Ecological Effects of Sargassum fusiforme Cultivation on Coastal Phytoplankton Community Structure and Water Quality: A Study Based on Microscopic Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.1.1. Sampling Site Selection
2.1.2. Sample Collection and Determination of Physical and Chemical Parameters
2.2. Phytoplankton Sample Analysis
2.3. Data Processing and Analysis
2.4. Reference Information on High-Throughput Sequencing
3. Results
3.1. Water Quality
3.1.1. April 2018 (Cultivation)
3.1.2. June 2018 (Non-Cultivation)
3.1.3. April 2019 (Cultivation)
3.1.4. June 2019 (Non-Cultivation)
3.2. Phytoplankton Species
3.3. Phytoplankton Abundance
3.4. Dominant Phytoplankton Species
3.5. Phytoplankton Diversity
3.6. Similarity Analysis of Community Composition
3.7. Relationship Between Dominant Phytoplankton Species and Environmental Factors
4. Discussion
4.1. S. fusiforme Cultivation Improves Water Quality
4.2. Impact of S. fusiforme Cultivation on Phytoplankton Community
4.3. Comparison of Microscopic Observation and High-Throughput Sequencing in Analyzing Phytoplankton Communities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, E.K.; Park, C.S. Seaweed cultivation and utilization of Korea. Algae 2020, 35, 107–121. [Google Scholar] [CrossRef]
- Zhang, C.; McIntosh, K.D.; Sienkiewicz, N.; Stelzer, E.A.; Graham, J.L.; Lu, J. Using cyanobacteria and other phytoplankton to assess trophic conditions: A qPCR-based, multi-year study in twelve large rivers across the United States. Water Res. 2023, 235, 119679. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 2013, 3, 961–968. [Google Scholar] [CrossRef]
- Costanza, R.; De Groot, R.; Sutton, P.; Van der Ploeg, S.; Anderson, S.J.; Kubiszewski, I.; Farber, S.; Turner, R.K. Changes in the global value of ecosystem services. Environ. Chang. 2014, 26, 152–158. [Google Scholar] [CrossRef]
- Radulovich, R.; Neori, A.; Valderrama, D.; Forster, J. Farming of seaweeds. In Seaweed Sustainability: Food and Non-Food Applications; Tiwari, B.K., Troy, D.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 27–59. [Google Scholar]
- Sondak, C.F.A.; Ang, P.O., Jr.; Beardall, J.; Bellgrove, A.; Boo, S.M.; Gerung, G.S.; Hepburn, C.D.; Hong, D.D.; Hu, Z.; Kawai, H.; et al. Carbon dioxide mitigation potential of seaweed aquaculture beds (SABs). J. Appl. Phycol. 2017, 29, 2363–2373. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Wernberg, T. Rise of turfs: A new battlefront for globally declining kelp forests. Bioscience 2018, 68, 64–76. [Google Scholar] [CrossRef]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Marilaure, G.; Zhang, J. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef]
- Kim, J.H.; Moon, H.; Han, M.J.; Ji, E.J.; Na, Y.L.; Jin, W.K.; Ji, C.O.; Park, G.H.; Lee, S.E.; Lee, M.H. The photosynthetic uptake of inorganic carbon from Pyropia seaweed aquaculture beds: Scaling up population-level estimations. Aquaculture 2024, 593, 741293. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, D.; Li, H.; Li, Z. Evaluation of the correlation of Sargassum fusiforme cultivation and biodiversity and network structure of marine bacteria in the coastal waters of Dongtou Island of China. Aquaculture 2021, 544, 737057. [Google Scholar] [CrossRef]
