Simple Summary
Growing rice requires a large amount of fresh water, but with increasing water shortages, improving the efficient use of water is essential. Many efforts have focused on the traits of stomata, tiny pores on leaves that control water loss and gas exchange, but other non-stomatal leaf traits, such as leaf structure, internal water movement, and biochemical processes, also play a crucial role. This review examines how both stomatal and non-stomatal leaf traits interact to influence water use efficiency (WUE) in rice. The findings suggest that optimising stomatal traits alone is not enough because non-stomatal factors significantly affect how efficiently leaves use water. By combining advanced technologies, such as high-throughput plant screening, genetic analysis, and computer modelling, researchers can identify the best mix of traits to develop rice varieties that use water more efficiently without reducing their yield. This integrated breeding approach can help create climate-resilient rice and improve the WUE in other cereal crops, supporting global food security in the face of water scarcity.
Abstract
Globally, rice cultivation consumes large amounts of fresh water, and urgent improvements in water use efficiency (WUE) are needed to ensure sustainable production, given increasing water scarcity. While stomatal traits have been a primary focus for enhancing WUE, complex interactions between stomatal and non-stomatal leaf traits remain poorly understood. In this review, we present an analysis of stomatal and non-stomatal leaf traits influencing WUE in rice. The data suggests that optimising stomatal density and size will be insufficient to maximise WUE because non-stomatal traits such as mesophyll conductance, leaf anatomy, and biochemical composition significantly modulate the relationship between stomatal conductance and the photosynthetic rate. Integrating recent advances in high-throughput phenotyping, multi-omics technologies, and crop modelling, we suggest that combinations of seemingly contradictory traits can enhance WUE without compromising yield potential. We propose a multi-trait breeding framework that leverages both stomatal and non-stomatal adaptations to develop rice varieties with superior WUE and climate resilience. This integrated approach provides a roadmap for accelerating the development of water-efficient rice cultivars, with broad implications for improving WUE in other crops.
1. Introduction
Water scarcity threatens global food security, mainly because over half the world’s population depends on water-intensive crops like rice (Oryza sativa L.) [1,2]. There is substantial evidence that climate change is exacerbating water scarcity, and enhancing the water use efficiency (WUE) of rice crops has become a significant issue for sustaining agricultural productivity [3,4]. Consequently, in addition to testing alternative agronomic methods [5,6,7], researchers worldwide are seeking to breed rice varieties with greater WUE [8,9,10,11,12]. Plant leaves play a pivotal role in water exchange, acting as the primary site for carbon dioxide uptake and water vapour loss through transpiration [4]. Historically, efforts to improve WUE have focused predominantly on stomatal traits, such as stomatal density, size, and conductance. There is, however, mounting evidence that non-stomatal leaf traits, including photosynthetic efficiency, leaf anatomy, and biochemical composition, significantly influence WUE in rice [13,14,15].
Rice cultivation spans a diverse range of agroecosystems, from traditional continuously flooded paddies to water-limited upland and dryland environments. In flooded systems, WUE-related traits may enhance the efficient use of water resources and reduce water input requirements without imposing stress on the crop, whereas in upland systems, these traits are critical for survival, resilience, and yield stability under drought conditions. The physiological and anatomical mechanisms underlying WUE, such as stomatal behaviour, root architecture, and osmotic adjustment, therefore differ in their functional importance depending on the cultivation system. Recognising this spectrum is essential for developing rice varieties adapted to varying water regimes.
WUE can be broadly defined as the ratio of the biomass produced or the yield obtained to the amount of water used [9,12]. However, the concept of WUE encompasses multiple scales and definitions depending on the context of measurement. At the leaf level, WUE is typically expressed as the ratio of carbon assimilation to water loss through transpiration [10]. This can be quantified as intrinsic water use efficiency (WUEi), defined as the ratio of the net CO2 assimilation rate (An) to stomatal conductance (gs), or alternatively computed as the instantaneous water use efficiency (WUEinst), calculated by dividing An by the transpiration rate (E) [10,11,16]. These leaf-level measurements provide insights into the physiological basis of WUE and are influenced by stomatal and non-stomatal leaf traits. Measurement techniques for leaf-level WUE in rice include gas exchange analysis using infrared gas analysers, which allow for the simultaneous measurement of photosynthesis and transpiration rates [11,17] and carbon isotope discrimination (Δ13C) over the period of leaf development [18,19]. Additionally, chlorophyll fluorescence techniques can provide insights into photosynthetic efficiency, which is closely linked to WUE [20].
At the whole-plant level, WUE can be assessed as the ratio of biomass accumulation to cumulative water use [21,22,23,24]. This approach captures both productive and non-productive water losses. Field-scale measurements often focus on yield-based WUE, calculated as the crop yield per unit of water applied or lost through combined evaporation and transpiration [12]. Recent advances in high-throughput phenotyping, including thermal imaging and remote sensing techniques, enable more rapid and precise measurements of WUE-related traits in large populations [25]. These technologies, combined with genomic approaches, are accelerating the identification of genetic determinants of WUE in rice. Understanding the physiological mechanisms underlying WUE variability at the leaf and whole-plant level is essential for developing rice varieties with improved drought tolerance and reduced water requirements.
This review aims to critically evaluate the roles of stomatal and non-stomatal leaf traits in determining WUE in rice and explore their potential for improving water use through breeding. We also explore intricate interactions among these traits and their combined impact on water conservation and carbon assimilation. We examine the complex interplay of leaf traits to identify novel strategies for breeding rice varieties with a superior WUE without compromising their yield potential. Included in our consideration are (1) the varying scales of WUE measurement, from leaf-level gas exchange to whole-plant carbon isotope discrimination; (2) recent advances in the understanding of stomatal behaviour, mesophyll conductance, leaf anatomy, and metabolic adaptations to water stress; and (3) emerging technologies, such as high-throughput phenotyping and multi-omic approaches. By integrating these factors, we aim to provide a more holistic understanding of breeding programs for WUE in rice, including potential synergies and trade-offs. Finally, we consider how agronomic practices [26,27] and environmental factors interact with leaf traits to influence WUE under field conditions. This review was conducted through a systematic literature search of Web of Science, Scopus, and Google Scholar, covering studies from 1984 to 2025 focused on stomatal and non-stomatal leaf traits related to WUE in rice. Priority was given to peer-reviewed research encompassing both field and controlled-environment studies, particularly those addressing genetic, physiological, and anatomical analyses relevant to breeding for improved WUE.
2. Stomatal Leaf Traits and WUE in Rice
Stomatal leaf traits refer to the characteristics and properties of the stomata, including their density, size, distribution, and conductance, which are regulated by controlling the gas exchange between leaves and the atmosphere. As highlighted in a recent herbarium study of plant adaptation to climate change [28], there is extensive knowledge of the dozens of genes involved in stomatal development (in terms of density, size, and distribution).
2.1. Stomatal Density, Size, and Arrangement
Recent studies have demonstrated that modifying stomatal density and size through crop engineering offers a promising approach to enhancing WUE and mitigating water scarcity challenges in agricultural crops [4,29]. Rice exhibits unique stomatal structures compared to other plant species, with greater stomatal densities and smaller stomatal sizes. The stomatal complex in rice comprises two dumbbell-shaped guard cells surrounded by subsidiary cells that regulate the stomatal aperture [30,31].
Four distinct models of leaf stomata were described in mutant populations of the purple rice cultivar, Jao Hom Nin, which varied in stomatal density and size [3]. Rice varieties with a low stomatal density and a smaller stomatal size exhibited improved WUE and biomass production under both field and greenhouse conditions. Similarly, three purple rice mutants were adapted to severe water stress conditions during the reproductive stage of growth, which resulted in a low stomatal density and greater WUE under restricted water conditions [32]. Despite these findings, gaps remain regarding the physiological trade-offs of reduced stomatal density, particularly under fluctuating environmental conditions. While smaller, fewer stomata conserve water and they may limit CO2 uptake under high demand. These dynamics are not yet fully quantified across diverse environments and growth stages.
The genetic manipulation of stomatal traits has shown promise in improving WUE. The high-yielding rice variety IR64 was engineered to reduce stomatal density by enhancing the expression of OsEPF1, a gene involved in rice epidermal patterning [33]. The resulting plants exhibited reduced stomatal conductance and enhanced water conservation. These traits were interpreted as having improved drought tolerance while maintaining or improving yields under elevated atmospheric CO2 and high-temperature conditions. A previous study explored the impact of the arrangement of leaf stomata on the physiological responses of rice to differing levels of water availability [34]. Yet, the implications of this spatial arrangement for canopy-level water and carbon fluxes remain underexplored. They proposed that a greater stomatal density in the upper leaves (the flag leaf and second leaf) with a reduced density but larger stomata in the lower leaves (third and fourth leaves) constituted an adaptive strategy (reduced water loss) for genotypes under conditions of limited water availability. The study concluded that this stomatal configuration could enhance water productivity in rice under aerobic conditions without a reduction in yield.
