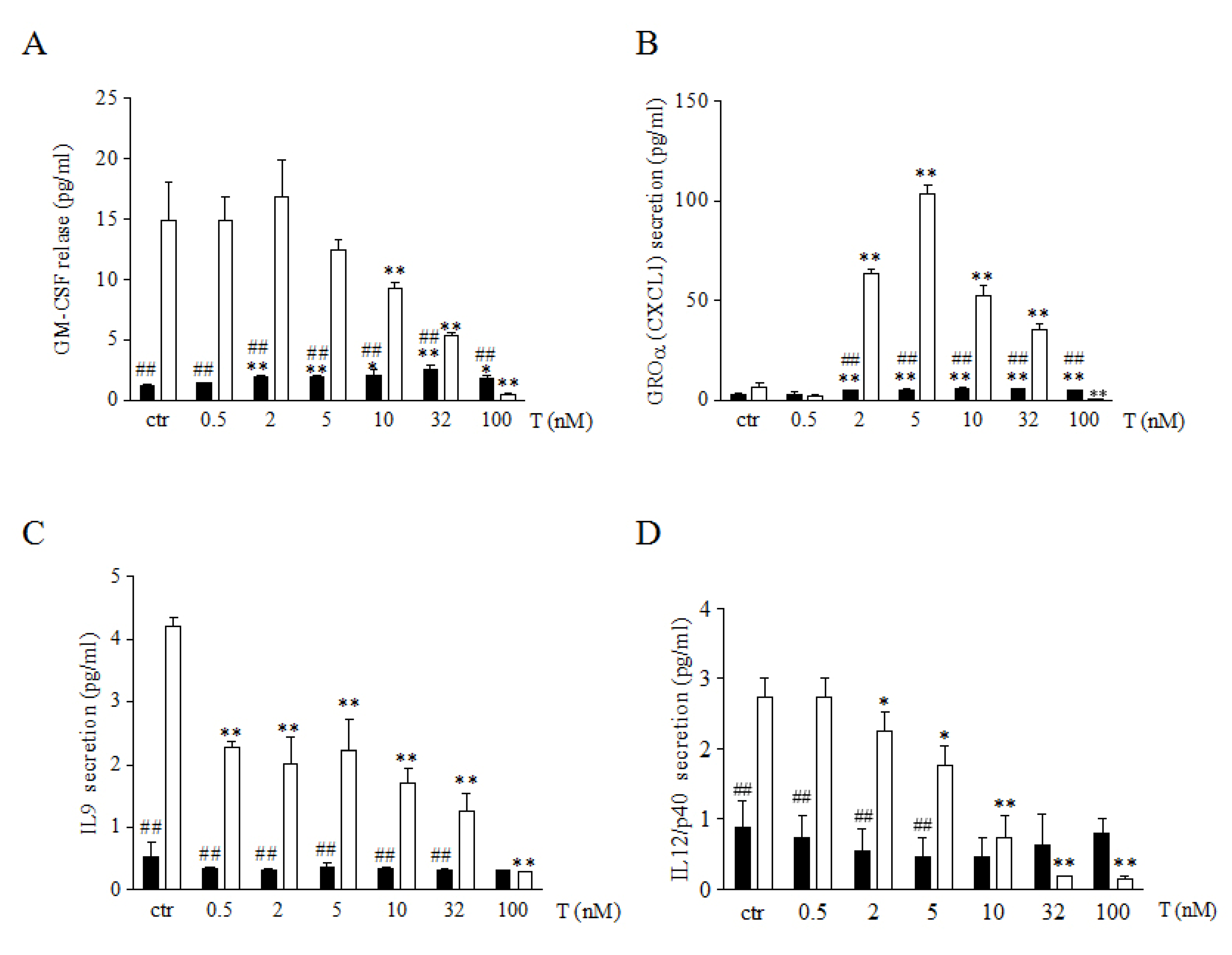
3.2. Testosterone Differently Modulates Myokine Release in Male and Female Human Skeletal Muscle Cells
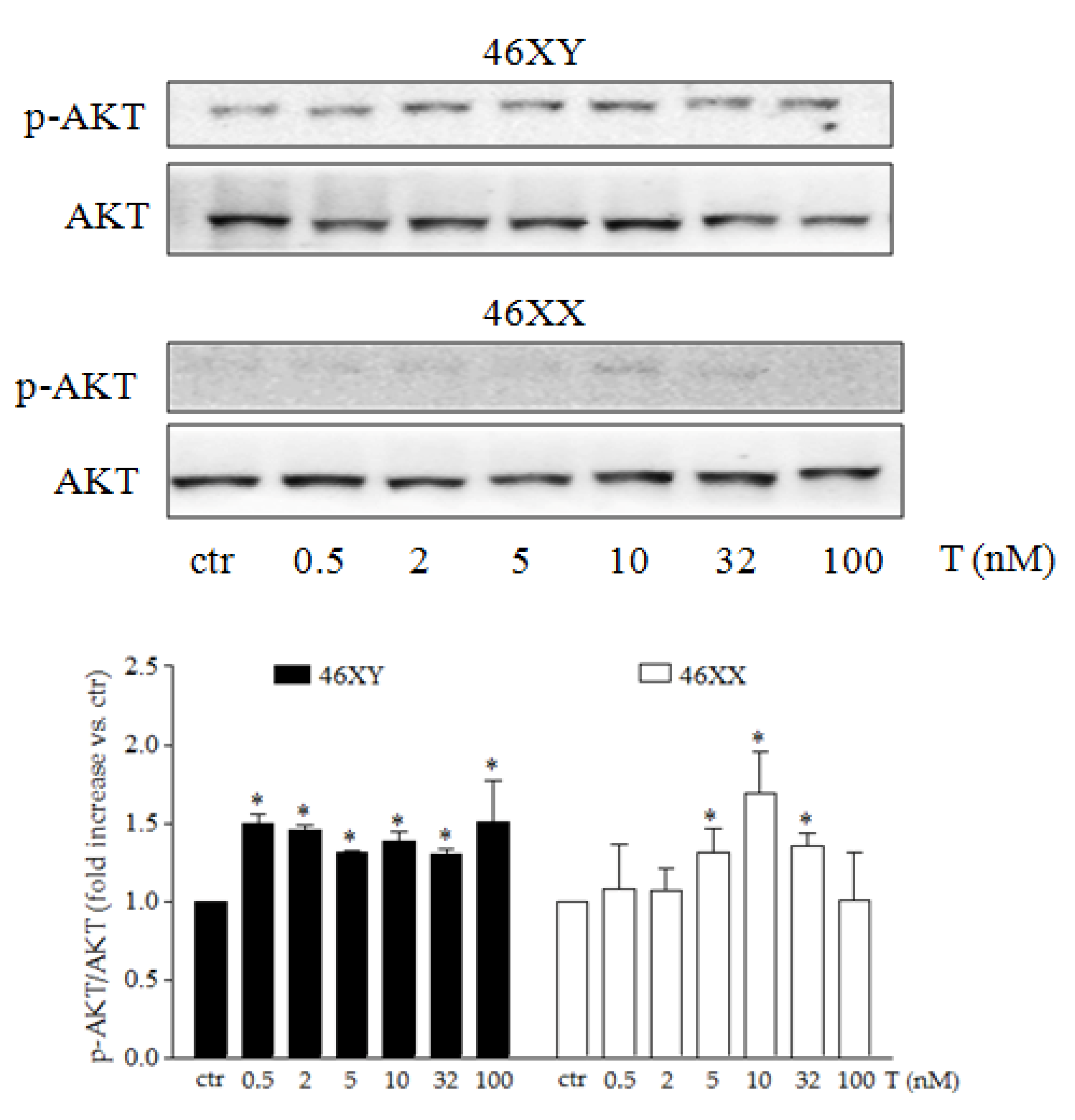
3.3. Testosterone Differently Modulates PI3K/AKT Activation Release in Male and Female Human Skeletal Muscle Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aizawa, K.; Iemitsu, M.; Otsuki, T.; Maeda, S.; Miyauchi, T.; Mesaki, N. Sex Differences in Steroidogenesis in Skeletal Muscle Following a Single Bout of Exercise in Rats. J. Appl. Physiol. 2008, 104, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Brameld, J.M.; Buttery, P.J.; Dawson, J.M.; Harper, J.M. Nutritional and Hormonal Control of Skeletal-Muscle Cell Growth and Differentiation. Proc. Nutr. Soc. 1998, 57, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef] [PubMed]
- DeWolfe, C.E.J.; Watt, M.C.; Romero-Sanchiz, P.; Stewart, S.H. Gender Differences in Physical Activity Are Partially Explained by Anxiety Sensitivity in Post-Secondary Students. J. Am. Coll. Health 2020, 68, 219–222. [Google Scholar] [CrossRef]
- Dominelli, P.B.; Molgat-Seon, Y. Sex, Gender and the Pulmonary Physiology of Exercise. Eur. Respir. Rev. 2022, 31, 210074. [Google Scholar] [CrossRef]
- Jones, B.A.; Haycraft, E.; Bouman, W.P.; Arcelus, J. The Levels and Predictors of Physical Activity Engagement Within the Treatment-Seeking Transgender Population: A Matched Control Study. J. Phys. Act. Health 2018, 15, 99–107. [Google Scholar] [CrossRef]
- Kim, Y.J.; Tamadon, A.; Park, H.T.; Kim, H.; Ku, S.-Y. The Role of Sex Steroid Hormones in the Pathophysiology and Treatment of Sarcopenia. Osteoporos. Sarcopenia 2016, 2, 140–155. [Google Scholar] [CrossRef]
- Manzano, R.; Toivonen, J.M.; Calvo, A.C.; Miana-Mena, F.J.; Zaragoza, P.; Muñoz, M.J.; Montarras, D.; Osta, R. Sex, Fiber-Type, and Age Dependent in Vitro Proliferation of Mouse Muscle Satellite Cells. J. Cell. Biochem. 2011, 112, 2825–2836. [Google Scholar] [CrossRef]
