Molecular and Genetic Pathogenesis of Oral Cancer: A Basis for Customized Diagnosis and Treatment
Simple Summary
Abstract
1. Clinical Aspects of Oral Cancer
1.1. Epidemiology
1.2. Diagnosis and Treatment
1.3. Prognosis
2. Biological Characteristics of Oral Cancer
2.1. Field Cancerization and Intratumor Heterogeneity
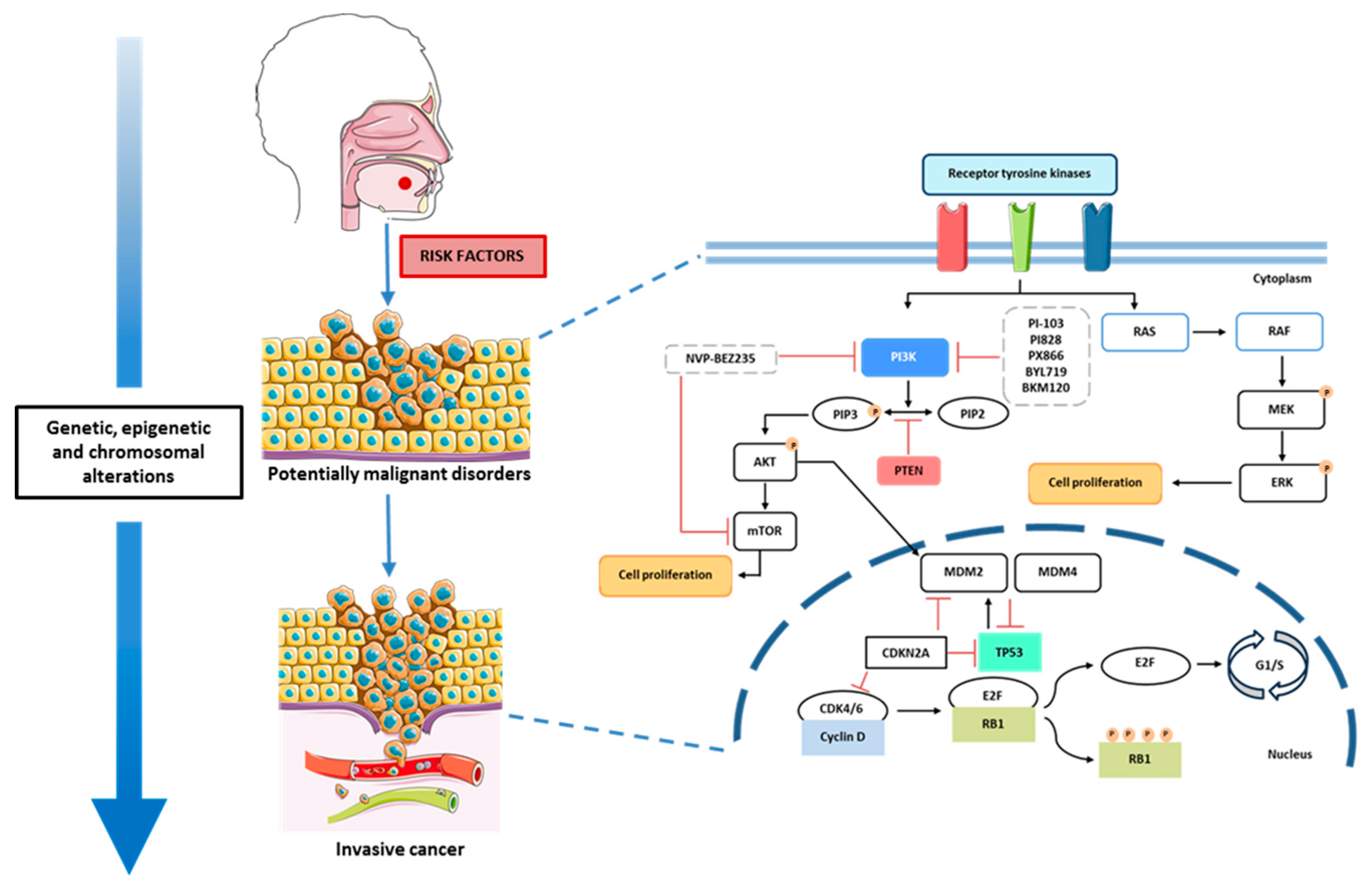
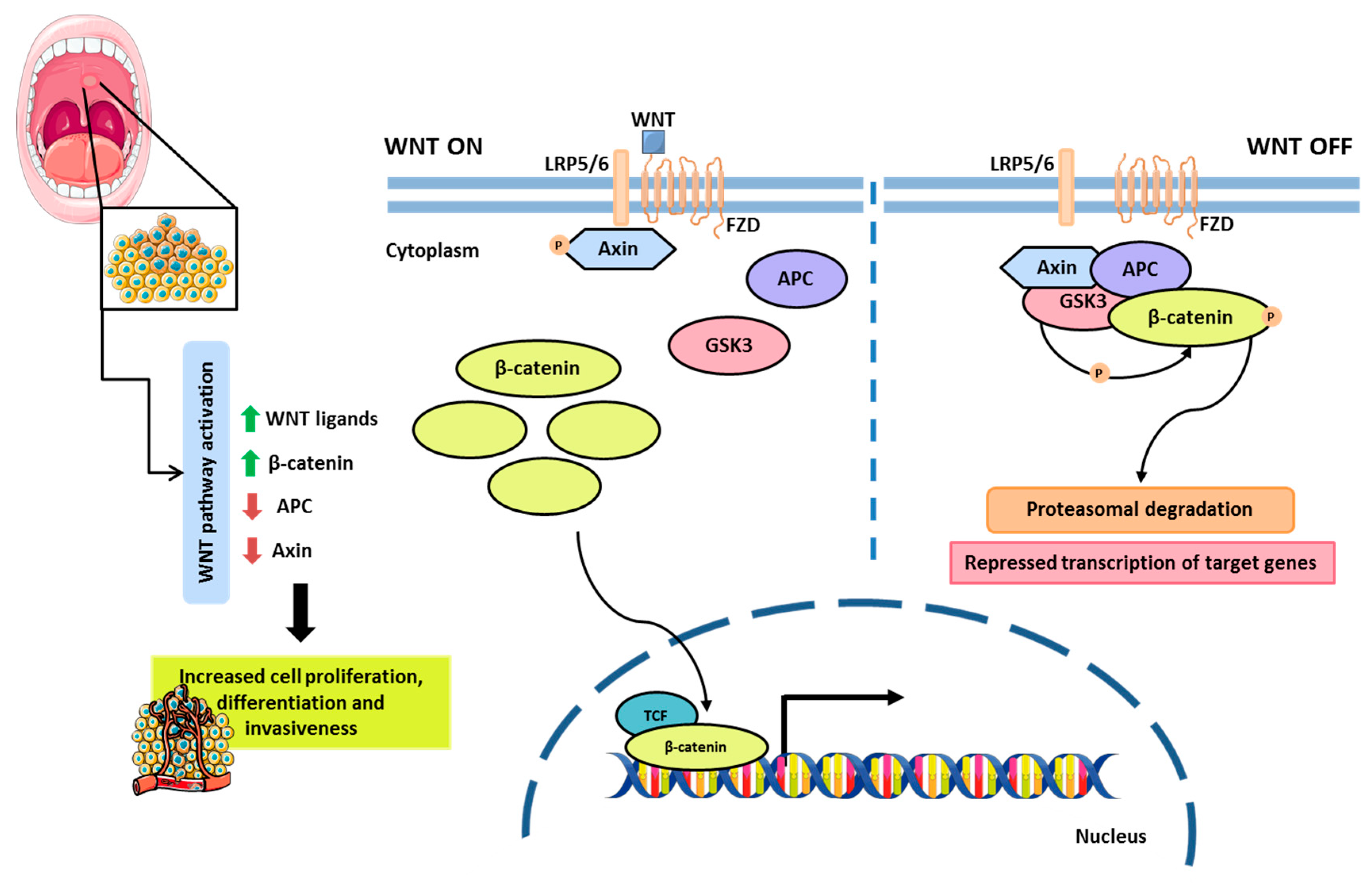
2.2. Molecular Pathogenesis of Oral Cancer
2.2.1. Cell Signaling Pathways and Therapeutic Targets
2.2.2. Genetic Mechanisms in Oral Cancer
2.2.3. Epigenetic Mechanisms in Oral Cancer
3. Liquid Biopsy in Oral Cancer
3.1. Circulating Tumor Cells
3.2. Cell-Free Nucleic Acids
3.3. Extracellular Vesicles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| cfDNA | Cell-free DNA |
| cfRNA | Cell-free RNA |
| CGH | Comparative Genomic Hybridization |
| CTCs | Circulating Tumor Cells |
| ctDNA | Circulating Tumor DNA |
| DNMTs | DNA methyltransferases |
| EMA | European Medicines Agency |
| EMT | Epithelial–mesenchymal transition |
| EVs | Extracellular Vesicles |
| FDA | Food and Drug Administration |
| HDACs | Histone deacetylases |
| HDACi | Histone deacetylases inhibitors |
| HPV | Human papillomavirus |
| NGS | Next-generation sequencing |
| OPMD | Oral potentially malignant disorders |
| OSCC | Oral squamous cell carcinoma |
| PEITC | Phenethyl isothiocyanate |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Nocini, R.; Lippi, G.; Mattiuzzi, C. Biological and Epidemiologic Updates on Lip and Oral Cavity Cancers. Ann. Cancer Epidemiol. 2020, 4, 1. [Google Scholar] [CrossRef]
- Chamoli, A.; Gosavi, A.S.; Shirwadkar, U.P.; Wangdale, K.V.; Behera, S.K.; Kurrey, N.K.; Kalia, K.; Mandoli, A. Overview of Oral Cavity Squamous Cell Carcinoma: Risk Factors, Mechanisms, and Diagnostics. Oral Oncol. 2021, 121, 105451. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Available online: https://www.iarc.who.int/ (accessed on 12 February 2025).
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Sundermann, B.V.; Uhlmann, L.; Hoffmann, J.; Freier, K.; Thiele, O.C. The Localization and Risk Factors of Squamous Cell Carcinoma in the Oral Cavity: A Retrospective Study of 1501 Cases. J. Cranio Maxillofac. Surg. 2018, 46, 177–182. [Google Scholar] [CrossRef]
- Lin, W.J.; Jiang, R.S.; Wu, S.H.; Chen, F.J.; Liu, S.A. Smoking, Alcohol, and Betel Quid and Oral Cancer: A Prospective Cohort Study. J. Oncol. 2011, 2011, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wyss, A.B.; Hashibe, M.; Lee, Y.C.A.; Chuang, S.C.; Muscat, J.; Chen, C.; Schwartz, S.M.; Smith, E.; Zhang, Z.F.; Morgenstern, H.; et al. Smokeless Tobacco Use and the Risk of Head and Neck Cancer: Pooled Analysis of US Studies in the Inhance Consortium. Am. J. Epidemiol. 2016, 184, 703–716. [Google Scholar] [CrossRef]