While much of the current understanding of stomatal traits in rice comes from controlled environments, field-based evaluations reveal important cultivar-specific responses and environmental interactions influencing WUE under real-world drought conditions. Field measurements across diverse genotypes have shown considerable variability in stomatal conductance (gs) (discussed further in Section 2.2), density, and size. For example, a study evaluating 64 rice accessions and high-yielding cultivars found that although the stomatal density did not directly correlate with gs, modern cultivars typically exhibited a higher density and moderate specific conductance, whereas traditional varieties had a lower density but larger stomata. These findings underscore the complex interactions among stomatal traits and highlight their potential for optimisation through breeding to improve photosynthesis and WUE in the field [35]. Complementing this, a multi-location field study demonstrated that drought-tolerant breeding lines, including Sahbhagi Dhan, consistently had longer flag leaves and a lower stomatal density compared to the drought-susceptible cultivar IR64. These traits were positively linked to the grain yield and harvest index under drought across India, Bangladesh, Nepal, and the Philippines, emphasizing the importance of validating stomatal traits under field conditions to guide breeding strategies for drought resilience and sustainable productivity [36]. Overall, field studies show that rice cultivars employ distinct stomatal strategies to manage water scarcity, with drought-tolerant varieties generally exhibiting a lower stomatal density and adaptive leaf traits that promote yield stability. These traits interact with complex environmental factors absent in controlled settings, underscoring the necessity of validating laboratory findings under real field conditions to ensure effective breeding.
2.2. Stomatal Conductance and Aperture
Leaf stomatal conductance (gs) is a critical physiological parameter influenced by a wide range of environmental and plant factors, including light, temperature, humidity, CO2 concentration, and plant water status [4,37]. The relationship between gs and WUE involves a trade-off between maximising CO2 uptake for photosynthesis and minimising water loss through transpiration. Recent studies have focused on understanding genetic and molecular mechanisms that regulate stomatal conductance and aperture in rice. A previous study revealed that the precise control of the stomatal aperture is crucial to maintaining an optimal balance between photosynthesis and water conservation [38]. The study identified genes such as EPF2, OSA1, and SLAC1 involved in stomatal regulation, enabling the enhancement of WUE through genetic manipulation. However, the long-term physiological impacts of manipulating these genes under fluctuating climate conditions remain poorly understood. In particular, the extent to which these molecular interventions affect gas exchange efficiency under combined heat and water stress is underexplored. Comparative evidence from model species like Arabidopsis suggests variation in stomatal responsiveness and downstream signaling efficiency, indicating that gene function may be context-dependent across crops [39].
2.3. Regulation of Transpiration and Carbon Dioxide Uptake
Abscisic acid (ABA) is widely believed to have a central role as a signal for the closure of stomata during water stress [37,40]. Other hormones, such as cytokinins and gibberellins, also contribute to stomatal regulation. Plant circadian rhythms reflect the significance of stomatal conductance, ensuring that stomatal opening and closing are synchronised with environmental light and temperature patterns [40]. This temporal coordination helps optimise WUE and CO2 uptake and contributes to plant growth and survival under varying environmental conditions. Similarly, ion transport and osmotic adjustments within stomatal guard cells facilitate rapid changes in the stomatal aperture, balancing the need for CO2 uptake with water conservation. Fine-tuning these processes enhances plant survival in diverse and changing environments [3,40]. The movement of ions such as potassium (K+), chloride (Cl−), and calcium (Ca2+), along with malate (C4H4O42−), causes water to enter guard cells, leading to the stomata opening for CO2 uptake during photosynthesis. Conversely, water follows when ions efflux from the guard cells, causing the stomata to close, thereby reducing water loss (Figure 1A). The fine-tuning of this mechanism is essential for balancing the demand for CO2 with the need for water conservation, especially under drought stress [41,42]. Despite this mechanistic clarity, limited studies have explored how these responses are modulated across rice genotypes with varying levels of limited water stress or drought resilience. Moreover, cross-species comparisons of hormonal regulation and ion channel dynamics remain scarce, leaving uncertainties about whether similar mechanisms operate with equal efficiency in monocot cereals versus dicot plants.
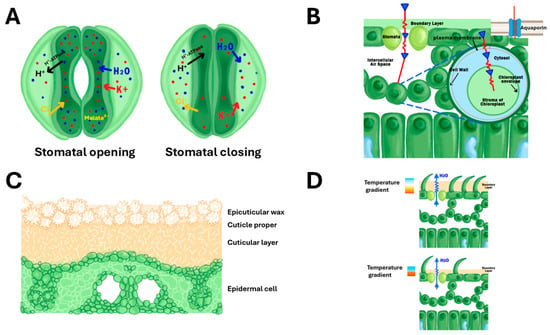
Figure 1.
Anatomical structures and physiological mechanisms regulating water use efficiency (WUE) in rice leaves. (A) Ion transport mechanisms in guard and subsidiary cells during stomatal dynamics. The left panel illustrates stomatal opening, initiated by activation of plasma membrane H+-ATPases, which drive H+ efflux and establish a membrane potential that facilitates the influx of K+, Cl−, and water (H2O), resulting in increased guard cell turgor and pore opening. Malate2− is indicated within the guard cell as a key osmotic anion. Right panel shows stomatal closing with efflux of ions, leading to guard cell shrinkage and reduced aperture. (B) Carbon dioxide diffusion pathway from atmosphere to chloroplast stroma, showing CO2 movement through the boundary layer, plasma membrane, cell wall, intercellular air space, and chloroplast envelope. CO2-permeable aquaporins in the plasma membrane facilitate efficient CO2 transport, enhancing mesophyll conductance for photosynthesis. (C) Cross-sectional view of leaf cuticle architecture showing the multilayer barrier structure: outer epicuticular wax crystals, cuticle proper, cuticular layer, and underlying epidermal cell. This waxy barrier regulates non-stomatal water loss and provides protection against environmental stress. (D) Comparison of leaf surface boundary layer effects on temperature gradients and water vapour (H2O) diffusion. Upper panel demonstrates how leaf pubescence (trichomes) creates a thicker boundary layer, increasing the temperature gradient and slowing water vapour diffusion, thereby improving water conservation and thermal regulation. Lower panel shows smooth leaf surface with thinner boundary layer and steeper temperature gradient from leaf surface to ambient air.
In addition to stomatal and cuticular transpiration, grasses such as rice also release water through hydathodes: specialised structures located at leaf margins that facilitate guttation under the conditions of a high amount of soil moisture and low transpiration demand. Hydathodes, unlike stomata, remain open and serve as low-resistance pathways for liquid exudation, playing a role in water release and nutrient exudation [43]. This process complements transpiration and may contribute to maintaining xylem flow when the evaporative demand is low. Furthermore, rice leaves contain well-defined vascular bundles composed of xylem and phloem, which are responsible for the transport of water, nutrients, and photosynthates. These vascular structures are closely associated with companion cells and are integral to leaf physiology, supporting both hydraulic conductance and photosynthate distribution [44].
3. Non-Stomatal Leaf Traits and WUE in Rice
Non-stomatal leaf traits in rice encompass the structural and biochemical characteristics of the leaf, which influence photosynthetic efficiency and WUE independent of stomatal behaviour.
3.1. The Role of ΦPSII in Photosynthetic Efficiency
In this review, photosynthetic efficiency is categorised as a non-stomatal leaf trait because it primarily reflects the biochemical and physiological processes within the leaf, including the efficiency of the photosynthetic machinery, such as chloroplasts, enzymes, and photosystems like ΦPSII in converting light energy into chemical energy. Photosynthetic efficiency refers to the ability of plants to convert light energy into chemical energy during photosynthesis [45]. Non-stomatal factors, such as the biochemical capacity of the mesophyll cells, chlorophyll content, and the efficiency of the photosynthetic machinery, play a pivotal role in determining photosynthetic efficiency [4,18,46,47]. These factors can enhance the plant’s ability to assimilate CO2 under varying environmental conditions, thereby optimising WUE. By improving photosynthetic efficiency, plants can achieve higher rates of CO2 assimilation per unit of water transpired. This balance is achieved by optimising stomatal conductance, so that stomata remain partially open to allow CO2 uptake while reducing excessive water loss. Improved mesophyll conductance, Rubisco efficiency, or enhanced photoprotection mechanisms can also contribute to a higher intrinsic WUE under drought stress [48]. This balance between CO2 uptake and water conservation is essential for plant productivity and resilience, making photosynthetic efficiency a key target for crop improvement strategies [49].
3.2. Leaf Anatomy
Mesophyll structure, vein density, and bulliform cells significantly impact CO2 diffusion, light distribution, and water management within leaves [50]. Mesophyll conductance (gm), an important factor influencing CO2 diffusion, is discussed in more detail in Section 3.5. Leaf thickness and cell arrangements influence light distribution and absorption, while chloroplast positioning optimises light harvesting and minimises photodamage. However, these anatomical features often involve trade-offs. For instance, it has been reported that an increased vein density in rice is correlated with greater photosynthetic rates but a reduced WUE [51], highlighting the complex interplay between structural traits and physiological efficiency.