- Javed, A.A.; Mayhew, A.J.; Shea, A.K.; Raina, P. Association Between Hormone Therapy and Muscle Mass in Postmenopausal Women: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2019, 2, e1910154. [Google Scholar] [CrossRef]
- Di Luigi, L.; Antinozzi, C.; Duranti, G.; Dimauro, I.; Sgrò, P. Sex-Chromosome-Related Dimorphism in Steroidogenic Enzymes and Androgen Receptor in Response to Testosterone Treatment: An In Vitro Study on Human Primary Skeletal Muscle Cells. Int. J. Mol. Sci. 2023, 24, 17382. [Google Scholar] [CrossRef]
- Aizawa, K.; Iemitsu, M.; Maeda, S.; Jesmin, S.; Otsuki, T.; Mowa, C.N.; Miyauchi, T.; Mesaki, N. Expression of Steroidogenic Enzymes and Synthesis of Sex Steroid Hormones from DHEA in Skeletal Muscle of Rats. Am. J. Physiol.-Endocrinol. Metab. 2007, 292, E577–E584. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-T.; Weng, Z.-X.; Lin, J.D.; Meng, Z.-X. Myokines: Metabolic Regulation in Obesity and Type 2 Diabetes. Life Metab. 2024, 3, loae006. [Google Scholar] [CrossRef]
- Das, D.K.; Graham, Z.A.; Cardozo, C.P. Myokines in Skeletal Muscle Physiology and Metabolism: Recent Advances and Future Perspectives. Acta Physiol. 2020, 228, e13367. [Google Scholar] [CrossRef] [PubMed]
- Sgrò, P.; Antinozzi, C.; Wasson, C.W.; Del Galdo, F.; Dimauro, I.; Di Luigi, L. Sexual Dimorphism in Sex Hormone Metabolism in Human Skeletal Muscle Cells in Response to Different Testosterone Exposure. Biology 2024, 13, 796. [Google Scholar] [CrossRef]
- Handelsman, D.J.; Hirschberg, A.L.; Bermon, S. Circulating Testosterone as the Hormonal Basis of Sex Differences in Athletic Performance. Endocr. Rev. 2018, 39, 803–829. [Google Scholar] [CrossRef]
- Badiu, C. Degroot’s Endocrinology, 8th Edition. Acta Endocrinol. 2022, 18, 406. [Google Scholar] [CrossRef]
- Antinozzi, C.; Duranti, G.; Ceci, R.; Lista, M.; Sabatini, S.; Caporossi, D.; Di Luigi, L.; Sgrò, P.; Dimauro, I. Hydrogen Peroxide Stimulates Dihydrotestosterone Release in C2C12 Myotubes: A New Perspective for Exercise-Related Muscle Steroidogenesis? Int. J. Mol. Sci. 2022, 23, 6566. [Google Scholar] [CrossRef]