- Dongtou District Local Chronicles Compilation Committee; Dongtou District Local Chronicles Research Office. Dongtou Yearbook (2019); Huanding, Z., Ed.; Thread-Bound Book Publishing House: Beijing, China, 2019. [Google Scholar]
- Liu, J.; Ling, S.Y.; Chen, Q.J.; Shen, Y.Z.; Zhang, J. Different extraction methods bring about distinct physicochemical properties and antioxidant activities of Sargassum fusiforme fucoidans. Int. J. Biol. Macromol. 2020, 155, 1385–1392. [Google Scholar] [CrossRef]
- Mao, W.; Li, B.; Gu, Q.; Fang, Y.; Xing, H. Preliminary studies on the chemical characterization and antihyperlipidemic activity of polysaccharide from the brown alga Sargassum fusiforme. Hydrobiologia 2004, 512, 263–266. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, W.; Lu, J.; Yu, Y.; Wu, B. Analysis of the monosaccharide composition of water-soluble polysaccharides from Sargassum fusiforme by high performance liquid chromatography/electrospray ionisation mass spectrometry. Food Chem. 2014, 145, 976–983. [Google Scholar] [CrossRef]
- Saetan, U.; Nontasak, P.; Palasin, K.; Saelim, H.; Wonglapsuwan, M.; Mayakun, J.; Pongparadon, S.; Chotigeat, W. Potential health benefits of fucoidan from the brown seaweeds Sargassum plagiophyllum and Sargassum polycystum. J. Appl. Phycol. 2021, 33, 3357–3364. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, M.; Lin, L.; Thring, R.W.; Yu, H.; Zhang, X.; Zhao, M. Allelopathic interactions between the macroalga Hizikia fusiformis (Harvey) and the harmful blooms-forming dinoflagellate Karenia mikimotoi. Harmful Algae 2017, 65, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, N.; Li, Y. Effect of the extracts of Sargassum fusiforme on red tide microalgae in East China Sea. Front. Mar. Sci. 2021, 8, 628095. [Google Scholar] [CrossRef]
- Sun, S.; Hu, S.; Zhang, B.; Sun, X.; Xu, N. Allelopathic effects and potential allelochemical of Sargassum fusiforme on red tide microalga Heterosigma akashiwo. Mar. Pollut. Bull. 2021, 170, 112673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, J.; Liu, J. Study review of Hizikia fusiformis. Mar. Fish. Res. 2002, 23, 67–74. [Google Scholar]
- Falkowski, P.G. Ocean science: The power of plankton. Nature 2012, 483, S17–S20. [Google Scholar] [CrossRef]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef]
- Carstensen, J.; Klais, R.; Cloern, J.E. Phytoplankton blooms in estuarine and coastal waters: Seasonal patterns and key species. Estuar. Coast. Shelf Sci. 2015, 162, 98–109. [Google Scholar] [CrossRef]
- Pohnert, G.; Poulin, R.X.; Baumeister, H.; Tim, U. The making of a plankton toxin. Science 2018, 361, 1308–1309. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Yarish, C.; Hwang, E.K.; Park, M.; Kim, Y. The role of seaweed aquaculture: Cultivation technologies, challenges and its ecosystem services. Algae 2017, 32, 1–13. [Google Scholar] [CrossRef]
- Parsons, M.L.; Brandt, A.L.; Turner, R.E.; Morrison, W.L.; Ralabais, N.N. Characterization of common phytoplankton on the Louisiana shelf. Mar. Pollut. Bull. 2021, 168, 112458. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, L.; Li, Y.; Lin, Q.; He, C.; Huang, S.; Li, H.; Zhang, X.; Liu, B.; Ge, F.; et al. The changing characteristics of phytoplankton community and biomass in subtropical shallow lakes: Coupling effects of land use patterns and lake morphology. Water Res. 2021, 200, 117235. [Google Scholar] [CrossRef]