Aquaporins, a family of integral membrane proteins, play a dual role in regulating both water transport and CO2 diffusion across leaf tissues, thereby influencing photosynthetic efficiency and WUE in rice. While CO2 is abundant in the atmosphere, its diffusion into the chloroplasts, where photosynthesis occurs, is limited by mesophyll conductance, which includes movement through intercellular air spaces, cell walls, and membranes. Certain aquaporins, particularly plasma membrane intrinsic proteins (PIPs), facilitate this process by enabling CO2 to cross cell membranes more efficiently [52]. In parallel, aquaporins influence leaf hydraulic conductance, which refers to the efficiency of water movement from the xylem to the sites of evaporation within the leaf. This conductance affects stomatal behaviour and transpiration, thereby indirectly modulating photosynthesis and WUE [50,53]. Environmental factors such as nitrogen availability can regulate aquaporin expression and activity, further linking nutrient status to water and carbon dynamics in leaves [48]. A balanced distribution of aquaporins in the mesophyll, bundle sheath, and vascular tissues is considered optimal for improving WUE and enhancing crop resilience under climate stress [54,55].
Studies have emphasised the significance of internal leaf anatomy, including aerenchyma and intercellular air spaces, in regulating CO2 diffusion to the sites of photosynthesis. CO2 must first dissolve in the thin water layer lining the mesophyll cell walls before diffusing into the symplast and reaching the chloroplasts. Therefore, a greater intercellular surface area, supported by well-developed aerenchyma, enhances both CO2 diffusion efficiency and evaporative capacity. This anatomical feature contributes to gm and plays a critical role in photosynthetic performance and WUE, particularly under stress conditions (Figure 1B) [56,57,58].
Studies have further demonstrated that a midvein-deficient mutant shows a higher vein density and photosynthetic efficiency but a significantly lower WUE. Although the study did not include water stress conditions, the mutants are still recommended as promising rice breeding material due to their enhanced photosynthetic efficiency [51]. In a glasshouse experiment with limited water (60% field capacity), it has been observed that high-leaf-mass-area (LMA) mutants exhibited a higher WUE and a more significant total number of veins than low-LMA mutants, which had a lower WUE. However, high-LMA mutants also showed a lower vein density (veins per centimetre) and fewer bulliform cells than their low-LMA counterparts [14].
Abaxial stomatal dominance, where stomata are mainly located on the leaf’s lower surface, helps reduce water loss by limiting stomatal exposure to direct sunlight and wind. This spatial trait can enhance WUE in rice, especially under drought or high vapor pressure conditions. When combined with other anatomical traits like a reduced stomatal density and thicker cuticles (as discussed in Section 3.3), abaxial dominance offers a promising target for breeding rice varieties with improved drought resilience and efficient gas exchange [33,59].
3.3. Leaf Cuticles and Epicuticular Wax
Leaf cuticles and epicuticular wax (EW) are critical components in regulating non-stomatal water loss and enhancing WUE in rice. In rice, the β-Ketoacyl-CoA synthase (KCS) enzyme is essential for the elongation of very long-chain fatty acids, which are major components of cuticular wax [60]. It has been reported that an increased expression of the OsCUT1 gene in rice resulted in improved drought tolerance and wax synthesis without reducing yield [61]. On the other hand, the OsGL1-1 gene plays a crucial role in cuticular wax deposition and drought resistance in rice. Mutants with reduced OsGL1-1 expression showed decreased cuticular wax deposition, increased water loss, and enhanced sensitivity to drought compared to wild-type plants [62]. Furthermore, previous research has demonstrated that EW traits contribute to rice resistance against pests, evidenced by EW rice mutants exhibiting reduced resistance to the rice water weevil (Coleoptera: Curculionidae) and the fall armyworm (Lepidoptera: Noctuidae) (Figure 1C) [63].
3.4. Metabolomic Changes in Leaves
Drought stress triggers complex metabolic responses in plants, regulated by gene activity, which impact vital processes like photosynthesis and respiration and assimilate translocation [64]. These changes help the plant adjust osmotically, manage reactive oxygen species (ROS), and stabilise cell membranes, which are crucial for maintaining function during drought.
Drought-tolerant rice cultivars often accumulate greater concentrations of osmoprotectants, including low-molecular-weight osmolytes such as glycine betaine and proline, as well as organic acids and soluble sugars [64,65]. These compounds help sustain cellular functions and contribute to defence mechanisms under water-limited conditions. Additionally, increased concentrations of antioxidants, phenolic compounds, and flavonoids have been observed in drought-stressed rice leaves, with flavonoids like kaempferol and quercetin playing a significant role in enhancing drought tolerance and modulating WUE [66].
3.5. Mesophyll Conductance (gm) and Intrinsic WUE
gm is a key physiological parameter that controls the rates of CO2 assimilation [46,67]. Von Caemmerer and Evans (2010) found that increasing photosynthesis without altering stomatal function can improve WUE and that enhancing gs could boost photosynthesis if water is not limited [68]. gm is measured as the rate of CO2 diffusion from intercellular spaces to chloroplasts, which substantially affects WUE. Varieties with greater gm also show improved carbon assimilation per unit of water transpired [15]. Additionally, they mentioned that manipulating gm could increase CO2 availability at Rubisco, the key enzyme in photosynthesis that catalyses the fixation of CO2 into organic molecules in the Calvin cycle, potentially improving photosynthesis and WUE [68]. Furthermore, Flexas et al. (2013) stated that to simultaneously improve photosynthesis and WUE, genetic manipulations of gm should be performed without parallel changes in gs [69]. It was suggested that the optimal trait for enhancing WUE is an increased ratio of gm to gs [69].
For rice, gm plays a significant role in transpiration efficiency (TE) and could affect WUE. Upland rice and O. glaberrima showed a stronger response of TE to the gm/gs ratio under drought conditions, suggesting a link between gm and WUE. Furthermore, it suggested that increasing rice’s gm/gs ratio, potentially by modifying leaf anatomy, could enhance both TE and WUE under water-limited conditions [70].
Strategies like genetic manipulation or selective breeding can significantly improve WUE without compromising photosynthetic capacity. The α subunit of a heterotrimeric G protein regulates gm and drought tolerance in rice, suggesting that modifying G protein signalling could enhance WUE in rice cultivars [71].
3.6. Leaf Canopy Architecture
Rice varieties with a higher total leaf area and optimised canopy structure exhibited improved WUE under water-limited conditions [34]. Rice mutants with LMA showed an increased WUE, increased photosynthetic capacity, and superior carboxylation efficiency compared to low-LMA mutants under both well-watered and water-limited conditions [14,72].
A glasshouse study conducted on the Azucena (tropical japonica) and IR64 (India) rice varieties revealed a correlation between increased leaf width and lower Δ13C. Leaves with higher widths have excellent leaf boundary layers that resist gaseous diffusion, which would lower the Δ13C and increase the WUE [73].
A higher crop density creates dense canopies and increases the thickness of the boundary layer, the layer of still air that surrounds each leaf. This reduces evaporative demand and water loss and potentially improves WUE. However, it may also limit CO2 diffusion, affecting photosynthesis [74,75].
Erect leaves, which form smaller angles with the stem, allow for greater light penetration to lower canopy layers, thereby enhancing the overall photosynthetic efficiency and reducing excessive transpiration in the upper canopy. This spatial leaf arrangement has been demonstrated in rice genotypes to improve light interception and optimise water use, contributing to a higher WUE [76,77]. Furthermore, studies on spatial leaf arrangement and canopy architecture in rice show that coordinated leaf positioning around the stem improves the light distribution throughout the canopy, enhances carbon assimilation, and reduces water loss. This trait is particularly beneficial in high-density planting systems, in which maximizing light use efficiency and conserving water are critical for sustaining productivity under variable environmental conditions [78,79].
3.7. Leaf Pubescence and Boundary Layer Resistance
Leaf hairiness (pubescence) has been understudied in rice despite its potential to enhance water conservation and photosynthetic efficiency. Rice varieties show differences in leaf hairiness, from smooth to densely hairy surfaces. These hairs form a boundary layer that slows water vapour diffusion, reducing transpiration and enhancing light reflectance, which can protect the leaf from excessive radiation (Figure 1D) [75,80].
The genetic feasibility of transferring this trait between rice varieties and the physiological benefits of leaf hairiness in terms of WUE in rice have been studied by introducing a gene from chromosome 6 from hairy-leafed Oryza nivara into the less hairy IR24 variety, creating an introgression line with the hairy leaf trait (IL-hairy). The IL-hairy plants exhibited warmer leaf surfaces under sunlight due to the increased boundary layer resistance conferred by the leaf hairs and lower transpiration rates under moderate and high light intensities, leading to a significantly higher photosynthetic WUE [75].
3.8. Carbon Fixation Efficiency
Carbon fixation efficiency is discussed in this review as a non-stomatal trait because it primarily reflects the internal biochemical capacity of the plant to assimilate carbon, beyond the regulation of gas exchange at the stomatal level. While stomatal conductance indeed influences CO2 availability in the intercellular spaces, the efficiency with which CO2 is fixed into carbohydrates, governed by enzymes like Rubisco, chloroplast function, and mesophyll conductance, is largely non-stomatal in nature.