- Di Luigi, L.; Sgrò, P.; Duranti, G.; Sabatini, S.; Caporossi, D.; Del Galdo, F.; Dimauro, I.; Antinozzi, C. Sildenafil Reduces Expression and Release of IL-6 and IL-8 Induced by Reactive Oxygen Species in Systemic Sclerosis Fibroblasts. Int. J. Mol. Sci. 2020, 21, 3161. [Google Scholar] [CrossRef]
- Antinozzi, C.; Marampon, F.; Corinaldesi, C.; Vicini, E.; Sgrò, P.; Vannelli, G.B.; Lenzi, A.; Crescioli, C.; Di Luigi, L. Testosterone Insulin-like Effects: An in Vitro Study on the Short-Term Metabolic Effects of Testosterone in Human Skeletal Muscle Cells. J. Endocrinol. Investig. 2017, 40, 1133–1143. [Google Scholar] [CrossRef]
- Bhasin, S.; Woodhouse, L.; Storer, T.W. Proof of the Effect of Testosterone on Skeletal Muscle. J. Endocrinol. 2001, 170, 27–38. [Google Scholar] [CrossRef]
- Rizk, J.; Sahu, R.; Duteil, D. An Overview on Androgen-Mediated Actions in Skeletal Muscle and Adipose Tissue. Steroids 2023, 199, 109306. [Google Scholar] [CrossRef] [PubMed]
- Griggs, R.C.; Kingston, W.; Jozefowicz, R.F.; Herr, B.E.; Forbes, G.; Halliday, D. Effect of Testosterone on Muscle Mass and Muscle Protein Synthesis. J. Appl. Physiol. 1989, 66, 498–503. [Google Scholar] [CrossRef] [PubMed]
- La Colla, A.; Pronsato, L.; Milanesi, L.; Vasconsuelo, A. 17β-Estradiol and Testosterone in Sarcopenia: Role of Satellite Cells. Ageing Res. Rev. 2015, 24, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Serra, C.; Sandor, N.L.; Jang, H.; Lee, D.; Toraldo, G.; Guarneri, T.; Wong, S.; Zhang, A.; Guo, W.; Jasuja, R.; et al. The Effects of Testosterone Deprivation and Supplementation on Proteasomal and Autophagy Activity in the Skeletal Muscle of the Male Mouse: Differential Effects on High-Androgen Responder and Low-Androgen Responder Muscle Groups. Endocrinology 2013, 154, 4594–4606. [Google Scholar] [CrossRef]
- Sinha-Hikim, I.; Cornford, M.; Gaytan, H.; Lee, M.L.; Bhasin, S. Effects of Testosterone Supplementation on Skeletal Muscle Fiber Hypertrophy and Satellite Cells in Community-Dwelling Older Men. J. Clin. Endocrinol. Metab. 2006, 91, 3024–3033. [Google Scholar] [CrossRef]