- Hashibe, M.; Brennan, P.; Benhamou, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Maso, L.D.; Daudt, A.W.; Fabianova, E.; Wünsch-Filho, V.; et al. Alcohol Drinking in Never Users of Tobacco, Cigarette Smoking in Never Drinkers, and the Risk of Head and Neck Cancer: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium. J. Natl. Cancer Inst. 2007, 99, 777–789. [Google Scholar] [CrossRef]
- Blot, W.J.; McLaughlin, J.K.; Winn, D.M.; Austin, D.F.; Greenberg, R.S.; Preston-Martin, S.; Bernstein, L.; Schoenberg, J.B.; Stemhagen, A.; Fraumeni, J.F. Smoking and Drinking in Relation to Oral and Pharyngeal Cancer. Cancer Res. 1988, 48, 3282–3287. [Google Scholar]
- Zhang, L.W.; Li, J.; Cong, X.; Hu, X.S.; Li, D.; Wu, L.L.; Hua, H.; Yu, G.Y.; Kerr, A.R. Incidence and Mortality Trends in Oral and Oropharyngeal Cancers in China, 2005–2013. Cancer Epidemiol. 2018, 57, 120–126. [Google Scholar] [CrossRef]
- Gormley, M.; Creaney, G.; Schache, A.; Ingarfield, K.; Conway, D.I. Reviewing the Epidemiology of Head and Neck Cancer: Definitions, Trends and Risk Factors. Br. Dent. J. 2022, 233, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Velleuer, E.; Dietrich, R. Fanconi Anemia: Young Patients at High Risk for Squamous Cell Carcinoma. Mol. Cell. Pediatr. 2014, 1, 9. [Google Scholar] [CrossRef]
- Nosé, V.; Lazar, A.J. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Familial Tumor Syndromes. Head Neck Pathol. 2022, 16, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Debta, P.; Dixit, A. Oral Potentially Malignant Disorders: Etiology, Pathogenesis, and Transformation Into Oral Cancer. Front. Pharmacol. 2022, 13, 825266. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; Ángel González-Moles, M.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral Potentially Malignant Disorders: Nomenclature and Classification; LAP Lambert Academic Publishing: East Finchley, UK, 2021. [Google Scholar]
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; Pardiñas López, S.; Shanti, R.M. Potentially Malignant Disorders of the Oral Cavity and Oral Dysplasia: A Systematic Review and Meta-Analysis of Malignant Transformation Rate by Subtype. Head Neck 2020, 42, 539–555. [Google Scholar] [CrossRef]
- NCCN Guidelines. Available online: www.nccn.org/guidelines (accessed on 12 February 2025).
- Yu, C.; Li, Q.; Zhang, Y.; Wen, Z.-F.; Dong, H.; Mou, Y. Current Status and Perspective of Tumor Immunotherapy for Head and Neck Squamous Cell Carcinoma. Front. Cell Dev. Biol. 2022, 10, 941750. [Google Scholar] [CrossRef]
- Oral Cavity (Mouth) and Oropharyngeal (Throat) Cancer. Available online: www.cancer.org (accessed on 14 February 2025).