- Shan, K.; Song, L.; Chen, W.; Li, L.; Liu, L.; Wu, Y.; Jia, Y.; Zhou, Q.; Peng, L. Analysis of environmental drivers influencing interspecific variations and associations among bloom-forming cyanobacteria in large, shallow eutrophic lakes. Harmful Algae 2019, 84, 84–94. [Google Scholar] [CrossRef]
- Huo, S.; Li, X.; Zhang, H.; Ma, C.; He, Z. Combining morphological and metabarcoding approaches reveals the freshwater eukaryotic phytoplankton community. Environ. Sci. Eur. 2020, 32, 37. [Google Scholar] [CrossRef]
- Navas-Parejo, J.C.C.; Corzo, A.; Paspati, S. Seasonal cycles of phytoplankton biomass and primary production in a tropical temporarily open-closed estuarine lagoon—The effect of an extreme climatic event. Sci. Total Environ. 2020, 723, 138014. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ning, D. Stochastic Community Assembly: Does It Matter in Microbial Ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervollkommnung der Quantitativen Phytoplankton-Methodik. Mitt. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, D.; Li, T.; Qiao, L.; Xu, N. Effects of large-scale Sargassum fusiforme culture on phytoplankton community structure and water quality. Front. Mar. Sci. 2022, 9, 907726. [Google Scholar] [CrossRef]
- Chai, Z.Y.; He, Z.L.; Deng, Y.Y.; Yang, Y.F.; Tang, Y.Z. Cultivation of seaweed Gracilaria lemaneiformis enhanced biodiversity in a eukaryotic plankton community as revealed via metagenomic analyses. Mol. Ecol. 2018, 27, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- HJ 442.3-2008; Technical Specification for Offshore Environmental Monitoring—Part 3: Offshore Seawater Quality Monitoring. Ministry of Ecology and Environment of the People’s Republic of China. China Environmental Science Press: Beijing, China, 2008.
- GB/T 12763.6–2007; Specification for Oceanographic Survey—Part 6: Marine Hydrological Observation. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China; Standardization Administration of China. Standards Press of China: Beijing, China, 2008.
- Guo, Y. Flora Algarum Marinarum Sinicarum, Vol. 5: Bacillariophyta; Science Press: Beijing, China, 2003. [Google Scholar]
- Lin, Y. Flora Algarum Marinarum Sinicarum, Vol. 6, Dinophyta, Fascicle 1: Dinophyceae—Ceratiaceae; Science Press: Beijing, China, 2009. [Google Scholar]
- Hu, J.; Yang, Y.; Chi, S.; Shen, Q.; Hu, J. Spatial variation of phytoplankton community structure and its relationship with environmental factors at the Mangshan pumping station. Acta Ecol. Sin. 2017, 37, 1054–1062. [Google Scholar][Green Version]
- Ye, L.A.; Wang, L.B.; Jiang, Z.F.; Li, Y.X.; Li, R.X. Nutrient Distributions in the East China Sea and Changes over the Last 25 Years. Appl. Ecol. Environ. Res. 2020, 18, 973–985. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful Algal Blooms and Eutrophication: Nutrient Sources, Composition, and Consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Howarth, R.W. Coastal nitrogen pollution: A review of sources and trends globally and regionally. Harmful Algae 2008, 8, 14–20. [Google Scholar] [CrossRef]