- Egner, I.M.; Bruusgaard, J.C.; Eftestøl, E.; Gundersen, K. A Cellular Memory Mechanism Aids Overload Hypertrophy in Muscle Long after an Episodic Exposure to Anabolic Steroids. J. Physiol. 2013, 591, 6221–6230. [Google Scholar] [CrossRef]
- Englund, D.A.; Peck, B.D.; Murach, K.A.; Neal, A.C.; Caldwell, H.A.; McCarthy, J.J.; Peterson, C.A.; Dupont-Versteegden, E.E. Resident Muscle Stem Cells Are Not Required for Testosterone-Induced Skeletal Muscle Hypertrophy. Am. J. Physiol.-Cell Physiol. 2019, 317, C719–C724. [Google Scholar] [CrossRef]
- Noirez, P.; Ferry, A. Effect of Anabolic/Androgenic Steroids on Myosin Heavy Chain Expression in Hindlimb Muscles of Male Rats. Eur. J. Appl. Physiol. 2000, 81, 155–158. [Google Scholar] [CrossRef]
- Woodhouse, L.J.; Reisz-Porszasz, S.; Javanbakht, M.; Storer, T.W.; Lee, M.; Zerounian, H.; Bhasin, S. Development of Models to Predict Anabolic Response to Testosterone Administration in Healthy Young Men. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E1009–E1017. [Google Scholar] [CrossRef][Green Version]
- Lazure, F.; Blackburn, D.M.; Corchado, A.H.; Sahinyan, K.; Karam, N.; Sharanek, A.; Nguyen, D.; Lepper, C.; Najafabadi, H.S.; Perkins, T.J.; et al. Myf6/MRF4 Is a Myogenic Niche Regulator Required for the Maintenance of the Muscle Stem Cell Pool. EMBO Rep. 2020, 21, e49499. [Google Scholar] [CrossRef]
- Velders, M.; Diel, P. How Sex Hormones Promote Skeletal Muscle Regeneration. Sports Med. 2013, 43, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Abou-Khalil, R.; Le Grand, F.; Pallafacchina, G.; Valable, S.; Authier, F.-J.; Rudnicki, M.A.; Gherardi, R.K.; Germain, S.; Chretien, F.; Sotiropoulos, A.; et al. Autocrine and Paracrine Angiopoietin 1/Tie-2 Signaling Promotes Muscle Satellite Cell Self-Renewal. Cell Stem Cell 2009, 5, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Hindi, S.M.; Kumar, A. TRAF6 Regulates Satellite Stem Cell Self-Renewal and Function during Regenerative Myogenesis. J. Clin. Investig. 2015, 126, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Ogura, Y.; Tajrishi, M.M.; Kumar, A. Elevated Levels of TWEAK in Skeletal Muscle Promote Visceral Obesity, Insulin Resistance, and Metabolic Dysfunction. FASEB J. 2015, 29, 988–1002. [Google Scholar] [CrossRef]