- Cheraghlou, S.; Schettino, A.; Zogg, C.K.; Judson, B.L. Changing Prognosis of Oral Cancer: An Analysis of Survival and Treatment between 1973 and 2014. Laryngoscope 2018, 128, 2762–2769. [Google Scholar] [CrossRef]
- Baxi, S.S.; Pinheiro, L.C.; Patil, S.M.; Pfister, D.G.; Oeffinger, K.C.; Elkin, E.B. Causes of Death in Long-Term Survivors of Head and Neck Cancer. Cancer 2014, 120, 1507–1513. [Google Scholar] [CrossRef]
- Leõn, X.; Martínez, V.; Lõpez, M.; García, J.; Venegas, M.D.P.; Esteller, E.; Quer, M. Second, Third, and Fourth Head and Neck Tumors. A Progressive Decrease in Survival. Head Neck 2012, 34, 1716–1719. [Google Scholar] [CrossRef]
- Leãn, X.; Martínez, V.; López, M.; García, J.; Quer, M. Risk of Third and Fourth Tumors in Patients with Head and Neck Cancer. Head Neck 2010, 32, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Osazuwa-Peters, N.; Simpson, M.C.; Zhao, L.; Boakye, E.A.; Olomukoro, S.I.; Deshields, T.; Loux, T.M.; Varvares, M.A.; Schootman, M. Suicide Risk among Cancer Survivors: Head and Neck versus Other Cancers. Cancer 2018, 124, 4072–4079. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, H.M.; Morton, R.P. Deterioration in Quality-of-Life of Late (10-Year) Survivors of Head and Neck Cancer. Clin. Otolaryngol. 2006, 31, 204–211. [Google Scholar] [CrossRef]
- Braakhuis, B.J.M.; Tabor, M.P.; Kummer, J.A.; Leemans, C.R.; Brakenhoff, R.H. A Genetic Explanation of Slaughter’s Concept of Field Cancerization: Evidence and Clinical Implications. Cancer Res. 2003, 63, 1727–1730. [Google Scholar]
- Curtius, K.; Wright, N.A.; Graham, T.A. An Evolutionary Perspective on Field Cancerization. Nat. Rev. Cancer 2018, 18, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, D.P.; Southwick, H.W.; Smejkal, W. Field Cancerization in Oral Stratified Squamous Epithelium; Clinical Implications of Multicentric Origin. Cancer 1953, 6, 963–968. [Google Scholar] [CrossRef]
- Ha, P.K.; Califano, J.A. The Molecular Biology of Mucosal Field Cancerization of the Head and Neck. Crit. Rev. Oral. Biol. Med. 2003, 14, 363–369. [Google Scholar] [CrossRef]
- Tabor, M.P.; Braakhuis, B.J.M.; van der Wal, J.E.; van Diest, P.J.; Leemans, C.R.; Brakenhoff, R.H.; Kummer, J.A. Comparative Molecular and Histological Grading of Epithelial Dysplasia of the Oral Cavity and the Oropharynx. J. Pathol. 2003, 199, 354–360. [Google Scholar] [CrossRef]
- Tabor, M.P.; Brakenhoff, R.H.; van Houten, V.M.; Kummer, J.A.; Snel, M.H.; Snijders, P.J.; Snow, G.B.; Leemans, C.R.; Braakhuis, B.J. Persistence of Genetically Altered Fields in Head and Neck Cancer Patients: Biological and Clinical Implications. Clin. Cancer Res. 2001, 7, 1523–1532. [Google Scholar]
- Peralta-Mamani, M.; Terrero-Pérez, Á.; Tucunduva, R.M.A.; Rubira, C.M.F.; Santos, P.S.d.S.; Honório, H.M.; Rubira-Bullen, I.R.F. Occurrence of Field Cancerization in Clinically Normal Oral Mucosa: A Systematic Review and Meta-Analysis. Arch. Oral Biol. 2022, 143, 105544. [Google Scholar] [CrossRef]
- Gabusi, A.; Gissi, D.B.; Querzoli, G.; Sangiovanni, A.; Rossi, R.; Lucchi, E.; Tarsitano, A.; Montebugnoli, L.; Foschini, M.P.; Morandi, L. DNA Methylation Analysis from Oral Brushing Reveals a Field Cancerization Effect in Proliferative Verrucous Leukoplakia. Pathologica 2024, 116, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Jagannathan, N. Oral Field Cancerization: An Update on Current Concepts. Oncol. Rev. 2014, 8, 13–19. [Google Scholar] [CrossRef]
- Gabusi, A.; Morandi, L.; Asioli, S.; Foschini, M.P. Oral Field Cancerization: History and Future Perspectives. Pathologica 2017, 109, 60–65. [Google Scholar] [PubMed]
- van Oijen, M.G.; Slootweg, P.J. Oral Field Cancerization: Carcinogen-Induced Independent Events or Micrometastatic Deposits? Cancer Epidemiol. Biomark. Prev. 2000, 9, 249–256. [Google Scholar]
- Simple, M.; Suresh, A.; Das, D.; Kuriakose, M.A. Cancer Stem Cells and Field Cancerization of Oral Squamous Cell Carcinoma. Oral. Oncol. 2015, 51, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Mroz, E.A.; Tward, A.M.; Hammon, R.J.; Ren, Y.; Rocco, J.W. Intra-Tumor Genetic Heterogeneity and Mortality in Head and Neck Cancer: Analysis of Data from The Cancer Genome Atlas. PLoS Med. 2015, 12, e1001786. [Google Scholar] [CrossRef]
- Mroz, E.A.; Rocco, J.W. The Challenges of Tumor Genetic Diversity. Cancer 2017, 123, 917–927. [Google Scholar] [CrossRef]
- Gabusi, A.; Gissi, D.B.; Montebugnoli, L.; Asioli, S.; Tarsitano, A.; Marchetti, C.; Balbi, T.; Helliwell, T.R.; Foschini, M.P.; Morandi, L. Prognostic Impact of Intra-Field Heterogeneity in Oral Squamous Cell Carcinoma. Virchows Arch. 2020, 476, 585–595. [Google Scholar] [CrossRef]
- Mroz, E.A.; Tward, A.D.; Pickering, C.R.; Myers, J.N.; Ferris, R.L.; Rocco, J.W. High Intratumor Genetic Heterogeneity Is Related to Worse Outcome in Patients with Head and Neck Squamous Cell Carcinoma. Cancer 2013, 119, 3034–3042. [Google Scholar] [CrossRef]
- Rocco, J.W. Mutant Allele Tumor Heterogeneity (MATH) and Head and Neck Squamous Cell Carcinoma. Head Neck Pathol. 2015, 9, 1–5. [Google Scholar] [CrossRef]
- Gabusi, A.; Gissi, D.B.; Tarsitano, A.; Asioli, S.; Marchetti, C.; Montebugnoli, L.; Foschini, M.P.; Morandi, L. Intratumoral Heterogeneity in Recurrent Metastatic Squamous Cell Carcinoma of the Oral Cavity: New Perspectives Afforded by Multiregion DNA Sequencing and MtDNA Analysis. J. Oral. Maxillofac. Surg. 2019, 77, 440–455. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.K. Reviews Molecular Pathogenesis of Oral Squamous Carcinoma. Mol Pathol. 2000, 53, 165–172. [Google Scholar] [CrossRef]
- Karunakaran, K.; Muniyan, R. Genetic Alterations and Clinical Dimensions of Oral Cancer: A Review. Mol. Biol. Rep. 2020, 47, 9135–9148. [Google Scholar] [CrossRef]