- Gillanders, B.M.; Kingsford, M.J. Impact of changes in flow of freshwater on estuarine and open coastal habitats and the associated organisms. Oceanogr. Mar. Biol. Annu. Rev. 2002, 40, 233–309. [Google Scholar]
- Smayda, T.J. Complexity in the Eutrophication-Harmful Algal Bloom Relationship, with Comment on the Importance of Grazing. Harmful Algae. 2008, 8, 140–151. [Google Scholar] [CrossRef]
- Shang, T.; Lin, L.; Chen, B.; Wang, M.; Qin, W.; Dai, C.; Yu, H.; Li, J.; Thring, R.W.; Ma, Z.; et al. Cell density-dependent suppression on the development and photosynthetic activities of Sargassum fusiforme embryos by dinoflagellate Karenia mikimotoi. Harmful Algae 2020, 96, 101842. [Google Scholar] [CrossRef]
- Tian, S.; Chen, B.; Wu, M.; Cao, C.; Gu, Z.; Zheng, T.; Zou, D.; Ma, Z. Are There Environmental Benefits Derived from Coastal Aquaculture of Sargassum fusiforme? Aquaculture 2023, 563, 738909. [Google Scholar] [CrossRef]
- Gu, H.; Sun, J.; Kooistra, W.H.C.F.; Zeng, R.; Zeng, N. Phylogenetic Position and Morphology of Thecae and Cysts of Scrippsiella (Dinophyceae) Species in the East China Sea. Phycologia 2008, 47, 615–625. [Google Scholar]
- Lin, S.J.; Ji, N.J.; Luo, H. Recent Progress in Marine Harmful Algal Bloom Research. Oceanol. Limnol. Sin. 2019, 50, 477–489. [Google Scholar]
- Paerl, H.W.; Huisman, J. Climate Change: A Catalyst for Global Expansion of Harmful Cyanobacterial Blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.O.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful Algal Blooms and Climate Change: Learning from the Past and Present to Forecast the Future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef]
- Dzhembekova, N.; Rubino, F.; Belmonte, M.; Zlateva, I.; Slabakova, N.; Ivanova, P.; Slabakova, V.; Nagai, S.; Moncheva, S. Distribution of Different Scrippsiella acuminata (Dinophyta) Cyst Morphotypes in Surface Sediments of the Black Sea: A Basin Scale Approach. Front. Mar. Sci. 2022, 9, 864214. [Google Scholar] [CrossRef]
- Rubino, F.; Saracino, O.D.; Moscatello, S.; Belmonte, G. An integrated water/sediment approach to study plankton (a case study in the southern Adriatic Sea). J. Mar. Syst. 2009, 78, 536–546. [Google Scholar] [CrossRef]
- National Climate Center. El Niño Has Occurred and Is Expected to Persist Until Spring [EB/OL]; China Meteorological Administration: Beijing, China, 2019.
- Li, H.; Xu, A.N.; Yang, Z.; Lei, Y.; Chen, J.; Zhan, Z.; Xu, K.; Li, T.; Xie, S. Species Diversity and Ecological Distribution of Phytoplankton in the Nanji Islands Marine Nature Reserve. Oceanol. Limnol. Sin. 2010, 41, 215–221. [Google Scholar]
- Wenzhou Municipal People’s Government. Bulletin on the Ecological Status of Wenzhou’s Coastal Waters (2017) [R]; Wenzhou Municipal People’s Government: Wenzhou, China, 2019. (In Chinese)
- Guo, S.; Feng, Y.; Wang, L.; Dai, M.; Liu, Z.; Bai, Y.; Sun, J. Seasonal Variation in the Phytoplankton Community of a Continental-Shelf Sea: The East China Sea. J. Mar. Syst. 2014, 135, 122–132. [Google Scholar] [CrossRef]
- Shen, A.; Su, S.; Li, H.; Kang, W.; Jia, R. Spatio-Temporal Variability of Surface Phytoplankton Community Structure in Relation to Different Water Systems in the East China Sea Coast. Estuar. Coast. Shelf Sci. 2023, 287, 108368. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and Ecosystem Functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Hautier, Y.; Tilman, D.; Isbell, F.; Seabloom, E.W.; Borer, E.T.; Reich, P.B. Anthropogenic Environmental Changes Affect Ecosystem Stability via Biodiversity. Science 2015, 348, 336–340. [Google Scholar] [CrossRef]