- Jiménez-Amilburu, V.; Salmerón, C.; Codina, M.; Navarro, I.; Capilla, E.; Gutiérrez, J. Insulin-like Growth Factors Effects on the Expression of Myogenic Regulatory Factors in Gilthead Sea Bream Muscle Cells. Gen. Comp. Endocrinol. 2013, 188, 151–158. [Google Scholar] [CrossRef]
- Sinha-Hikim, I.; Artaza, J.; Woodhouse, L.; Gonzalez-Cadavid, N.; Singh, A.B.; Lee, M.I.; Storer, T.W.; Casaburi, R.; Shen, R.; Bhasin, S. Testosterone-Induced Increase in Muscle Size in Healthy Young Men Is Associated with Muscle Fiber Hypertrophy. Am. J. Physiol.-Endocrinol. Metab. 2002, 283, E154–E164. [Google Scholar] [CrossRef]
- Jiao, S.; Ren, H.; Li, Y.; Zhou, J.; Duan, C.; Lu, L. Differential Regulation of IGF-I and IGF-II Gene Expression in Skeletal Muscle Cells. Mol. Cell. Biochem. 2013, 373, 107–113. [Google Scholar] [CrossRef]
- Machida, S.; Booth, F.W. Insulin-like Growth Factor 1 and Muscle Growth: Implication for Satellite Cell Proliferation. Proc. Nutr. Soc. 2004, 63, 337–340. [Google Scholar] [CrossRef]
- Korbecki, J.; Kupnicka, P.; Barczak, K.; Bosiacki, M.; Ziętek, P.; Chlubek, D.; Baranowska-Bosiacka, I. The Role of CXCR1, CXCR2, CXCR3, CXCR5, and CXCR6 Ligands in Molecular Cancer Processes and Clinical Aspects of Acute Myeloid Leukemia (AML). Cancers 2023, 15, 4555. [Google Scholar] [CrossRef]
- Masuda, S.; Tanaka, M.; Inoue, T.; Ohue-Kitano, R.; Yamakage, H.; Muranaka, K.; Kusakabe, T.; Shimatsu, A.; Hasegawa, K.; Satoh-Asahara, N. Chemokine (C-X-C Motif) Ligand 1 Is a Myokine Induced by Palmitate and Is Required for Myogenesis in Mouse Satellite Cells. Acta Physiol. 2018, 222, e12975. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, L.; Zhao, Z.; Lu, W.; Fan, J.; Gao, B.; Luo, Z.; Jie, Q.; Shi, X.; Yang, L. Cytokines CCL2 and CXCL1 May Be Potential Novel Predictors of Early Bone Loss. Mol. Med. Rep. 2020, 22, 4716–4724. [Google Scholar] [CrossRef] [PubMed]