- Roda, D.; Veiga, P.; Melo, J.B.; Carreira, I.M.; Ribeiro, I.P. Principles in the Management of Glioblastoma. Genes 2024, 15, 501. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Sekar, R.; Dhayasankar, P.S.; Ali, E.M.; Abdelsalam, S.A.; Balaraman, S.; Chellappan, B.V.; Metwally, A.M.; Abdallah, B.M. PI3K/AKT Signaling Pathway Mediated Autophagy in Oral Carcinoma—A Comprehensive Review. Int. J. Med. Sci. 2024, 21, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Harsha, C.; Banik, K.; Ang, H.L.; Girisa, S.; Vikkurthi, R.; Parama, D.; Rana, V.; Shabnam, B.; Khatoon, E.; Kumar, A.P.; et al. Targeting AKT/MTOR in Oral Cancer: Mechanisms and Advances in Clinical Trials. Int. J. Mol. Sci. 2020, 21, 3285. [Google Scholar] [CrossRef] [PubMed]
- Cívico-Ortega, J.L.; González-Ruiz, I.; Ramos-García, P.; Cruz-Granados, D.; Samayoa-Descamps, V.; González-Moles, M.Á. Prognostic and Clinicopathological Significance of Epidermal Growth Factor Receptor (EGFR) Expression in Oral Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 11888. [Google Scholar] [CrossRef]
- Aggarwal, S.; John, S.; Sapra, L.; Sharma, S.C.; Das, S.N. Targeted Disruption of PI3K/Akt/MTOR Signaling Pathway, via PI3K Inhibitors, Promotes Growth Inhibitory Effects in Oral Cancer Cells. Cancer Chemother. Pharmacol. 2019, 83, 451–461. [Google Scholar] [CrossRef]
- Su, Y.-C.; Yu, C.-C.; Hung, S.-K.; Lin, H.-Y.; Chan, M.W.-Y.; Huang, H.-B.; Lee, C.-C. Effect of NVP-BEZ235, Dual Phosphatidylinositol 3-Kinase/Mammalian Target of Rapamycin Inhibitor, on Radiosensitivity of Oral Cancer Cell Line through G2/M Phase Checkpoint Regulation. J. Clin. Oncol. 2014, 32, e13558. [Google Scholar] [CrossRef]
- Hsu, C.M.; Lin, P.M.; Lin, H.C.; Tsai, Y.T.; Tsai, M.S.; Li, S.H.; Wu, C.Y.; Yang, Y.H.; Lin, S.F.; Yang, M.Y. NVP-BEZ235 Attenuated Cell Proliferation and Migration in the Squamous Cell Carcinoma of Oral Cavities and P70s6K Inhibition Mimics Its Effect. Int. J. Mol. Sci. 2018, 19, 3546. [Google Scholar] [CrossRef]
- Chuang, F.C.; Wang, C.C.; Chen, J.H.; Hwang, T.Z.; Yeh, S.A.; Su, Y.C. PI3k Inhibitors (BKM120 and BYL719) as Radiosensitizers for Head and Neck Squamous Cell Carcinoma during Radiotherapy. PLoS ONE 2021, 16, e0245715. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Li, Y.; Zhou, Q.; Xu, Z.; Sun, C.; Tan, X.; Lu, L. Cetuximab Inhibits Oral Squamous Cell Carcinoma Invasion and Metastasis via Degradation of Epidermal Growth Factor Receptor. J. Oral. Pathol. Med. 2014, 43, 250–257. [Google Scholar] [CrossRef]
- Naruse, T.; Yanamoto, S.; Matsushita, Y.; Sakamoto, Y.; Morishita, K.; Ohba, S.; Shiraishi, T.; Yamada, S.-I.; Asahina, I.; Umeda, M. Cetuximab for the Treatment of Locally Advanced and Recurrent/Metastatic Oral Cancer: An Investigation of Distant Metastasis. Mol. Clin. Oncol. 2016, 5, 246–252. [Google Scholar] [CrossRef]
- Tsuchihashi, H.; Naruse, T.; Yanamoto, S.; Okuyama, K.; Furukawa, K.; Omori, K.; Umeda, M. Selective Inhibition of PI3K110α as a Novel Therapeutic Strategy for Cetuximab-Resistant Oral Squamous Cell Carcinoma. Oncol. Rep. 2020, 44, 863–872. [Google Scholar] [CrossRef]
- Peng, Q.; Deng, Z.; Pan, H.; Gu, L.; Liu, O.; Tang, Z. Mitogen-Activated Protein Kinase Signaling Pathway in Oral Cancer (Review). Oncol. Lett. 2018, 15, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Ngan, H.L.; Law, C.H.; Choi, Y.C.Y.; Chan, J.Y.S.; Lui, V.W.Y. Precision Drugging of the MAPK Pathway in Head and Neck Cancer. NPJ Genom. Med. 2022, 7, 1–10. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, J.; Shi, Y.; Fang, X.; Tang, Z. MAPK Signaling Pathway in Oral Squamous Cell Carcinoma: Biological Function and Targeted Therapy. Cancers 2022, 14, 4625. [Google Scholar] [CrossRef]
- Gkouveris, I.; Nikitakis, N.; Karanikou, M.; Rassidakis, G.; Sklavounou, A. JNK1/2 Expression and Modulation of STAT3 Signaling in Oral Cancer. Oncol. Lett. 2016, 12, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Uppaluri, R.; Winkler, A.E.; Lin, T.; Law, J.H.; Haughey, B.H.; Nussenbaum, B.; Paniello, R.C.; Rich, J.T.; Diaz, J.A.; Michel, L.P.; et al. Biomarker and Tumor Responses of Oral Cavity Squamous Cell Carcinoma to Trametinib: A Phase II Neoadjuvant Window-of-Opportunity Clinical Trial. Clin. Cancer Res. 2017, 23, 2186–2194. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting P53 Pathways: Mechanisms, Structures, and Advances in Therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. P53 Signaling in Cancer Progression and Therapy. Cancer Cell Int. 2021, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.; Chaudhary, M.; Sharma, P.; Hande, A.; Patil, S.; Sonone, A. Expression of P53 at Invasive Front of Oral Squamous Cell Carcinoma and Negative Histopathological Surgical Margins to Establish Correlation at 3-Year Survival. J. Oral. Maxillofac. Pathol. 2020, 24, 582. [Google Scholar] [CrossRef]
- Shi, Y.; Ren, X.; Cao, S.; Chen, X.; Yuan, B.; Brasil Da Costa, F.H.; Rodriguez Rosario, A.E.; Corona, A.; Michikawa, C.; Veeramachaneni, R.; et al. TP53 Gain-of-Function Mutation Modulates the Immunosuppressive Microenvironment in Non-HPV-Associated Oral Squamous Cell Carcinoma. J. Immunother. Cancer 2023, 11, e006666. [Google Scholar] [CrossRef]
- Hyodo, T.; Kuribayashi, N.; Fukumoto, C.; Komiyama, Y.; Shiraishi, R.; Kamimura, R.; Sawatani, Y.; Yaguchi, E.; Hasegawa, T.; Izumi, S.; et al. The Mutational Spectrum in Whole Exon of P53 in Oral Squamous Cell Carcinoma and Its Clinical Implications. Sci. Rep. 2022, 12, 21695. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, Z.; Myers, J.N. TP53 Mutations in Head and Neck Squamous Cell Carcinoma and Their Impact on Disease Progression and Treatment Response. J. Cell. Biochem. 2016, 117, 2682–2692. [Google Scholar] [CrossRef] [PubMed]