- Isbell, F.; Craven, D.; Connolly, J.; Loreau, M.; Schmid, B.; Beierkuhnlein, C.; Bezemer, T.M.; Bonin, C.; Bruelheide, H.; de Luca, E.; et al. Biodiversity Increases the Resistance of Ecosystem Productivity to Climate Extremes. Nature 2015, 526, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity Loss and Its Impact on Humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.B.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.L.; Petchey, O.L.; et al. Biodiversity and Resilience of Ecosystem Functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef]
- Sylvers, P.H.; Gobler, C.J. Mitigation of Harmful Algal Blooms Caused by Alexandrium catenella and Reduction in Saxitoxin Accumulation in Bivalves Using Cultivable Seaweeds. J. Appl. Phycol. 2021, 33, 2345–2358. [Google Scholar] [CrossRef]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in Understanding Harmful Algal Blooms: Paradigm Shifts and New Technologies for Research, Monitoring, and Management. Annu. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef]
- Reguera, B.; Riobó, P.; Rodríguez, F.; Díaz, P.A.; Pizarro, G.; Paz, B. Dinophysis Toxins: Causative Organisms, Distribution and Fate in Shellfish. Mar. Drugs 2014, 12, 394–461. [Google Scholar] [CrossRef]
- Egge, J.K.; Aksnes, D.L. Silicate as regulating nutrient in phytoplankton competition. Mar. Ecol. Prog. Ser. 1992, 83, 281–289. [Google Scholar] [CrossRef]
- Song, L.; Wu, J. High-Throughput Sequencing and Comparative Analysis of Eukaryotic Phytoplankton Community in Liaodong Bay, China. Reg. Stud. Mar. Sci. 2020, 40, 10150. [Google Scholar] [CrossRef]
- Foulon, E.; Grulois, D.; Siano, R.; Worden, A.Z.; Derelle, E.; de Vargas, C. Revision of the Genus Micromonas Manton et Parke (Chlorophyta, Mamiellophyceae), of the Type Species M. pusilla (Butcher) Manton & Parke and of the Species M. commoda van Baren, Bachy and Worden and Description of Two New Species Based on the Genetic and Phenotypic Characterization of Cultured Isolates. Protist 2017, 168, 612–635. [Google Scholar]
- Sieburth, J.M.; Johnson, P.W.; Hargraves, P.E. Ultrastructure and Ecology of Aureococcus anophagefferens gen. et sp. nov. (Chrysophyceae), the Dominant Picoplankter During a Bloom in Narragansett Bay, Rhode Island, Summer. J. Phycol. 2017, 24, 416–425. [Google Scholar] [CrossRef]
- Lima-Mendez, G.; Faust, K.; Henry, N.; Decelle, J.; Colin, S.; Carcillo, F.; Chaffron, S.; Ignacio-Espinosa, J.C.; Roux, S.; Vincent, F.; et al. Determinants of Community Structure in the Global Plankton Interactome. Science 2015, 348, 1262073. [Google Scholar] [CrossRef]
- de Vargas, C.; Audic, S.; Henry, N.; Decelle, J.; Mahé, F.; Logares, R.; Lara, E.; Berney, C.; Le Bescot, N.; Probert, I.; et al. Eukaryotic Plankton Diversity in the Sunlit Ocean. Science 2015, 348, 1261605. [Google Scholar] [CrossRef] [PubMed]
- Giner, C.R.; Forn, I.; Romac, S.; Logares, R.; de Vargas, C.; Massana, R. Environmental Sequencing Provides Reasonable Estimates of the Relative Abundance of Specific Taxa in Marine Protist Communities. Environ. Microbiol. 2016, 18, 2759–2770. [Google Scholar]