Δ13C and the carbon isotope ratio (δ13C) are widely used indicators of WUE in C3 plants like rice. Δ13C reflects the degree of discrimination against 13CO2 during photosynthesis, which is influenced both by stomatal behaviour and the biochemical processes involved in carbon fixation [18,81]. Higher Δ13C values indicate greater discrimination against 13C, typically associated with higher stomatal conductance and a lower WUE [82]. Conversely, δ13C, which is derived from plant tissue and reflects the integrated effects of carbon assimilation over time, is negatively correlated with Δ13C [83]. A higher δ13C value suggests reduced stomatal conductance and an improved carbon fixation efficiency per unit water lost, thus serving as a reliable proxy for assessing WUE in rice, especially under water-limited conditions [84].
4. Integrating Stomatal and Non-Stomatal Traits for WUE Improvement
Improving WUE in rice requires an integrated approach that considers both stomatal and non-stomatal leaf traits. This will enable the development of more effective strategies for sustainable rice production under water-limited conditions.
4.1. Interactions and Trade-Offs Among Leaf Traits
Reducing stomatal density can enhance water conservation [33], but it may also limit CO2 uptake, potentially impacting photosynthetic efficiency. Conversely, increasing gm can improve photosynthetic rates but may lead to greater water loss if not balanced with appropriate stomatal control [47]. Hence, some studies have highlighted the importance of considering multiple traits simultaneously. It has been demonstrated that rice varieties with both optimised stomatal traits and enhanced gm exhibit superior WUE compared to those improved for either trait alone [85]. Further, it has been found that stomatal traits modulated the relationship between leaf nitrogen content and WUE, emphasizing the need for integrated trait selection in breeding programs [86].
4.2. Breeding Strategies for Optimising WUE Through Leaf Traits
Optimising WUE in rice through breeding involves selecting specific leaf traits that improve water management and photosynthetic performance. This multi-trait approach combines traditional breeding with advanced genetic and genomic tools, focusing on stomatal behaviour, leaf morphology, physiological traits, photosynthetic efficiency, and biochemical pathways to develop more water-efficient, productive rice varieties.
Advances in quantitative trait loci (QTL) mapping have allowed the identification of genetic regions linked to desirable leaf traits, such as leaf area, thickness, and orientation. By correlating genetic variations with these traits, QTL mapping helps breeders efficiently integrate these traits into new plant varieties [87,88,89].
Marker-assisted selection (MAS) allows for the rapid integration of WUE-related traits by using molecular markers associated with key traits like stomatal conductance, leaf morphology, and Rubisco efficiency. Genomic selection takes this a step further by utilising genome-wide data to predict and select for high WUE traits, streamlining the breeding process and reducing the time needed to develop new varieties. This approach leverages high-throughput genotyping and phenotyping to build predictive models that can accurately identify the best candidates for breeding programs focused on improving WUE [90,91,92].
Recent studies have highlighted the importance of integrating both stomatal and non-stomatal traits to enhance WUE in rice. Stomatal morphology and physiology operate in a coordinated manner, influencing both transpiration efficiency and carbon assimilation [49]. Environmental drivers shape stomatal traits, which in turn interact with broader leaf structural features to modulate WUE at the ecosystem level [93]. Complementing these findings, another study [13] has shown that rice genotypes with low-frequency, large-size stomata exhibited a higher intrinsic WUE and more responsive gas exchange under varying vapour pressure deficits. This study also linked epidermal patterning and the expression of key regulatory genes (e.g., ERECTA, TMM, and YODA) to stomatal development, reinforcing the need to integrate leaf surface traits with internal physiological and anatomical features. Collectively, these findings support a holistic breeding approach that considers the interplay of multiple leaf traits to develop rice cultivars resilient to climate variability and water-limited environments.
Understanding the heritability of stomatal and non-stomatal leaf traits is critical for effective breeding strategies to improve WUE in rice. Heritability measures the proportion of total phenotypic variation in a trait attributable to genetic variation among individuals within a population. It quantifies a trait’s potential to respond to selection and is a key parameter in plant breeding programs [94]. Key leaf anatomical traits, such as mesophyll porosity and vein density, have been reported to exhibit high broad-sense heritability values ranging from 0.743 to 0.996, based on multi-model genome-wide association studies (GWAS) in diverse rice accessions [95]. Similarly, a high level of heritability was observed for vein density, leaf width, and photosynthetic rate in 99 rice genotypes evaluated under field conditions, indicating strong genetic control and the potential for effective selection in breeding programs targeting WUE and yield improvement [96]. Advances in high-throughput phenotyping have enabled the identification of heritable image-based drought resistance traits (i-traits), with GWAS revealing 443 loci strongly associated with these traits, many co-localising with known drought resistance QTLs [97]. This demonstrates the feasibility of dissecting complex drought-related traits into simpler, heritable components for breeding applications. Furthermore, photosynthetic traits such as the maximum CO2 assimilation, carboxylation rate, electron transport rate, and triose phosphate utilisation (TPU) exhibit moderate to high broad-sense heritability (H2 = 0.63–0.73), indicating strong genetic control and selection potential for improving photosynthetic efficiency in rice [98]. Genetic advances of up to 17.7% suggest significant gains can be achieved through selection in breeding programs.
4.3. Agronomic Practices and Environmental Factors Influencing WUE
Anthropogenic water management is an important factor influencing plant performance. Inefficient irrigation methods lead to a low WUE and environmental issues. Several alternative rice production methods, such as aerobic rice, direct-seeded rice (DSR), alternate wetting and drying (AWD), and smart irrigation technologies, can improve WUE, though each has specific challenges like high costs or labour intensity [6,99]. Delayed permanent water (DPW) is an alternative water management practice extensively used in Australian rice cultivation to increase water productivity for drill-sown rice. In this method, the rice crop is sown and initially managed like conventional drill-sown rice, but the application of permanent water is delayed until at least 50 days after the first incidence of flush irrigation [100].
Further, it has been emphasised that to promote water-saving irrigation technology, countries should develop supportive policies, raise farmers’ awareness, and offer training and financial assistance tailoring water-saving technologies to specific regions, which can reduce irrigation water use and improve rice WUE, helping to address global water scarcity challenges [101]. As a country with a well-managed rice industry, the Australian government’s Climate-Smart Agriculture Program provides funding for innovative irrigation projects [102]. Educational campaigns like Brisbane’s “Every Drop Counts” raise awareness among farmers about water-saving practices [103]. Organizations such as Irrigation Australia offer training programs and financial subsidies to help farmers adopt new technologies [104].
Nutrient management such as applying balanced fertilisers, particularly nitrogen, in appropriate amounts can enhance plant growth and photosynthetic efficiency, leading to a better WUE [9]. Sowing techniques, such as direct seeding and having a proper transplanting density, can optimise plant establishment and water utilisation. Direct seeding generally achieves a higher WUE than traditional transplanting, though wet seeding by broadcast, a common method, results in a lower WUE compared to traditional transplanting [105]. Moreover, uncontrolled weeds compete with rice for water and nutrients. It has been found that while weed control does not affect total water consumption, it influences WUE through its impact on yield [106]. Higher temperatures can raise transpiration rates, but practices like mulching help regulate plant temperature and reduce water loss. Non-flooded plastic film mulching and wheat straw mulching are effective options for improving WUE in rice cultivation [7]. Therefore, optimising WUE in rice production requires multiple approaches, integrating breeding for better leaf traits, effective agronomic practices, and the consideration of environmental factors to achieve sustainable rice production in water-limited conditions.
4.4. WUE Optimization Strategies Between Traditional and Dryland Rice Farming Systems
Rice cultivation spans a diverse range of agroecosystems, from continuously flooded lowland paddies to water-limited upland or dryland fields. These contrasting environments impose distinct physiological constraints and selection pressures on rice plants, resulting in divergent strategies for optimising WUE. Figure 2 compares key physiological, anatomical, and agronomic traits of rice grown in traditional paddy systems versus dryland/upland systems.
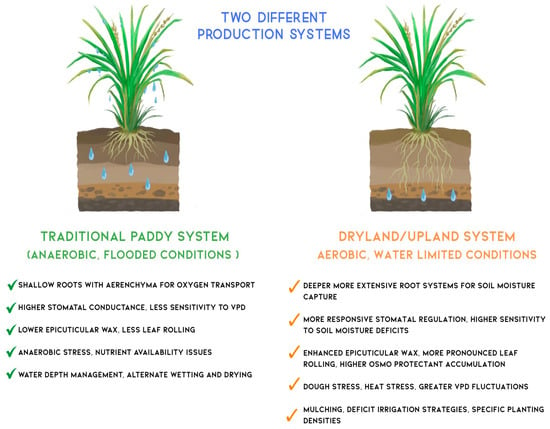
Figure 2.