- Sandulache, V.C.; Michikawa, C.; Kataria, P.; Gleber-Netto, F.O.; Bell, D.; Trivedi, S.; Rao, X.; Wang, J.; Zhao, M.; Jasser, S.; et al. High-Risk TP53 Mutations Are Associated with Extranodal Extension in Oral Cavity Squamous Cell Carcinoma. Clin. Cancer Res. 2018, 24, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.-Y.; Liu, K.Y.P.; Novack, R.; Mattu, P.S.; Ng, T.L.; Hoang, L.N.; Prisman, E.; Poh, C.F.; Ko, Y.C.K. Abnormal P53 Immunohistochemical Patterns Are Associated with Regional Lymph Node Metastasis in Oral Cavity Squamous Cell Carcinoma at Time of Surgery. Mod. Pathol. 2024, 37, 100614. [Google Scholar] [CrossRef]
- Chen, P.Y.; Lin, K.C.; Lin, J.P.; Tang, N.Y.; Yang, J.S.; Lu, K.W.; Chung, J.G. Phenethyl Isothiocyanate (PEITC) Inhibits the Growth of Human Oral Squamous Carcinoma Hsc-3 Cells through g 0/g 1 Phase Arrest and Mitochondria-Mediated Apoptotic Cell Death. Evid. Based Complement. Altern. Med. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Yeh, Y.T.; Yeh, H.; Su, S.H.; Lin, J.S.; Lee, K.J.; Shyu, H.W.; Chen, Z.F.; Huang, S.Y.; Su, S.J. Phenethyl Isothiocyanate Induces DNA Damage-Associated G2/M Arrest and Subsequent Apoptosis in Oral Cancer Cells with Varying P53 Mutations. Free. Radic. Biol. Med. 2014, 74, 1–13. [Google Scholar] [CrossRef]
- Thomas, S.; Balan, A.; Balaram, P. The Expression of Retinoblastoma Tumor Suppressor Protein in Oral Cancers and Precancers: A Clinicopathological Study. Dent. Res. J. 2015, 12, 307–314. [Google Scholar] [CrossRef]
- Zhou, Y.; Nakajima, R.; Shirasawa, M.; Fikriyanti, M.; Zhao, L.; Iwanaga, R.; Bradford, A.P.; Kurayoshi, K.; Araki, K.; Ohtani, K. Expanding Roles of the E2F-RB-P53 Pathway in Tumor Suppression. Biology 2023, 12, 1511. [Google Scholar] [CrossRef]
- Sabir, M.; Baig, R.M.; Ali, K.; Mahjabeen, I.; Saeed, M.; Kayani, M.A. Retinoblastoma (RB1) Pocket Domain Mutations and Promoter Hyper-Methylation in Head and Neck Cancer. Cell. Oncol. 2014, 37, 203–213. [Google Scholar] [CrossRef]
- Gleich, L.L.; Salamone, F.N. Molecular Genetics of Head and Neck Cancer. Cancer Control. 2002, 9, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Jayasurya, R.; Sathyan, K.M.; Lakshminarayanan, K.; Abraham, T.; Nalinakumari, K.R.; Abraham, E.K.; Krishnan Nair, M.; Kannan, S. Phenotypic Alterations in Rb Pathway Have More Prognostic Influence than P53 Pathway Proteins in Oral Carcinoma. Mod. Pathol. 2005, 18, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Berdugo, J.; Rooper, L.M.; Chiosea, S.I. RB1, P16, and Human Papillomavirus in Oropharyngeal Squamous Cell Carcinoma. Head Neck Pathol. 2021, 15, 1109–1118. [Google Scholar] [CrossRef]
- Knudsen, E.S.; Pruitt, S.C.; Hershberger, P.A.; Witkiewicz, A.K.; Goodrich, D.W. Cell Cycle and Beyond: Exploiting New RB1 Controlled Mechanisms for Cancer Therapy. Trends Cancer 2019, 5, 308–324. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Purwaningsih, N.M.S.; Khor, G.H.; Nik Mohd Rosdy, N.M.M.; Abdul Rahman, E.O. Wnt Pathway in Oral Cancer: A Review Update. Saudi Dent. J. 2021, 33, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Flores, T.; Betancur, D.; Peña-Oyarzún, D.; Torres, V.A. Wnt/β-Catenin Signaling in Oral Carcinogenesis. Int. J. Mol. Sci. 2020, 21, 4682. [Google Scholar] [CrossRef]
- Sato, K.; Okazaki, Y.; Tonogi, M.; Tanaka, Y.; Yamane, G.-Y. Expression of B-Catenin in Rat Oral Epithelial Dysplasia Induced by 4-Nitroquinoline 1-Oxide. Oral Oncol. 2002, 38, 772–778. [Google Scholar] [CrossRef]
- Campolo, M.; Scuderi, S.A.; Filippone, A.; Bova, V.; Lombardo, S.P.; Colarossi, L.; Sava, S.; Capra, A.P.; De Gaetano, F.; Portelli, M.; et al. EZH2 Inhibition to Counteract Oral Cancer Progression through Wnt/β-Catenin Pathway Modulation. Pharmaceuticals 2024, 17, 1102. [Google Scholar] [CrossRef] [PubMed]
- Georgaki, M.; Theofilou, V.I.; Pettas, E.; Stoufi, E.; Younis, R.H.; Kolokotronis, A.; Sauk, J.J.; Nikitakis, N.G. Understanding the Complex Pathogenesis of Oral Cancer: A Comprehensive Review. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2021, 132, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Vincent-Chong, V.K.; Anwar, A.; Karen-Ng, L.P.; Cheong, S.C.; Yang, Y.H.; Pradeep, P.J.; Rahman, Z.A.A.; Ismail, S.M.; Zaini, Z.M.; Prepageran, N.; et al. Genome Wide Analysis of Chromosomal Alterations in Oral Squamous Cell Carcinomas Revealed over Expression of MGAM and ADAM9. PLoS ONE 2013, 8, e54705. [Google Scholar] [CrossRef] [PubMed]
- Lese Martin, C.; Reshmi, S.C.; Ried, T.; Gottberg, W.; Wilson, J.W.; Reddy, J.K.; Khanna, P.; Johnson, J.T.; Myers, E.N.; Gollin, S.M.; et al. Chromosomal Imbalances in Oral Squamous Cell Carcinoma. Examination of 31 Cell Lines and Review of the Literature. Oral Oncol. 2008, 44, 369–382. [Google Scholar] [CrossRef]
- Pickering, C.R.; Zhang, J.; Yoo, S.Y.; Bengtsson, L.; Moorthy, S.; Neskey, D.M.; Zhao, M.; Ortega Alves, M.V.; Chang, K.; Drummond, J.; et al. Integrative Genomic Characterization of Oral Squamous Cell Carcinoma Identifies Frequent Somatic Drivers. Cancer Discov. 2013, 3, 770–781. [Google Scholar] [CrossRef]
- Jäwert, F.; Fehr, A.; Öhman, J.; Stenman, G.; Kjeller, G. Recurrent Copy Number Alterations Involving EGFR, CDKN2A, and CCND1 in Oral Premalignant Lesions. J. Oral. Pathol. Med. 2022, 51, 546–552. [Google Scholar] [CrossRef]
- Nakagaki, T.; Tamura, M.; Kobashi, K.; Koyama, R.; Fukushima, H.; Ohashi, T.; Idogawa, M.; Ogi, K.; Hiratsuka, H.; Tokino, T.; et al. Profiling Cancer-Related Gene Mutations in Oral Squamous Cell Carcinoma from Japanese Patients by Targeted Amplicon Sequencing. Oncotarget 2017, 8, 59113–59122. [Google Scholar] [CrossRef]
- Lan, T.; Ge, Q.; Zheng, K.; Huang, L.; Yan, Y.; Zheng, L.; Lu, Y.; Zheng, D. FAT1 Upregulates in Oral Squamous Cell Carcinoma and Promotes Cell Proliferation via Cell Cycle and DNA Repair. Front. Oncol. 2022, 12, 870055. [Google Scholar] [CrossRef]