- Piredda, R.; Tomasino, M.P.; DErchia, A.M.; Manzari, C.; Zingone, A. Diversity and Temporal Patterns of Planktonic Protist Assemblages at a Mediterranean Long Term Ecological Research Site. FEMS Microbiol. Ecol. 2017, 93, fiw200. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Chang, Z.; Li, J.; Chen, Z. Phytoplankton community succession in relation to water quality changes in the indoor industrial aquaculture system for Litopenaeus vannamei. Aquaculture 2020, 527, 735441. [Google Scholar] [CrossRef]
- Reeder, J.; Knight, R. Rapidly denoising pyrosequencing amplicon reads by exploiting rank-abundance distributions. Nat. Methods 2010, 7, 668–669. [Google Scholar] [CrossRef]
- Evans, N.T.; Olds, B.P.; Renshaw, M.A.; Turner, C.R.; Li, Y.; Jerde, C.L.; Mahon, A.R.; Pfrender, M.E.; Lamberti, G.A.; Lodge, D.M. Quantification of Mesocosm Fish and Amphibian Species Diversity via eDNA Metabarcoding. Mol. Ecol. Resour. 2016, 16, 29–41. [Google Scholar] [CrossRef]
- Alberdi, A.; Gilbert, M.T.P. A Guide to the Application of Hill Numbers to DNA-Based Diversity Analyses. Mol. Ecol. Resour. 2019, 19, 804–817. [Google Scholar] [CrossRef]
- Creer, S.; Deiner, K.; Frey, S.; Porazinska, D.; Taberlet, P.; Thomas, W.K.; Potter, C.; Bik, H.M. The Ecologist’s Field Guide to Sequence-Based Identification of Biodiversity. Methods Ecol. Evol. 2016, 7, 1008–1018. [Google Scholar] [CrossRef]
- Pedrós-Alió, C. The Rare Bacterial Biosphere. Annu. Rev. Mar. Sci. 2012, 4, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Behrenfeld, M.J.; Boss, E.S. Student’s Tutorial on Bloom Hypotheses in the Context of Phytoplankton Annual Cycles. Glob. Change Biol. 2018, 24, 55–77. [Google Scholar] [CrossRef] [PubMed]
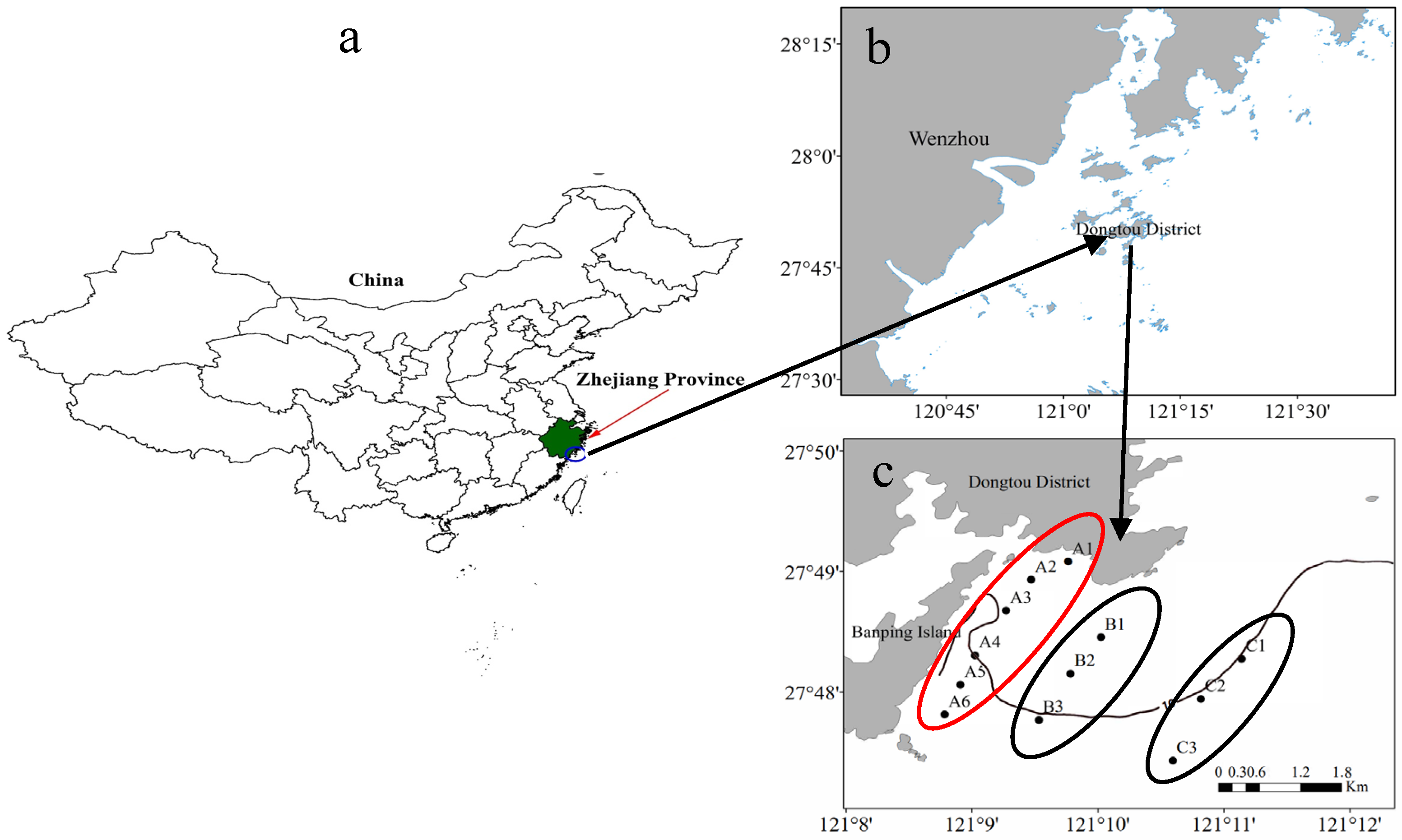

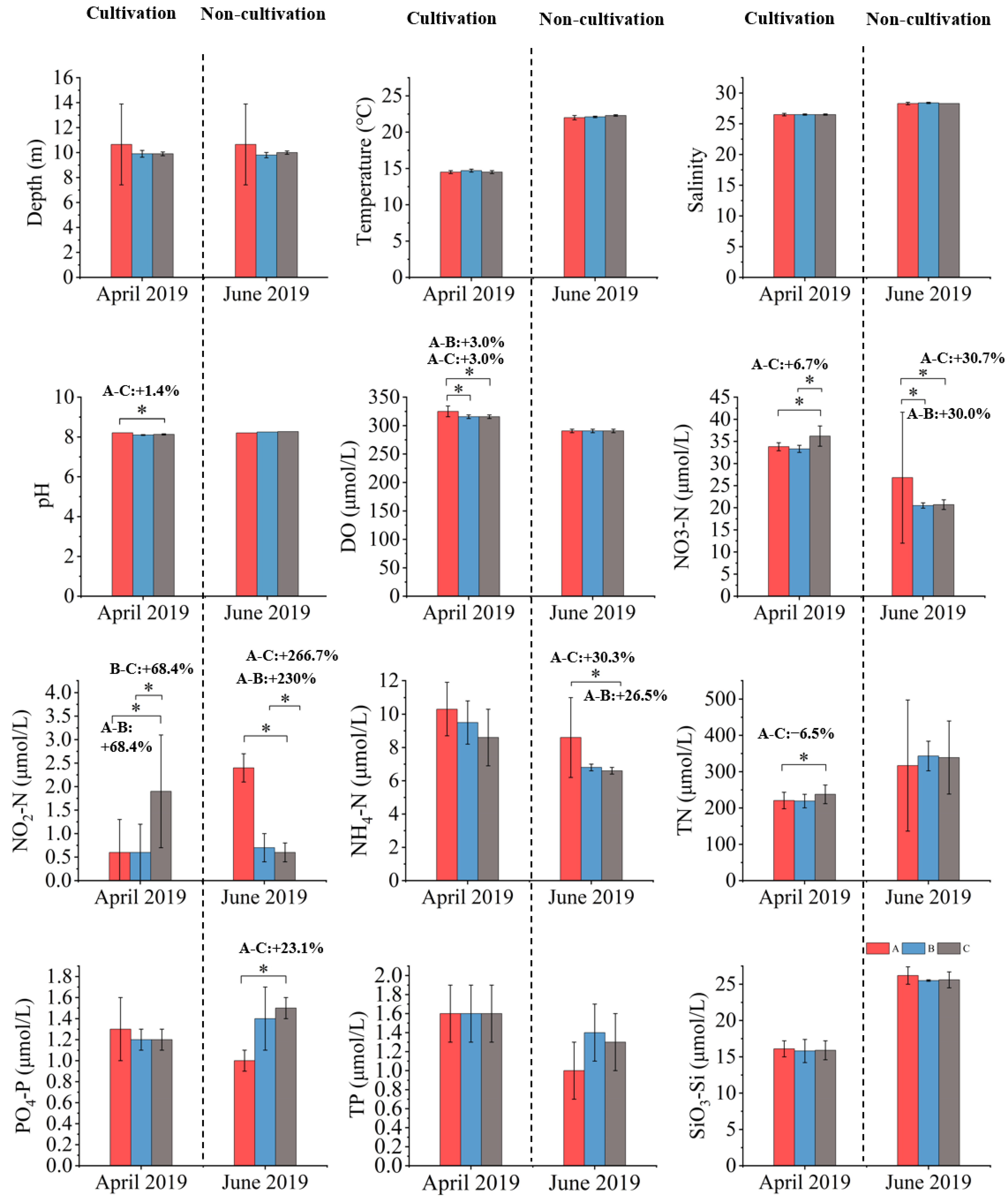



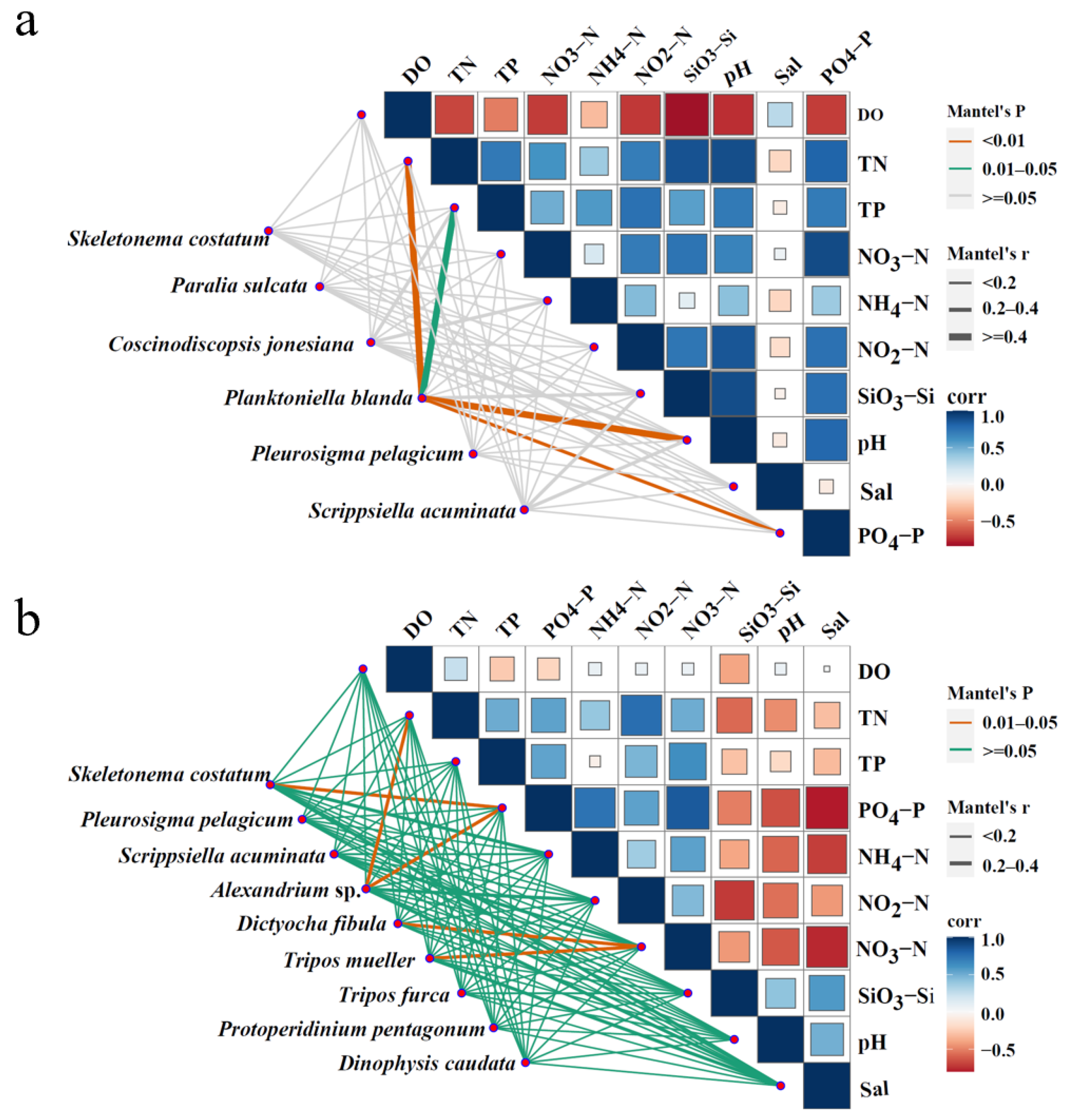
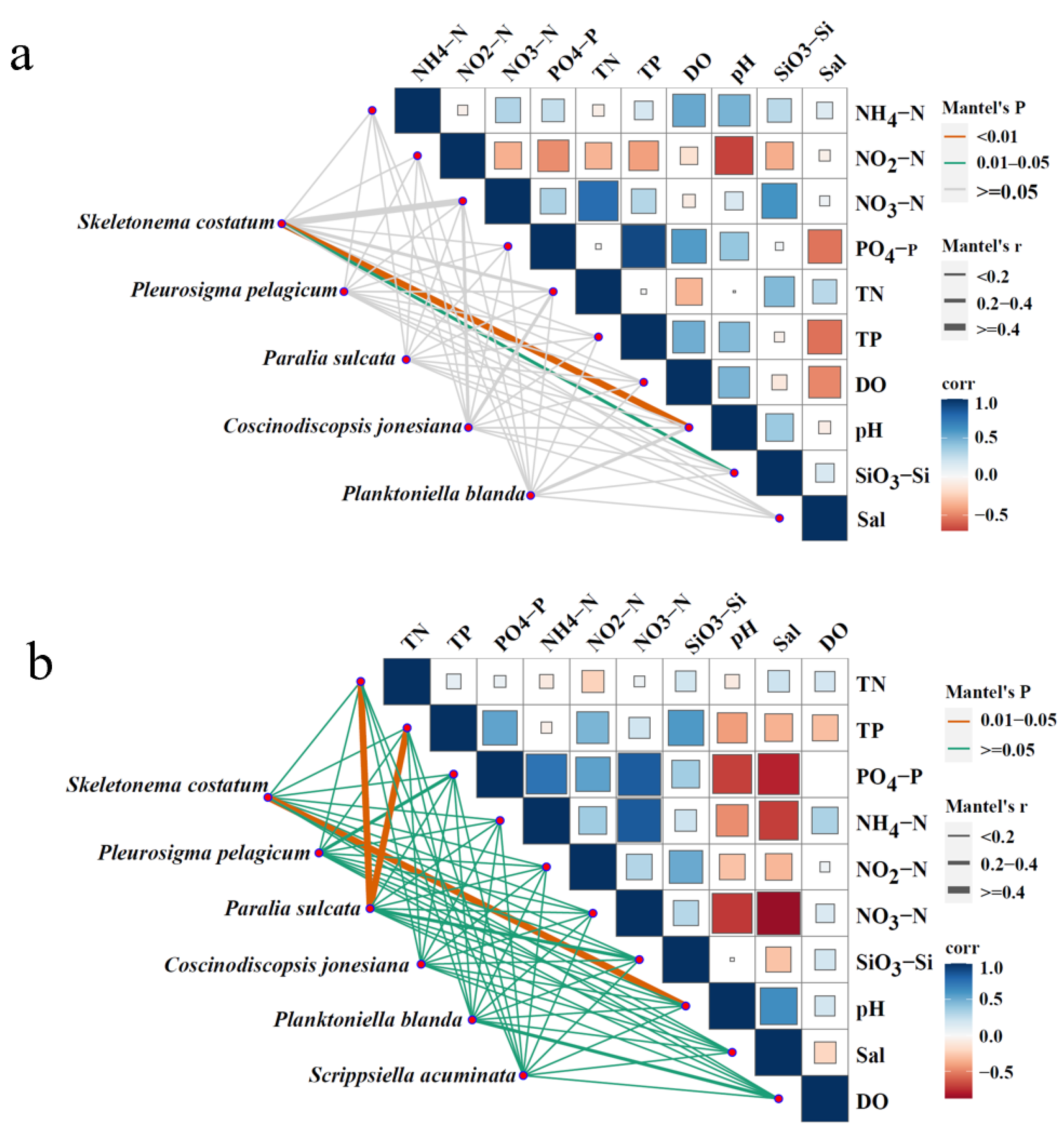
| Area | April 2018 | June 2018 | April 2019 | June 2019 |
|---|---|---|---|---|
| A | 1168 ± 564 | 15,982 ± 9531 | 632 ± 211 | 1522 ± 437 |
| B | 1167 ± 440 | 88,373 ± 10,855 * | 660 ± 221 | 1300 ± 433 |
| C | 1172 ± 122 | 358,673 ± 136,877 * | 640 ± 275 | 2090 ± 433 * |
| Phylum | Dominant Species | April | June | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | Mean | A | B | C | Mean | ||
| Bacillariophyta | Skeletonema costatum (Greville) Cleve 1873 | 0.17 * | 0.38 * | 0.74 | 0.37 | 0.45 * | 0.41 * | 0.97 | 0.83 |
| Planktoniella blanda (A.W.F.Schmidt) Syvertsen & Hasle 1993 | 0.26 | 0.15 * | 0.01 * | 0.14 | - | - | - | - | |
| Coscinodiscopsis jonesiana (Greville) E.A.Sar & I.Sunesen 2008 | 0.21 * | 0.08 | 0.06 | 0.14 | - | - | - | - | |
| Paralia sulcata (Ehrenberg) Cleve, 1873 | 0.06 | 0.23 * | 0.03 | 0.08 | - | - | - | - | |
| Pleurosigma pelagicum Cleve 1894 | 0.04 | 0.10 | - | 0.03 | 0.01 | - | - | - | |
| Dinophyta | Scrippsiella acuminata (Ehrenberg) Kretschmann, Elbrächter, Zinssmeister, S.Soehner, Kirsch, Kusber & Gottschling 2015 | 0.01 | 0.01 | 0.02 | 0.01 | 0.47 * | 0.44 * | 0.02 | 0.13 |
| Tripos muelleri Bory 1826 | - | - | - | - | 0.01 | 0.02 | - | - | |
| Alexandrium sp. | - | - | - | - | 0.01 | 0.01 | - | - | |
| Tripos furca (Ehrenberg) F.Gómez 2013 | - | - | - | - | - | 0.01 | - | - | |