Comparative analysis of physiological adaptations and management strategies in two distinct rice production systems. The diagram contrasts traditional irrigated paddy systems (left) operating under anaerobic, flooded conditions with dryland/upland systems (right) under aerobic, water-limited conditions. Traditional paddy system features shallow root architecture with aerenchyma tissue for oxygen transport under waterlogged conditions, higher stomatal conductance with reduced sensitivity to vapour pressure deficit (VPD), minimal epicuticular wax deposition, and water depth management through alternate wetting and drying practices. Dryland/upland system exhibits deeper, more extensive root systems for enhanced soil moisture capture, responsive stomatal regulation with a higher degree of sensitivity to soil moisture deficits, increased epicuticular wax accumulation and leaf rolling for water conservation, enhanced osmoprotectant production, and water-conserving management practices, including mulching and deficit irrigation strategies. Water droplets illustrate the different soil moisture distribution patterns characteristic of each system.
In traditional irrigated paddy systems, the presence of standing water creates a saturated microenvironment with a high level of humidity and low evaporative demand. This decouples plant water status from atmospheric vapor pressure deficit (VPD), reducing transpirational losses [107]. Consequently, irrigated rice typically develops shallow root systems and extensive aerenchyma tissue to facilitate internal oxygen transport under anaerobic conditions. Stomatal conductance in these systems tends to be relatively high and less responsive to VPD fluctuations. Additionally, non-stomatal adaptations such as epicuticular wax deposition and leaf rolling are minimal, as the consistently moist conditions reduce the need for such drought avoidance traits [6,108,109,110].
In contrast, dryland or upland rice is cultivated under aerobic conditions in which water availability is often intermittent and limiting. These environments favour more conservative water use strategies. Upland rice varieties typically exhibit deeper and more highly branched root systems to access subsoil moisture. They also demonstrate tighter stomatal regulation, with greater sensitivity to both soil water potential and atmospheric VPD. Non-stomatal adaptations are more pronounced, including increased epicuticular wax accumulation, enhanced leaf rolling during drought stress, and the production of osmoprotectants such as proline and soluble sugars that help maintain cellular integrity under dehydration [6].
Management practices to enhance WUE also differ significantly between the two systems. In irrigated paddy fields, techniques like AWD have been adopted to reduce water inputs while maintaining yield stability. This method intermittently drains fields to encourage deeper rooting and reduce methane emissions. In dryland systems, practices such as mulching, deficit irrigation, and optimised planting densities are employed to reduce surface evaporation, improve soil moisture retention, and enhance root–soil contact [111,112]. These contrasting strategies underscore the importance of context-specific breeding and agronomic interventions. While some core physiological principles of WUE, such as efficient stomatal control and root system architecture, are relevant across systems, their functional significance and genetic regulation vary with environmental and management conditions.
5. Future Perspectives and Research Directions
The exploration of WUE in rice is entering an exciting new era, driven by technological advances and interdisciplinary approaches. This last section examines emerging technologies that are revolutionising our ability to study leaf traits and WUE, from high-resolution imaging techniques to multi-omics approaches It also addresses the key challenges facing researchers and breeders in developing water-efficient rice cultivars and discusses the broader implications of this work for sustainable agriculture and climate resilience. By highlighting these future directions, we aim to provide a roadmap for researchers and emphasise the critical importance of continued innovation in this field.
5.1. Emerging Technologies and Approaches for Studying Leaf Traits and WUE
Recent technological advancements have revolutionised our ability to study leaf traits and WUE in rice at unprecedented levels of detail and scale. These innovations span a wide range of disciplines, from advanced imaging techniques to molecular biology approaches, offering researchers powerful new tools to unravel the complexities of plant–water interactions. Confocal microscopy offers high-resolution, 3D images of leaf tissues, focusing on cellular structures and stomatal dynamics [113]. Its advantage lies in cellular-level visualisation, but it is limited to small sample sizes and controlled environments. Thermal imaging with infrared thermography measures canopy temperature, indicating plant water status and transpiration rates. This method is rapid and non-invasive, yet sensitive to environmental fluctuations such as wind and ambient temperature [114,115]. X-ray micro-computed tomography (Micro-CT) and magnetic resonance imaging (MRI) provide non-destructive 3D imaging of leaf anatomy and water distribution. These offer exceptional anatomical insights but require costly equipment and may not be feasible for field-scale studies [116,117]. Chlorophyll fluorescence imaging, such as pulse amplitude modulation (PAM) fluorometry, assesses photosynthetic efficiency. While informative, its interpretation requires specialised knowledge, and results can vary depending on leaf age and environmental stress [118,119]. Field-based platforms use sensors and imaging systems on tractors [120], while greenhouses and growth chambers utilise automated systems to monitor conditions [121]. Unmanned aerial vehicles (UAVs) equipped with multispectral, hyperspectral, and thermal cameras capture data on plant health and water status from above [122]. UAVs enable the rapid coverage of large areas but depend on the weather and require data-processing expertise [123]. Hyperspectral imaging captures detailed spectral information to assess physiological and biochemical traits [124]. Light detection and ranging (LIDAR) creates high-resolution 3D maps of plant structures, helping to evaluate leaf area and canopy architecture, as well as how these factors influence light interception, transpiration, and overall water use [125]. Both tools provide deep insights into spatial traits, though data complexity and processing time remain significant challenges.
In addition to the stable carbon isotopes in Δ13C, oxygen and hydrogen isotopes (δ18O and δ2H) offer insights into water sources and usage dynamics [126]. Near-infrared spectroscopy (NIRS) can also analyse the chemical composition of plant tissues, which correlates with water content and WUE [127]. Raman spectroscopy analyses molecular compositions and structures to study biochemical changes in leaves [128]. These spectroscopic methods are fast and non-destructive but may require calibration against destructive sampling methods [129].
Genomic approaches like CRISPR-Cas9 and gene editing, along with transcriptomics, proteomics, and metabolomics, are powerful tools for studying WUE. CRISPR-Cas9 enables precise gene modifications to improve WUE, while transcriptomics and proteomics analyse gene expression and protein profiles to identify key WUE regulators [130]. Metabolomics examines the complete set of plant metabolites to understand metabolic pathways related to water use and stress responses [131]. These molecular approaches allow for targeted breeding and trait discovery, though they are resource-intensive and require validation under field conditions.
Big data and machine learning are transforming the study of WUE by using predictive modelling to combine environmental, genomic, and phenotypic data for optimising breeding strategies. Machine learning algorithms analyse large datasets to identify patterns affecting WUE, such as soil moisture and climate conditions [132]. Satellite imagery and geographic information systems (GIS) provide large-scale vegetation, soil, and climate data to monitor and manage WUE across regions. Smart irrigation systems utilise soil moisture sensors and weather forecasts to optimise water use and reduce waste [133]. While these digital tools offer scalability and precision, their effectiveness depends on data quality, infrastructure, and model accuracy.
Compared to traditional methods, such as manual trait measurements, gas exchange analysis using portable photosynthesis systems, or basic soil moisture probes, emerging technologies offer greater speed, scale, precision, and data integration. However, they also demand greater technical expertise, infrastructure, and investments. A balanced application that combines traditional and emerging methods may provide the most comprehensive insight into rice physiology and WUE improvement [134].
5.2. Challenges in Developing Water-Efficient Rice Cultivars
Despite significant advances in our understanding of WUE in rice, the development of water-efficient cultivars faces several complex challenges. These obstacles span genetic, physiological, and agronomic domains, necessitating a multidisciplinary approach to breeding and crop improvement. Key challenges include the trade-off between improving WUE and potential reductions in biomass or yield. Genetic complexity is another challenge, as WUE is influenced by many genes and their interactions with the environment [73,135]. Additionally, the diversity of rice-growing ecosystems, from flooded paddies to rainfed fields, necessitates a multifaceted approach to breeding [136]. Therefore, variations in soil type, fertility, and water-holding capacity further complicate efforts to develop effective cultivars.
High-throughput phenotyping for WUE can be challenging due to the technical difficulty and cost of advanced technologies, which may not be accessible to all breeding programs, especially in developing countries. Additionally, traditional rice farming practices and the historical significance of rice cultivation may lead to resistance among traditional farmers to adopt new cultivars due to concerns about their performance or a preference for established methods.
To address these challenges, multi-omics approaches offer promising tools for dissecting the complex genetic and physiological traits underlying WUE. A summary of these omics-based strategies and their applications in rice WUE research is provided in Table 1.

Table 1.
Multi-omics approaches for studying water use efficiency (WUE) in rice. The table summarises key technologies, their specific applications to WUE research, notable research findings, and representative references across different omics platforms. Genomics approaches focus on gene identification and editing; transcriptomics on expression profiling; proteomics on protein-level changes; metabolomics on biochemical responses; phenomics on high-throughput trait measurement; and integrative approaches on system-level understanding of WUE mechanisms.
The implications for sustainable agriculture and climate resilience are as follows. The development of water-efficient rice cultivars has far-reaching implications that extend beyond immediate agricultural productivity, touching on global issues of food security, environmental sustainability, and climate change adaptation. By addressing these critical challenges, advances in rice WUE have the potential to transform agricultural practices and contribute significantly to sustainable development goals. Developing and using water-efficient rice cultivars can reduce irrigation needs, conserving water resources and supporting agriculture in water-scarce areas. This approach lowers energy requirements for water distribution, promotes sustainable farming, and ensures stable yields despite variable water availability, enhancing food security and farmer incomes, especially in drought-prone regions. By improving crop resilience to water stress, these cultivars help farmers adapt to climate change, minimize crop failure risks, and reduce economic losses. Additionally, efficient water use can decrease methane emissions from flooded fields and benefit local ecosystems by lessening the depletion of natural water bodies [1,146,147].