- Mesgari, H.; Esmaelian, S.; Nasiri, K.; Ghasemzadeh, S.; Doroudgar, P.; Payandeh, Z. Epigenetic Regulation in Oral Squamous Cell Carcinoma Microenvironment: A Comprehensive Review. Cancers 2023, 15, 5600. [Google Scholar] [CrossRef]
- Hema, K.N.; Smitha, T.; Sheethal, H.; Mirnalini, S.A. Epigenetics in Oral Squamous Cell Carcinoma. J. Oral. Maxillofac. Pathol. 2017, 21, 252–259. [Google Scholar] [CrossRef]
- Flausino, C.S.; Daniel, F.I.; Modolo, F. DNA Methylation in Oral Squamous Cell Carcinoma: From Its Role in Carcinogenesis to Potential Inhibitor Drugs. Crit. Rev. Oncol. Hematol. 2021, 164, 103399. [Google Scholar] [CrossRef] [PubMed]
- Irimie, A.I.; Ciocan, C.; Gulei, D.; Mehterov, N.; Atanasov, A.G.; Dudea, D.; Berindan-Neagoe, I. Current Insights into Oral Cancer Epigenetics. Int. J. Mol. Sci. 2018, 19, 670. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Yang, J.; Wu, D.; Yu, S.; Liu, J.; Hu, T.; Luo, J.; Zhou, H. DNMT1-Targeting Remodeling Global DNA Hypomethylation for Enhanced Tumor Suppression and Circumvented Toxicity in Oral Squamous Cell Carcinoma. Mol. Cancer 2024, 23, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Towle, R.; Truong, D.; Hogg, K.; Robinson, W.P.; Poh, C.F.; Garnis, C. Global Analysis of DNA Methylation Changes during Progression of Oral Cancer. Oral. Oncol. 2013, 49, 1033–1042. [Google Scholar] [CrossRef]
- Kim, S.Y.; Han, Y.K.; Song, J.M.; Lee, C.H.; Kang, K.; Yi, J.M.; Park, H.R. Aberrantly Hypermethylated Tumor Suppressor Genes Were Identified in Oral Squamous Cell Carcinoma (OSCC). Clin. Epigenetics 2019, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dvojakovska, S.; Popovic-Monevska, D.; Grcev, A.; Pancevski, G.; Benedetti, A.; Popovski, V.; Dimovski, A.; Stamatoski, A. Promotor Hypermethylated Genes: Prospective Diagnostic Biomarkers in Oral Cancerogenesis. J. Cranio Maxillofac. Surg. 2018, 46, 1737–1740. [Google Scholar] [CrossRef]
- Neganova, M.E.; Klochkov, S.G.; Aleksandrova, Y.R.; Aliev, G. Histone Modifications in Epigenetic Regulation of Cancer: Perspectives and Achieved Progress. Semin. Cancer Biol. 2022, 83, 452–471. [Google Scholar] [CrossRef]
- Ruzic, D.; Djoković, N.; Srdić-Rajić, T.; Echeverria, C.; Nikolic, K.; Santibanez, J.F. Targeting Histone Deacetylases: Opportunities for Cancer Treatment and Chemoprevention. Pharmaceutics 2022, 14, 209. [Google Scholar] [CrossRef]
- Lian, B.; Chen, X.; Shen, K. Inhibition of Histone Deacetylases Attenuates Tumor Progression and Improves Immunotherapy in Breast Cancer. Front. Immunol. 2023, 14, 1164514. [Google Scholar] [CrossRef]
- Lee, H.Y.; Tang, D.W.; Liu, C.Y.; Cho, E.C. A Novel HDAC1/2 Inhibitor Suppresses Colorectal Cancer through Apoptosis Induction and Cell Cycle Regulation. Chem. Biol. Interact. 2022, 352, 109778. [Google Scholar] [CrossRef]
- Abbas, A.; Gupta, S. The Role of Histone Deacetylases in Prostate Cancer. Epigenetics 2008, 3, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Li, M.; Duan, Y.; Jiang, X.; Hou, X.; Xue, F.; Zhang, Y.; Luo, Y. HDAC Inhibitors Enhance the Anti-Tumor Effect of Immunotherapies in Hepatocellular Carcinoma. Front. Immunol. 2023, 14, 1170207. [Google Scholar] [CrossRef]
- Antrobus, J.; Mackinnon, B.; Melia, E.; Hughes, J.R.; Parsons, J.L. HDAC Inhibitors Can Enhance Radiosensitivity of Head and Neck Cancer Cells Through Suppressing DNA Repair. Cancers 2024, 16, 4108. [Google Scholar] [CrossRef] [PubMed]
- Al-Khafaji, A.S.K.; Wang, L.M.; Alabdei, H.H.; Liloglou, T. Effect of Valproic Acid on Histone Deacetylase Expression in Oral Cancer (Review). Oncol. Lett. 2024, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y. HDAC Inhibitor Apicidin Suppresses Murine Oral Squamous Cell Carcinoma Cell Growth in Vitro and in Vivo via Inhibiting HDAC8 Expression. Oncol. Lett. 2018, 16, 6552–6560. [Google Scholar] [CrossRef]
- Ahn, M.-Y.; Ahn, S.-G.; Yoon, J.-H. Apicidin, a Histone Deaceylase Inhibitor, Induces Both Apoptosis and Autophagy in Human Oral Squamous Carcinoma Cells. Oral. Oncol. 2011, 47, 1032–1038. [Google Scholar] [CrossRef]
- Xu, M.; Hou, Y.; Li, N.; Yu, W.; Chen, L. Targeting Histone Deacetylases in Head and Neck Squamous Cell Carcinoma: Molecular Mechanisms and Therapeutic Targets. J. Transl. Med. 2024, 22, 1–26. [Google Scholar] [CrossRef]
- Yan, H.; Bu, P. Non-Coding RNA in Cancer. Essays Biochem. 2021, 65, 625–639. [Google Scholar]
- Smolarz, B.; Durczyński, A.; Romanowicz, H.; Szyłło, K.; Hogendorf, P. MiRNAs in Cancer (Review of Literature). Int. J. Mol. Sci. 2022, 23, 2805. [Google Scholar] [CrossRef] [PubMed]
- Osan, C.; Chira, S.; Nutu, A.M.; Braicu, C.; Baciut, M.; Korban, S.S.; Berindan-Neagoe, I. The Connection between Micrornas and Oral Cancer Pathogenesis: Emerging Biomarkers in Oral Cancer Management. Genes 2021, 12, 1989. [Google Scholar] [CrossRef]
- Balakittnen, J.; Weeramange, C.E.; Wallace, D.F.; Duijf, P.H.G.; Cristino, A.S.; Kenny, L.; Vasani, S.; Punyadeera, C. Noncoding RNAs in Oral Cancer. Wiley Interdiscip. Rev. RNA 2023, 14, e1754. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, X.; Fang, B. mu A Variant of H19 Transcript Regulates EMT and Oral Cancer Progression. Oral. Dis. 2022, 28, 116–124. [Google Scholar] [CrossRef]
- Halajzadeh, J.; Dana, P.M.; Asemi, Z.; Mansournia, M.A.; Yousefi, B. An Insight into the Roles of PiRNAs and PIWI Proteins in the Diagnosis and Pathogenesis of Oral, Esophageal, and Gastric Cancer. Pathol. Res. Pract. 2020, 216, 153112. [Google Scholar] [CrossRef]
- Chamorro-Petronacci, C.; Perez-Sayáns, M.; Padín-Iruegas, M.E.; Marichalar-Mendia, X.; Gallas-Torreira, M.; García García, A. Differential Expression of SnoRNAs in Oral Squamous Cell Carcinomas: New Potential Diagnostic Markers. J. Enzym. Inhib. Med. Chem. 2018, 33, 424–427. [Google Scholar] [CrossRef]