| Protoperidinium pentagonum (Gran) Balech 1974 | - | - | - | - | - | 0.01 | - | - | |
| Dinophysis caudata Kent 1881 | - | - | - | - | - | 0.01 | - | - | |
| Chrysophyta | Dictyocha fibula Ehrenberg 1839 | - | - | - | - | 0.03 | 0.06 | - | 0.01 |
| Phylum | Dominant Species | April | June | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | Mean | A | B | C | Mean | ||
| Bacillariophyta | Skeletonema costatum (Greville) Cleve 1873 | 0.06 * | 0.13 | 0.19 | 0.12 | 0.36 | 0.38 | 0.46 | 0.38 |
| Pleurosigma pelagicum Cleve 1894 | 0.11 | 0.17 | 0.10 | 0.12 | 0.03 | 0.03 | 0.02 | 0.03 | |
| Paralia sulcata (Ehrenberg) Cleve, 1873 | 0.18 | 0.18 | 0.17 | 0.18 | 0.02 | 0.02 | 0.01 | 0.02 | |
| Coscinodiscopsis jonesiana (Greville) E.A.Sar & I.Sunesen 2008 | 0.16 | 0.20 | 0.23 | 0.19 | 0.23 | 0.23 | 0.26 | 0.24 | |
| Planktoniella blanda (A.W.F.Schmidt) Syvertsen & Hasle 1993 | 0.07 | 0.20 | 0.25 | 0.20 | 0.24 | 0.13 | 0.20 | 0.20 | |
| Pinnularia sp. | 0.05 | 0.02 | 0.01 | - | - | - | - | - | |
| Thalassiosira eccentrica (Ehrenberg) Cleve 1904 | 0.01 | - | - | - | - | - | - | - | |
| Chaetoceros lorenzianus Grunow 1863 | 0.02 | - | - | - | - | - | - | - | |
| Synedra sp. | - | 0.02 | - | - | - | - | - | - | |
| Surirella sp. | - | 0.01 | - | - | - | - | - | - | |
| Dinophyta | Scrippsiella acuminata (Ehrenberg) Kretschmann, Elbrächter, Zinssmeister, S.Soehner, Kirsch, Kusber & Gottschling 2015 | - | - | - | - | 0.01 | - | 0.01 | 0.01 |
| Diversity Indices | Area | April 2018 | June 2018 | April 2019 | June 2019 | p(S) |
|---|---|---|---|---|---|---|
| H′ | A | 1.566 ± 0.306 | 1.026 ± 0.187 | 1.826 ± 0.272 | 1.629 ± 0.231 | * |
| B | 1.630 ± 0.309 | 1.235 ± 0.222 | 1.949 ± 0.163 | 1.501 ± 0.266 | * | |
| C | 0.826 ± 0.069 * | 0.158 ± 0.052 | 1.501 ± 0.266 * | 1.307 ± 0.125 | * | |
| D | A | 1.025 ± 0.367 | 1.418 ± 0.187 | 1.221 ± 0.367 | 1.207 ± 0.387 | * |
| B | 1.093 ± 0.403 | 1.322 ± 0.185 | 1.229 ± 0.346 | 1.070 ± 0.252 | * | |
| C | 0.643 ± 0.086 * | 0.308 ± 0.066 * | 1.031 ± 0.183 * | 0.960 ± 0.143 * | * | |
| J | A | 0.764 ± 0.087 | 0.384 ± 0.073 | 0.884 ± 0.067 | 0.727 ± 0.058 | * |
| B | 0.765 ± 0.068 | 0.456 ± 0.082 | 0.902 ± 0.046 | 0.699 ± 0.065 | * | |
| C | 0.477 ± 0.015 * | 0.055 ± 0.019 * | 0.657 ± 0.114 * | 0.618 ± 0.026 * | * |
| Group | ANOSIM | |
|---|---|---|
| R | p | |
| 18-Apr-A vs. 18-Apr-B | 0.272 | 0.119 |
| 18-Apr-A vs. 18-Apr-C | 0.667 | 0.024 |
| 18-Jun-A vs. 18-Jun-B | 0.105 | 0.631 |
| 18-Jun-A vs. 18-Jun-C | 0.104 | 0.612 |
| 19-Apr-A vs. 19-Apr-B | 0.160 | 0.214 |
| 19-Apr-A vs. 19-Apr-C | 0.537 | 0.048 |
| 19-Jun-A vs. 19-Jun-B | 0.105 | 0.274 |
| 19-Jun-A vs. 19-Jun-C | 0.086 | 0.595 |
| Time | Environmental Variable | R2 | p | Significant |
|---|---|---|---|---|
| April 2018 | SiO3;–Si | 0.285 | 0.001 | Significant |
| April 2018 | TN | 0.285 | 0.002 | Significant |
| June 2018 | NO3–N | 0.194 | 0.065 | Marginal |
| June 2019 | SiO3–Si | 0.165 | 0.056 | Marginal |
| June 2019 | NO2;–N | 0.163 | 0.072 | Marginal |
| Comparison Aspect | Microscopic Observation | High-Throughput Sequencing [33] |
|---|---|---|
| Identification Method | Identifies species based on morphological characteristics. | Uses 18S rRNA V4 region universal primers (18sV4F and 18sV4R) and Illumina MiSeq PE250/PE300 sequencing. |