A comprehensive understanding of the interplay between stomatal and non-stomatal traits is vital for enhancing WUE in rice under climate variability. Stomatal traits directly influence transpiration and gas exchange, while non-stomatal traits, such as leaf morphology, cuticular wax load, and root architecture, contribute to water retention and drought adaptation. Together, these traits modulate the plant’s physiological response to biotic and abiotic stresses, offering complementary pathways to improve WUE without compromising yield potential. Integrating both trait types in breeding strategies supports the development of cultivars that are better adapted to diverse and water-limited environments, aligning with the broader goals of sustainable agriculture and climate resilience [13,93].
5.3. Interaction with Root Traits
Another important consideration that is often overlooked is that the WUE of rice involves a complex interaction between leaf and root traits. Deeper rooting in rice genotypes increases WUE under drought conditions by reducing the internal concentration of CO2 (Ci) within the leaf, highlighting variability in root traits and gas exchange parameters among genotypes. Extensive root systems help plants access more water, but efficient stomatal control is vital to prevent excessive water loss. Roots signal leaves to adjust stomatal conductance based on soil water availability. Good WUE enhances root growth by providing more photosynthates, further improving water acquisition [11]. It was found that the gas and transpiration rates decrease during soil drying before the leaf water potential declines, indicating the importance of root signaling [148]. Hence, the interaction of leaf and root traits in enhancing WUE is also an underexplored area of study that warrants further investigation.
To support future research and trait-based breeding strategies, Supplementary Table S1 provides a comprehensive list of rice genes associated with both stomatal and non-stomatal leaf traits affecting WUE. The table includes gene functions, locus identifiers (IDs), and directional associations with WUE, offering a valuable resource for researchers aiming to link physiological traits with genetic targets. Locus IDs correspond to the Rice Annotation Project and were verified using the SNP-Seek database from the International Rice Research Institute [149]. Genes are organised into two main categories: (A) stomatal traits [13,33,38,150,151,152,153,154] and (B) non-stomatal leaf traits [61,62,92,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178], encompassing key functional areas such as stomatal development and regulation, cuticular wax biosynthesis, leaf morphology, stress response pathways, and metabolic adaptations.
6. Enhanced WUE in Rice
The concept map in Figure 3 encapsulates the complex nature of WUE in rice, highlighting the intricate relationships between stomatal and non-stomatal traits. This visual representation underscores the complexity of WUE as a trait and the need for integrated approaches in future research and breeding efforts. Stomatal traits such as density, size, and conductance interact closely with non-stomatal factors like leaf anatomy, photosynthetic efficiency, and metabolic adaptations. The integration and optimisation branch of the map emphasises the importance of considering trade-offs between these traits and leveraging emerging technologies for crop improvement. This holistic view guides future research directions, suggesting that advancements in WUE will likely come from a synergistic approach that combines traditional breeding with cutting-edge technologies like CRISPR gene editing and high-throughput phenotyping. The map also highlights the critical role of environmental interactions, reminding researchers of the need to develop rice varieties that maintain a high WUE across diverse and changing climatic conditions.
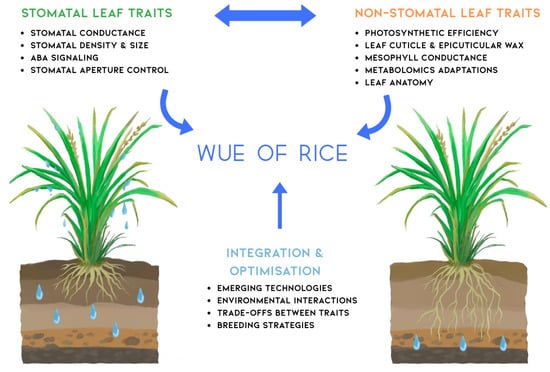
Figure 3.
Conceptual framework illustrating the integrated approach to water use efficiency (WUE) improvement in rice through stomatal and non-stomatal leaf traits. The diagram demonstrates how stomatal leaf traits (left side: stomatal conductance, density and size, ABA signaling pathways, and aperture control mechanisms) and non-stomatal leaf traits (right side: photosynthetic efficiency, leaf cuticle and epicuticular wax deposition, mesophyll conductance, metabolic adaptations, and leaf anatomy) both contribute to overall WUE in rice. The bidirectional arrow between trait categories indicates their interactive and complementary nature in determining water use efficiency. The central integration and optimisation component emphasizes the requirement for holistic approaches incorporating emerging technologies (multi-omics and high-throughput phenotyping), environmental interactions (genotype × environment effects), trade-offs between traits (balancing water conservation with carbon assimilation), and strategic breeding approaches (marker-assisted selection and genomic selection) to develop water-efficient rice cultivars. The rice plants represent the two major cultivation systems (traditional paddy and dryland/upland) discussed throughout the review, highlighting that WUE optimisation strategies must be adapted to specific production environments. This framework provides a roadmap for researchers and breeders to systematically approach WUE improvement through coordinated manipulation of multiple physiological and anatomical traits.
Moving forward, the challenge lies in translating this complex understanding into practical breeding strategies and agronomic practices. Interdisciplinary collaboration will be key, bringing together expertise in plant physiology, genetics, agronomy, and data science. By addressing the interconnected aspects of WUE illustrated in this concept map, researchers can develop more resilient and water-efficient rice varieties, contributing significantly to sustainable agriculture in the face of global water scarcity and climate change.
7. Conclusions
There is an intricate relationship between stomatal and non-stomatal leaf traits, and both collectively have an impact on WUE in rice. Understanding the complex interactions among these traits is essential for developing rice varieties with an improved WUE, as well as for implementing agronomic practices that optimise water management in rice cultivation. Further investigations will reveal specific beneficial varieties for special agronomic practices. By focusing on both stomatal and non-stomatal leaf traits, researchers can develop climate-resilient rice varieties that ensure food security for future generations. Insights from this review can guide breeding programs to develop new crop varieties with an enhanced WUE, ensuring sustainable production in water-scarce regions. Precision agriculture practices can be optimised based on specific leaf traits linked to efficient water use. The review offers a foundation for future research aimed at elucidating the mechanisms of WUE, facilitating the discovery of novel genetic targets and pathways.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology14070843/s1. Table S1. Comprehensive list of rice genes involved in (A) stomatal and (B) non-stomatal leaf traits affecting water use efficiency. The table includes genes spanning key functional categories including stomatal development and regulation, cuticular wax biosynthesis, leaf morphology, stress response pathways, and metabolic adaptations. Locus IDs correspond to the Rice Annotation Project and were verified via the SNP-Seek database from the International Rice Research Institute [149]. Directional trends in the WUE Association column indicate: ↑ = positive association with increased WUE (gene overexpression or enhanced function improves water use efficiency); ↓ = negative association with WUE (gene overexpression or enhanced function reduces water use efficiency); ± = variable or context-dependent association (effects depend on environmental conditions, genetic background, or developmental stage).
Author Contributions
Conceptualisation, V.B.J. and M.A.; writing-original draft preparation, Y.F. and V.B.J.; writing—review and editing, Y.F., V.B.J., M.A. and M.K.; supervision, V.B.J.; project administration, V.B.J.; funding acquisition, V.B.J.; investigation, Y.F., V.B.J., M.A. and M.K.; Validation, formal analysis, Y.F., V.B.J., M.A. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from AgriFutures Australia (Project No. PRP-013062 and PRO-016914).
Data Availability Statement
Data sharing is not applicable to this article as it is a review of the existing literature. No new data were created or analysed.
Acknowledgments
We would like to express our gratitude to AgriFutures Australia for providing the stipend and for funding this research project. We would also like to thank Swinburne University of Technology for the Tuition Fee Scholarship. The three figures used in this publication were graphically illustrated by Samantha and Elisha Butardo.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| WUE | water use efficiency |
| gs | stomatal conductance |
| Δ13C | carbon isotope discrimination |
| gm | mesophyll conductance |
References
- Food and Agriculture Organization of the United Nations (FAO). Water Scarcity—One of the Greatest Challenges of Our Time. Available online: https://www.fao.org/newsroom/story/Water-Scarcity-One-of-the-greatest-challenges-of-our-time/en (accessed on 20 February 2024).
- World Health Organization (WHO). 1 in 3 People Globally Do Not Have Access to Safe Drinking Water—UNICEF, WHO. Available online: https://www.who.int/news/item/18-06-2019-1-in-3-people-globally-do-not-have-access-to-safe-drinking-water-unicef-who (accessed on 27 December 2022).