- Christianto, A.; Baba, T.; Takahashi, F.; Inui, K.; Inoue, M.; Suyama, M.; Ono, Y.; Ohkawa, Y.; Morohashi, K. Sex Differences in Metabolic Pathways Are Regulated by Pfkfb3 and Pdk4 Expression in Rodent Muscle. Commun. Biol. 2021, 4, 1264. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Laurent, M.; Boonen, S.; Vanderschueren, D.; Claessens, F. Androgens and Skeletal Muscle: Cellular and Molecular Action Mechanisms Underlying the Anabolic Actions. Cell. Mol. Life Sci. 2012, 69, 1651–1667. [Google Scholar] [CrossRef] [PubMed]
- Schaiter, A.; Hentschel, A.; Kleefeld, F.; Schuld, J.; Umathum, V.; Procida-Kowalski, T.; Nelke, C.; Roth, A.; Hahn, A.; Krämer, H.H.; et al. Molecular Composition of Skeletal Muscle in Infants and Adults: A Comparative Proteomic and Transcriptomic Study. Sci. Rep. 2024, 14, 22965. [Google Scholar] [CrossRef]

| Gene Name | Forward 5′–3′ | Reverse 5′–3′ |
|---|---|---|
| MYF-6 | GAAGATCCCACCGACCCTCCTGGC | GAGGCTAGACCTAAGCCACTCGCA |
| Β-ACTIN | AAC CTGAACCCCAAGGCC | AGCCTGGATAGCAACGTACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgrò, P.; Antinozzi, C.; Duranti, G.; Dimauro, I.; Radak, Z.; Di Luigi, L. Sex Differences in Human Myogenesis Following Testosterone Exposure. Biology 2025, 14, 855. https://doi.org/10.3390/biology14070855
Sgrò P, Antinozzi C, Duranti G, Dimauro I, Radak Z, Di Luigi L. Sex Differences in Human Myogenesis Following Testosterone Exposure. Biology. 2025; 14(7):855. https://doi.org/10.3390/biology14070855
Chicago/Turabian StyleSgrò, Paolo, Cristina Antinozzi, Guglielmo Duranti, Ivan Dimauro, Zsolt Radak, and Luigi Di Luigi. 2025. "Sex Differences in Human Myogenesis Following Testosterone Exposure" Biology 14, no. 7: 855. https://doi.org/10.3390/biology14070855
APA StyleSgrò, P., Antinozzi, C., Duranti, G., Dimauro, I., Radak, Z., & Di Luigi, L. (2025). Sex Differences in Human Myogenesis Following Testosterone Exposure. Biology, 14(7), 855. https://doi.org/10.3390/biology14070855