| Accuracy of Phytoplankton Identification | Depends on morphological traits, making it difficult to distinguish morphologically similar species. Identifies Chlorophyta, Cyanophyta, Chrysophyta, Dinophyta, and Bacillariophyta. | Identifies up to genus or species level. Detects small, morphologically similar taxa including Bacillariophyta, Dinophyta, Chlorophyta, Cryptophyta, Chrysophyta, Ochrophyta, Rhodophyta, and Cyanophyta. |
| Number of Species and Dominant Species Identification | Identifies 75 species. Accurately quantifies larger-sized diatoms and dinoflagellate. Clearly reflects the influence of S. costatum dominance. | Detects approximately 601 OTUs. Identifies dominant species across a broad range of phytoplankton size classes, including microphytoplankton (>20 µm), nanophytoplankton (2–20 µm), and picophytoplankton (0.2–2 µm), and reveals taxonomic and abundance patterns |
| Abundance Metric | Uses cell counts (cells/L), which directly reflect actual abundance. | Uses OTU counts to reflect relative abundance of species. |
| Community Diversity and Stability | H′, D, and J indices in the cultivation and adjacent areas were higher, indicating that S. fusiforme cultivation enhances diversity and community stability. | α- and β-diversity indices indicate that S. fusiforme cultivation enhances community diversity and ecological stability. |
| Influence of Environmental Factors on Community Structure | N, P, Si, and DO are key environmental factors that influence community structure. | N, P, Si, and DO also influence community structure. |
| Time and Labor Cost | Requires taxonomic expertise. Time-consuming and labor-intensive. | Standardized process enables batch analysis and requires bioinformatics expertise. |
| Ecological Interpretation Capability | Reflects the spatial and temporal distributions of major species and helps interpret community dynamics. | Reveals taxon diversity and network structures, especially for difficult-to-identify taxa, providing insights into ecological processes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Jiang, R.; Han, Q.; Li, Z.; Mao, Z.; Jiao, H. Ecological Effects of Sargassum fusiforme Cultivation on Coastal Phytoplankton Community Structure and Water Quality: A Study Based on Microscopic Analysis. Biology 2025, 14, 844. https://doi.org/10.3390/biology14070844
Zhang Y, Jiang R, Han Q, Li Z, Mao Z, Jiao H. Ecological Effects of Sargassum fusiforme Cultivation on Coastal Phytoplankton Community Structure and Water Quality: A Study Based on Microscopic Analysis. Biology. 2025; 14(7):844. https://doi.org/10.3390/biology14070844
Chicago/Turabian StyleZhang, Yurong, Rijin Jiang, Qingxi Han, Zimeng Li, Zhen Mao, and Haifeng Jiao. 2025. "Ecological Effects of Sargassum fusiforme Cultivation on Coastal Phytoplankton Community Structure and Water Quality: A Study Based on Microscopic Analysis" Biology 14, no. 7: 844. https://doi.org/10.3390/biology14070844
APA StyleZhang, Y., Jiang, R., Han, Q., Li, Z., Mao, Z., & Jiao, H. (2025). Ecological Effects of Sargassum fusiforme Cultivation on Coastal Phytoplankton Community Structure and Water Quality: A Study Based on Microscopic Analysis. Biology, 14(7), 844. https://doi.org/10.3390/biology14070844