- Pitaloka, M.K.; Caine, R.S.; Hepworth, C.; Harrison, E.L.; Sloan, J.; Chutteang, C.; Phunthong, C.; Nongngok, R.; Toojinda, T.; Ruengphayak, S.; et al. Induced Genetic Variations in Stomatal Density and Size of Rice Strongly Affects Water Use Efficiency and Responses to Drought Stresses. Front. Plant Sci. 2022, 13, 801706. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of Stomatal Density and Morphology on Water-Use Efficiency in a Changing World. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef]
- Chapagain, T.; Riseman, A.; Yamaji, E. Achieving more with less water: Alternate wet and dry irrigation (AWDI) as an alternative to the conventional water management practices in rice farming. J. Agric. Sci. 2011, 3, 3. [Google Scholar] [CrossRef]
- Mallareddy, M.; Thirumalaikumar, R.; Balasubramanian, P.; Naseeruddin, R.; Nithya, N.; Mariadoss, A.; Eazhilkrishna, N.; Choudhary, A.K.; Deiveegan, M.; Subramanian, E. Maximizing water use efficiency in rice farming: A comprehensive review of innovative irrigation management technologies. Water 2023, 15, 1802. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Yang, J.; Zhang, J. Yield, grain quality and water use efficiency of rice under non-flooded mulching cultivation. Field Crops Res. 2008, 108, 71–81. [Google Scholar] [CrossRef]
- Borrell, A.; Garside, A.; Fukai, S. Improving efficiency of water use for irrigated rice in a semi-arid tropical environment. Field Crops Res. 1997, 52, 231–248. [Google Scholar] [CrossRef]
- Fukai, S.; Mitchell, J. Factors determining water use efficiency in aerobic rice. Crop Environ. 2022, 1, 24–40. [Google Scholar] [CrossRef]
- Gago, J.; Douthe, C.; Florez-Sarasa, I.; Escalona, J.M.; Galmes, J.; Fernie, A.R.; Medrano, H. Opportunities for improving leaf water use efficiency under climate change conditions. Plant Sci. 2014, 226, 108–119. [Google Scholar] [CrossRef]
- Gobu, R.; Dash, G.K.; Lal, J.P.; Swain, P.; Mahender, A.; Anandan, A.; Ali, J. Unlocking the Nexus between Leaf-Level Water Use Efficiency and Root Traits Together with Gas Exchange Measurements in Rice (Oryza sativa L.). Plants 2022, 11, 1270. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Ramachandra, A.; Vijayaraghavareddy, P.; Purushothama, C.; Nagaraju, S.; Sreeman, S. Decoding stomatal characteristics regulating water use efficiency at leaf and plant scales in rice genotypes. Planta 2024, 260, 56. [Google Scholar] [CrossRef]
- Reddy, S.H.; Singhal, R.K.; DaCosta, M.V.J.; Kambalimath, S.K.; Rajanna, M.P.; Muthurajan, R.; Sevanthi, A.M.; Mohapatra, T.; Sarla, N.; Chinnusamy, V.; et al. Leaf mass area determines water use efficiency through its influence on carbon gain in rice mutants. Physiol. Plant. 2020, 169, 194–213. [Google Scholar] [CrossRef]
- Xiong, D.; Flexas, J. From one side to two sides: The effects of stomatal distribution on photosynthesis. New Phytol. 2020, 228, 1754–1766. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.; Rebetzke, G.; Farquhar, G. Improving intrinsic water-use efficiency and crop yield. Crop Sci. 2002, 42, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, S.O.; Habila, D.G.; Mamadou, F.; Abolanle, B.M.; Olatunbosun, A.N. Grain yield and leaf gas exchange in upland NERICA rice under repeated cycles of water deficit at reproductive growth stage. Agric. Water Manag. 2022, 264, 107507. [Google Scholar] [CrossRef]
- Centritto, M.; Lauteri, M.; Monteverdi, M.C.; Serraj, R. Leaf gas exchange, carbon isotope discrimination, and grain yield in contrasting rice genotypes subjected to water deficits during the reproductive stage. J. Exp. Bot. 2009, 60, 2325–2339. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, J.; Wang, Y.; Agathokleous, E.; Hamoud, Y.A.; Qiu, R.; Hong, C.; Tian, M.; Shaghaleh, H.; Guo, X. Relationships between stable isotope natural abundances (δ13C and δ15N) and water use efficiency in rice under alternate wetting and drying irrigation in soils with high clay contents. Front. Plant Sci. 2022, 13, 1077152. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J.; Peng, S.; Xiong, D. Leaf rolling precedes stomatal closure in rice (Oryza sativa) under drought conditions. J. Exp. Bot. 2023, 74, 6650–6661. [Google Scholar] [CrossRef]
- Blankenagel, S.; Yang, Z.; Avramova, V.; Schön, C.-C.; Grill, E. Generating Plants with Improved Water Use Efficiency. Agronomy 2018, 8, 194. [Google Scholar] [CrossRef]
- Bramley, H.; Turner, N.C.; Siddique, K.H.M. Water Use Efficiency. In Genomics and Breeding for Climate-Resilient Crops: Vol. 2 Target Traits; Kole, C., Ed.; Springer: Berlin, Heidelberg, 2013; pp. 225–268. [Google Scholar] [CrossRef]
- Brendel, O. The relationship between plant growth and water consumption: A history from the classical four elements to modern stable isotopes. Ann. For. Sci. 2021, 78, 47. [Google Scholar] [CrossRef]
- Petrík, P.; Petek-Petrik, A.; Mukarram, M.; Schuldt, B.; Lamarque, L.J. Leaf physiological and morphological constraints of water-use efficiency in C3 plants. AoB Plants 2023, 15, plad047. [Google Scholar] [CrossRef] [PubMed]
- Pandya, P.; Parmar, S.; Vadalia, D.; Prajapati, G. Thermal Imaging and Its Application in Irrigation Water Management. AgriTech Today 2023, 1, 40. [Google Scholar]
- Akram, H.; Ali, A.; Sattar, A.; Rehman, H.; Bibi, A. Impact of water deficit stress on various physiological and agronomic traits of three basmati rice (Oryza sativa L.) cultivars. J. Anim. Plant Sci. 2013, 23, 1415–1423. [Google Scholar]
- Yadav, N.; Sevanthi, A.C.; Pandey, R.; Chinnusamy, V.; Singh, A.K.; Singh, N.K. Physiological response and agronomic performance of drought tolerance mutants of Aus rice cultivar Nagina 22 (Oryza sativa L). Field Crops Res. 2023, 290, 108760. [Google Scholar] [CrossRef]
- Lang, P.L.; Erberich, J.M.; Lopez, L.; Weiß, C.L.; Amador, G.; Fung, H.F.; Latorre, S.M.; Lasky, J.R.; Burbano, H.A.; Expósito-Alonso, M. Century-long timelines of herbarium genomes predict plant stomatal response to climate change. Nat. Ecol. Evol. 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, U.; Caine, R.; Atkinson, J.; Harrison, E.; Wells, D.; Chater, C.; Gray, J.; Swarup, R.; Murchie, E. IRice plants overexpressing OsEPF1 show reduced stomatal density and increased root cortical aerenchyma formation. Sci. Rep. 2019, 9, 5584. [Google Scholar] [CrossRef]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress. Adv. Exp. Med. Biol. 2018, 1081, 215–232. [Google Scholar] [CrossRef]
- Laza, M.R.C.; Kondo, M.; Ideta, O.; Barlaan, E.; Imbe, T. Quantitative trait loci for stomatal density and size in lowland rice. Euphytica 2010, 172, 149–158. [Google Scholar] [CrossRef]
- Phunthong, C.; Pitaloka, M.K.; Chutteang, C.; Ruengphayak, S.; Arikit, S.; Vanavichit, A. Rice mutants, selected under severe drought stress, show reduced stomatal density and improved water use efficiency under restricted water conditions. Front. Plant Sci. 2024, 15, 1307653. [Google Scholar] [CrossRef]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef]
- Ramachandra, A.; PRABHU, B.; Sheshshayee, M. Relevance of Stomatal Traits in Determining the Water Use and Water Use Efficiency in Rice Genotypes Adapted to Different Cultivation Systems. Mysore J. Agric. Sci. 2023, 57, 33–42. [Google Scholar]
- Ohsumi, A.; Tomomi, K.; Koki, H.; Takeshi, H.; Shiraiwa, T. Genotypic Variation of Stomatal Conductance in Relation to Stomatal Density and Length in Rice (Oryza sativa L.). Plant Prod. Sci. 2007, 10, 322–328. [Google Scholar] [CrossRef]
- Kumar, S.; Tripathi, S.; Singh, S.P.; Prasad, A.; Akter, F.; Syed, M.A.; Badri, J.; Das, S.P.; Bhattarai, R.; Natividad, M.A.; et al. Rice breeding for yield under drought has selected for longer flag leaves and lower stomatal density. J. Exp. Bot. 2021, 72, 4981–4992. [Google Scholar] [CrossRef]