- Cristóbal, I.; Caramés, C.; Rubio, J.; Sanz-Alvarez, M.; Luque, M.; Madoz-Gúrpide, J.; Rojo, F.; García-Foncillas, J. Functional and Clinical Impact of Circrnas in Oral Cancer. Cancers 2020, 12, 1041. [Google Scholar] [CrossRef]
- Deorah, S.; Singh, A.; Gupta, S. Beyond Tissue: Liquid Biopsy’s Promise in Unmasking Oral Cancer. Oral Oncol. Rep. 2024, 9, 100162. [Google Scholar] [CrossRef]
- Baby, N.T.; Abdullah, A.; Kannan, S. The Scope of Liquid Biopsy in the Clinical Management of Oral Cancer. Int. J. Oral. Maxillofac. Surg. 2022, 51, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, Y.J. Liquid Biopsy for Early Detection and Therapeutic Monitoring of Hepatocellular Carcinoma. J. Liver Cancer 2022, 22, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Tomasik, B.; Skrzypski, M.; Bieńkowski, M.; Dziadziuszko, R.; Jassem, J. Current and Future Applications of Liquid Biopsy in Non-Small-Cell Lung Cancer-A Narrative Review. Transl. Lung Cancer Res. 2023, 12, 594–614. [Google Scholar] [CrossRef]
- Gattuso, G.; Crimi, S.; Lavoro, A.; Rizzo, R.; Musumarra, G.; Gallo, S.; Facciponte, F.; Paratore, S.; Russo, A.; Bordonaro, R.; et al. Liquid Biopsy and Circulating Biomarkers for the Diagnosis of Precancerous and Cancerous Oral Lesions. Noncoding RNA 2022, 8, 60. [Google Scholar] [CrossRef]
- Campos, C.D.M.; Jackson, J.M.; Witek, M.A.; Soper, S.A. Molecular Profiling of Liquid Biopsy Samples for Precision Medicine. Cancer J. 2018, 24, 93–103. [Google Scholar] [CrossRef]
- Shinde, A.; Reddy, M.G.; Anjali, A.K. Liquid Biopsy: A Comprehensive Review. Oral. Maxillofac. Pathol. J. 2024, 15, 87–90. [Google Scholar]
- Sharma, Y.K.; Gawande, M.; Reche, A.; Bardia, M.R. Circulating Tumor Cells in Oral Cancer. Cureus 2024, 16, e51684. [Google Scholar] [CrossRef]
- Ju, S.; Chen, C.; Zhang, J.; Xu, L.; Zhang, X.; Li, Z.; Chen, Y.; Zhou, J.; Ji, F.; Wang, L. Detection of Circulating Tumor Cells: Opportunities and Challenges. Biomark. Res. 2022, 10, 1–25. [Google Scholar] [CrossRef]
- Tretyakova, M.S.; Menyailo, M.E.; Schegoleva, A.A.; Bokova, U.A.; Larionova, I.V.; Denisov, E.V. Technologies for Viable Circulating Tumor Cell Isolation. Int. J. Mol. Sci. 2022, 23, 15979. [Google Scholar] [CrossRef] [PubMed]
- Geng, N.; Lin, W.; Zhang, D.; Cao, W.; Feng, C.; Chen, S. Detection of Circulating Tumor Cells in Peripheral Blood of Patients with Tongue Squamous Cell Carcinoma and Its Relationship with Clinical Features and Prognosis: A Retrospective Study. Discov. Oncol. 2024, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khandare, J.; Qayyumi, B.; Bharde, A.; Aland, G.; Jayant, S.; Tripathi, S.; Singh, N.; Badave, R.; D’Souza, A.; Singh, B.; et al. Correlation of CTCs with Disease Progression in Indian Oral Cancer Patients. J. Clin. Oncol. 2020, 38, e15541. [Google Scholar] [CrossRef]
- Qayyumi, B.; Bharde, A.; Aland, G.; D’Souza, A.; Jayant, S.; Singh, N.; Tripathi, S.; Badave, R.; Kale, N.; Singh, B.; et al. Circulating Tumor Cells as a Predictor for Poor Prognostic Factors and Overall Survival in Treatment Naïve Oral Squamous Cell Carcinoma Patients. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2022, 134, 73–83. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, S.; Li, G.; Yi, Y.; Fu, H.; Gao, Y.; Sun, M. Detection of Peripheral Blood Circulating Tumor Cells in Oral Squamous Cell Carcinoma and Its Clinical Significance. Hua Xi Kou Qiang Yi Xue Za Zhi/West. China J. Stomatol. 2021, 39, 591–597. [Google Scholar] [CrossRef]
- Telekes, A.; Horváth, A. The Role of Cell-Free DNA in Cancer Treatment Decision Making. Cancers 2022, 14, 6115. [Google Scholar] [CrossRef]
- Medina, J.E.; Dracopoli, N.C.; Bach, P.B.; Lau, A.; Scharpf, R.B.; Meijer, G.A.; Andersen, C.L.; Velculescu, V.E. Cell-Free DNA Approaches for Cancer Early Detection and Interception. J. Immunother. Cancer 2023, 11, e006013. [Google Scholar] [CrossRef]
- Roberto, T.M.; Jorge, M.A.; Francisco, G.V.; Noelia, T.; Pilar, R.G.; Andrés, C. Strategies for Improving Detection of Circulating Tumor DNA Using next Generation Sequencing. Cancer Treat. Rev. 2023, 119, 102595. [Google Scholar]
- Dao, J.; Conway, P.J.; Subramani, B.; Meyyappan, D.; Russell, S.; Mahadevan, D. Using CfDNA and CtDNA as Oncologic Markers: A Path to Clinical Validation. Int. J. Mol. Sci. 2023, 24, 13219. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, Y. Circulating Tumor DNA Methylation Detection as Biomarker and Its Application in Tumor Liquid Biopsy: Advances and Challenges. MedComm 2024, 5, e766. [Google Scholar] [CrossRef]
- Song, P.; Wu, L.R.; Yan, Y.H.; Zhang, J.X.; Chu, T.; Kwong, L.N.; Patel, A.A.; Zhang, D.Y. Limitations and Opportunities of Technologies for the Analysis of Cell-Free DNA in Cancer Diagnostics. Nat. Biomed. Eng. 2022, 6, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.H.; Chang, K.W.; Kao, S.Y.; Cheng, H.W.; Liu, C.J. Increased Plasma Circulating Cell-Free DNA Could Be a Potential Marker for Oral Cancer. Int. J. Mol. Sci. 2018, 19, 3303. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Sborchia, M.; Bye, H.; Roman-Escorza, M.; Amar, A.; Henley-Smith, R.; Odell, E.; McGurk, M.; Simpson, M.; Ng, T.; et al. Mutation Detection in Saliva from Oral Cancer Patients. Oral. Oncol. 2024, 151, 106717. [Google Scholar] [CrossRef]
- Kumar, P.; Gupta, S.; Das, B.C. Saliva as a Potential Non-Invasive Liquid Biopsy for Early and Easy Diagnosis/Prognosis of Head and Neck Cancer. Transl. Oncol. 2024, 40, 101827. [Google Scholar] [CrossRef]
- Rashid, S.; Puttagunta, P.; Pamulapati, S.; Yang, J.; Pocha, S.; Saba, N.F.; Teng, Y. Leveraging Saliva for Insights into Head and Neck Cancer. Int. J. Mol. Sci. 2024, 25, 13514. [Google Scholar] [CrossRef]
- Rapado-González, Ó.; López-Cedrún, J.L.; Lago-Lestón, R.M.; Abalo, A.; Rubin-Roger, G.; Salgado-Barreira, Á.; López-López, R.; Muinelo-Romay, L.; Suárez-Cunqueiro, M.M. Integrity and Quantity of Salivary Cell-Free DNA as a Potential Molecular Biomarker in Oral Cancer: A Preliminary Study. J. Oral. Pathol. Med. 2022, 51, 429–435. [Google Scholar] [CrossRef]
- Devaraji, M.; Ravikumar, L. The Role of Liquid Biopsy in Early Detection and Monitoring of Oral Cancer. Oral. Oncol. Rep. 2024, 11, 100618. [Google Scholar] [CrossRef]
- Rapado-González, Ó.; Salta, S.; López-López, R.; Henrique, R.; Suárez-Cunqueiro, M.M.; Jerónimo, C. DNA Methylation Markers for Oral Cancer Detection in Non- and Minimally Invasive Samples: A Systematic Review. Clin. Epigenetics 2024, 16, 105. [Google Scholar] [CrossRef] [PubMed]
- MacLellan, S.A.; Lawson, J.; Baik, J.; Guillaud, M.; Poh, C.F.Y.; Garnis, C. Differential Expression of MiRNAs in the Serum of Patients with High-Risk Oral Lesions. Cancer Med. 2012, 1, 268–274. [Google Scholar] [CrossRef]
- Zahran, F.; Ghalwash, D.; Shaker, O.; Al-Johani, K.; Scully, C. Salivary MicroRNAs in Oral Cancer. Oral. Dis. 2015, 21, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, X.; Sun, Z. Screening and Validation of Plasma Long Non-Coding RNAs as Biomarkers for the Early Diagnosis and Staging of Oral Squamous Cell Carcinoma. Oncol. Lett. 2021, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wang, J.; Tang, Y.; Zhang, S.; Xiong, F.; Guo, C.; Zhou, Y.; Li, Z.; Li, X.; Li, Y.; et al. Upregulation of Long Non-Coding RNA LOC284454 May Serve as a New Serum Diagnostic Biomarker for Head and Neck Cancers. BMC Cancer 2020, 20, 917. [Google Scholar] [CrossRef]
- Roi, A.; Boia, S.; Rusu, L.-C.; Roi, C.I.; Boia, E.R.; Riviș, M. Circulating MiRNA as a Biomarker in Oral Cancer Liquid Biopsy. Biomedicines 2023, 11, 965. [Google Scholar] [CrossRef]
- Tang, H.; Wu, Z.; Zhang, J.; Su, B. Salivary LncRNA as a Potential Marker for Oral Squamous Cell Carcinoma Diagnosis. Mol. Med. Rep. 2013, 7, 761–766. [Google Scholar] [CrossRef]
- Irmer, B.; Chandrabalan, S.; Maas, L.; Bleckmann, A.; Menck, K. Extracellular Vesicles in Liquid Biopsies as Biomarkers for Solid Tumors. Cancers 2023, 15, 1307. [Google Scholar] [CrossRef]
- Asleh, K.; Dery, V.; Taylor, C.; Davey, M.; Djeungoue-Petga, M.A.; Ouellette, R.J. Extracellular Vesicle-Based Liquid Biopsy Biomarkers and Their Application in Precision Immuno-Oncology. Biomark. Res. 2023, 11, 1–37. [Google Scholar] [CrossRef]
- Zhao, Z.; Wijerathne, H.; Godwin, A.K.; Soper, S.A. Isolation and Analysis Methods of Extracellular Vesicles (EVs). Extracell. Vesicles Circ. Nucl. Acids 2021, 2, 80–103. [Google Scholar]
- Leung, L.L.; Riaz, M.K.; Qu, X.; Chan, J.; Meehan, K. Profiling of Extracellular Vesicles in Oral Cancer, from Transcriptomics to Proteomics. Semin. Cancer Biol. 2021, 74, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Kalele, K.; Nyahatkar, S.; Mirgh, D.; Muthuswamy, R.; Adhikari, M.D.; Anand, K. Exosomes: A Cutting-Edge Theranostics Tool for Oral Cancer. ACS Appl. Bio. Mater. 2024, 7, 1400–1415. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, Y.; Zhang, M.; Xie, R.; Duan, N.; Liu, H.; Qin, Y.; Ma, J.; Li, Z.; Ye, P.; et al. Oral Squamous Cell Carcinoma-Derived EVs Promote Tumor Progression by Regulating Inflammatory Cytokines and the IL-17A-Induced Signaling Pathway. Int. Immunopharmacol. 2023, 118, 110094. [Google Scholar] [CrossRef] [PubMed]
- Hang, Y.; Huang, J.Y.; Ding, M.; Shen, Y.; Zhou, Y.Z.; Cai, W. Extracellular Vesicles Reshape the Tumor Microenvironment to Improve Cancer Immunotherapy: Current Knowledge and Future Prospects. Int. Immunopharmacol. 2024, 140, 112820. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Bala, S. Extracellular Vesicles in Oral Squamous Carcinoma Carry Oncogenic MiRNA Profile and Reprogram Monocytes via NF-ΚB Pathway. Oncotarget 2018, 9, 34838–34854. [Google Scholar] [CrossRef]
- Languino, L.R.; Singh, A.; Prisco, M.; Inman, G.J.; Luginbuhl, A.; Curry, J.M.; South, A.P. Exosome-Mediated Transfer from the Tumor Microenvironment Increases TGFβ Signaling in Squamous Cell Carcinoma. Am. J. Transl. Res. 2016, 8, 2432–2437. [Google Scholar]
- Dickman, C.T.D.; Lawson, J.; Jabalee, J.; Maclellan, S.A.; Lepard, N.E.; Bennewith, K.L.; Garnis, C. Selective Extracellular Vesicle Exclusion of MiR-142-3p by Oral Cancer Cells Promotes Both Internal and Extracellular Malignant Phenotypes. Oncotarget 2017, 8, 15252–15266. [Google Scholar] [CrossRef]
- Bano, A.; Vats, R.; Verma, D.; Yadav, P.; Kamboj, M.; Bhardwaj, R. Exploring Salivary Exosomes as Early Predictors of Oral Cancer in Susceptible Tobacco Consumers: Noninvasive Diagnostic and Prognostic Applications. J. Cancer Res. Clin. Oncol. 2023, 149, 15781–15793. [Google Scholar] [CrossRef]
- Wang, J.; Jing, J.; Zhou, C.; Fan, Y. Emerging Roles of Exosomes in Oral Diseases Progression. Int. J. Oral. Sci. 2024, 16, 1–16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barroso, L.; Veiga, P.; Melo, J.B.; Carreira, I.M.; Ribeiro, I.P. Molecular and Genetic Pathogenesis of Oral Cancer: A Basis for Customized Diagnosis and Treatment. Biology 2025, 14, 842. https://doi.org/10.3390/biology14070842
Barroso L, Veiga P, Melo JB, Carreira IM, Ribeiro IP. Molecular and Genetic Pathogenesis of Oral Cancer: A Basis for Customized Diagnosis and Treatment. Biology. 2025; 14(7):842. https://doi.org/10.3390/biology14070842
Chicago/Turabian StyleBarroso, Leonor, Pedro Veiga, Joana Barbosa Melo, Isabel Marques Carreira, and Ilda Patrícia Ribeiro. 2025. "Molecular and Genetic Pathogenesis of Oral Cancer: A Basis for Customized Diagnosis and Treatment" Biology 14, no. 7: 842. https://doi.org/10.3390/biology14070842
APA StyleBarroso, L., Veiga, P., Melo, J. B., Carreira, I. M., & Ribeiro, I. P. (2025). Molecular and Genetic Pathogenesis of Oral Cancer: A Basis for Customized Diagnosis and Treatment. Biology, 14(7), 842. https://doi.org/10.3390/biology14070842