- Malini, M.; Karwa, S.; Priyadarsini, P.; Kumar, P.; Nagar, S.; Kumar, M.; Kumar, S.; Chinnusamy, V.; Pandey, R.; Pal, M. Abscisic-Acid-Modulated Stomatal Conductance Governs High-Temperature Stress Tolerance in Rice Accessions. Agriculture 2023, 13, 545. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Tang, Y.; Zhu, X.-G. Stomata conductance as a goalkeeper for increased photosynthetic efficiency. Curr. Opin. Plant Biol. 2022, 70, 102310. [Google Scholar] [CrossRef]
- Villalobos-López, M.A.; Arroyo-Becerra, A.; Quintero-Jiménez, A.; Iturriaga, G. Biotechnological advances to improve abiotic stress tolerance in crops. Int. J. Mol. Sci. 2022, 23, 12053. [Google Scholar] [CrossRef]
- Yari Kamrani, Y.; Shomali, A.; Aliniaeifard, S.; Lastochkina, O.; Moosavi-Nezhad, M.; Hajinajaf, N.; Talar, U. Regulatory role of circadian clocks on ABA production and signaling, stomatal responses, and water-use efficiency under water-deficit conditions. Cells 2022, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, T.C.; O’Toole, J.C.; Yambao, E.B.; Turner, N.C. Influence of osmotic adjustment on leaf rolling and tissue death in rice (Oryza sativa L.). Plant Physiol. 1984, 75, 338–341. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Su, Y.; Shen, H. Rice Responses to Abiotic Stress: Key Proteins and Molecular Mechanisms. Int. J. Mol. Sci. 2025, 26, 896. [Google Scholar] [CrossRef]
- Singh, S. Guttation: Quantification, Microbiology and Implications for Phytopathology. In Progress in Botany; Lüttge, U., Beyschlag, W., Cushman, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 75, pp. 187–214. [Google Scholar] [CrossRef]
- Aloni, R. Leaf Development and Vascular Differentiation. In Vascular Differentiation and Plant Hormones; Aloni, R., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 141–162. [Google Scholar] [CrossRef]
- Li, R.; He, Y.; Chen, J.; Zheng, S.; Zhuang, C. Research progress in improving photosynthetic efficiency. Int. J. Mol. Sci. 2023, 24, 9286. [Google Scholar] [CrossRef]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriqui, M.; Diaz-Espejo, A.; Douthe, C.; Dreyer, E.; Ferrio, J.P.; Gago, J.; et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012, 193–194, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Díaz-Espejo, A.; Conesa, M.; Coopman, R.; Douthe, C.; Gago, J.; Gallé, A.; Galmés, J.; Medrano, H.; Ribas-Carbo, M. Mesophyll conductance to CO2 and Rubisco as targets for improving intrinsic water use efficiency in C3 plants. Plant Cell Environ. 2016, 39, 965–982. [Google Scholar] [CrossRef]
- Ye, Z.-P.; Ling, Y.; Yu, Q.; Duan, H.-L.; Kang, H.-J.; Huang, G.-M.; Duan, S.-H.; Chen, X.-M.; Liu, Y.-G.; Zhou, S.-X. Quantifying light response of leaf-scale water-use efficiency and its interrelationships with photosynthesis and stomatal conductance in C3 and C4 species. Front. Plant Sci. 2020, 11, 374. [Google Scholar] [CrossRef]
- Haworth, M.; Marino, G.; Loreto, F.; Centritto, M. Integrating stomatal physiology and morphology: Evolution of stomatal control and development of future crops. Oecologia 2021, 197, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Terashima, I.; Hanba, Y.T.; Tholen, D.; Niinemets, Ü. Leaf Functional Anatomy in Relation to Photosynthesis. Plant Physiol. 2010, 155, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.; Zhang, N.; Tan, T.; Zhang, Z.; Wang, R.; Wu, L. Leaf microstructure and photosynthetic characteristics of a rice midvein-deficient mutant dl-14. Biol. Plant. 2022, 66, 172–177. [Google Scholar] [CrossRef]
- Chen, J.; Yue, K.; Shen, L.; Zheng, C.; Zhu, Y.; Han, K.; Kai, L. Aquaporins and CO2 diffusion across biological membrane. Front. Physiol. 2023, 14, 1205290. [Google Scholar] [CrossRef]
- Ding, L.; Li, Y.; Gao, L.; Lu, Z.; Wang, M.; Ling, N.; Shen, Q.; Guo, S. Aquaporin Expression and Water Transport Pathways inside Leaves Are Affected by Nitrogen Supply through Transpiration in Rice Plants. Int. J. Mol. Sci. 2018, 19, 256. [Google Scholar] [CrossRef]
- Raza, Q.; Rashid, M.A.R.; Waqas, M.; Ali, Z.; Rana, I.A.; Khan, S.H.; Khan, I.A.; Atif, R.M. Genomic diversity of aquaporins across genus Oryza provides a rich genetic resource for development of climate resilient rice cultivars. BMC Plant Biol. 2023, 23, 172. [Google Scholar] [CrossRef]
- Nguyen, M.X.; Moon, S.; Jung, K.-H. Genome-wide expression analysis of rice aquaporin genes and development of a functional gene network mediated by aquaporin expression in roots. Planta 2013, 238, 669–681. [Google Scholar] [CrossRef]
- Flexas, J.; Cano, F.J.; Carriquí, M.; Coopman, R.E.; Mizokami, Y.; Tholen, D.; Xiong, D. CO2 Diffusion Inside Photosynthetic Organs. In The Leaf: A Platform for Performing Photosynthesis; Adams Iii, W.W., Terashima, I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 163–208. [Google Scholar] [CrossRef]
- Oguchi, R.; Onoda, Y.; Terashima, I.; Tholen, D. Leaf Anatomy and Function. In The Leaf: A Platform for Performing Photosynthesis; Adams Iii, W.W., Terashima, I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 97–139. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakazono, M. Cavity Tissue for the Internal Aeration in Plants. In Responses of Plants to Soil Flooding; Sakagami, J.-I., Nakazono, M., Eds.; Springer Nature: Singapore, 2024; pp. 105–117. [Google Scholar] [CrossRef]
- Muir, C.D. Making pore choices: Repeated regime shifts in stomatal ratio. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151498. [Google Scholar] [CrossRef]
- Wang, X.; Guan, Y.; Zhang, D.; Dong, X.; Tian, L.; Qu, L.Q. A β-Ketoacyl-CoA Synthase Is Involved in Rice Leaf Cuticular Wax Synthesis and Requires a CER2-LIKE Protein as a Cofactor. Plant Physiol. 2016, 173, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, Y.; Zhang, H.; Huang, J. A β-ketoacyl-CoA Synthase OsCUT1 Confers Increased Drought Tolerance in Rice. Rice Sci. 2022, 29, 353–362. [Google Scholar] [CrossRef]
- Qin, B.-X.; Tang, D.; Huang, J.; Li, M.; Wu, X.-R.; Lu, L.-L.; Wang, K.-J.; Yu, H.-X.; Chen, J.-M.; Gu, M.-H. Rice OsGL1-1 is involved in leaf cuticular wax and cuticle membrane. Mol. Plant 2011, 4, 985–995. [Google Scholar] [CrossRef]
- Bernaola, L.; Butterfield, T.S.; Tai, T.H.; Stout, M.J. Epicuticular Wax Rice Mutants Show Reduced Resistance to Rice Water Weevil (Coleoptera: Curculionidae) and Fall Armyworm (Lepidoptera: Noctuidae). Environ. Entomol. 2021, 50, 948–957. [Google Scholar] [CrossRef]
- Pradhan, A.K.; Shandilya, Z.M.; Lahkar, L.; Hasnu, S.; Kalita, J.; Borgohain, D.; Tanti, B. Chapter 26—Comparative Metabolomics Approach Towards Understanding Chemical Variation in Rice Under Abiotic Stress. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 537–550. [Google Scholar]
- Hassanein, A.; Ibrahim, E.; Ali, R.A.; Hashem, H. Differential metabolic responses associated with drought tolerance in egyptian rice. J. Appl. Biol. Biotechnol. 2021, 9, 37–46. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.-A.; Asaf, S.; Lubna; Waqas, M.; Park, J.-R.; Asif, S.; Kim, N.; Lee, I.-J.; Kim, K.-M. Drought and UV radiation stress tolerance in rice is improved by overaccumulation of non-enzymatic antioxidant flavonoids. Antioxidants 2022, 11, 917. [Google Scholar] [CrossRef] [PubMed]
- Pons, T.L.; Flexas, J.; von Caemmerer, S.; Evans, J.R.; Genty, B.; Ribas-Carbo, M.; Brugnoli, E. Estimating mesophyll conductance to CO2: Methodology, potential errors, and recommendations. J. Exp. Bot. 2009, 60, 2217–2234. [Google Scholar] [CrossRef]
- von Caemmerer, S.; Evans, J.R. Enhancing C3 photosynthesis. Plant Physiol. 2010, 154, 589–592. [Google Scholar] [CrossRef]
- Flexas, J.; Niinemets, Ü.; Gallé, A.; Barbour, M.M.; Centritto, M.; Diaz-Espejo, A.; Douthe, C.; Galmés, J.; Ribas-Carbo, M.; Rodriguez, P.L. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynth. Res. 2013, 117, 45–59. [Google Scholar] [CrossRef]
- Ouyang, W.; Struik, P.C.; Yin, X.; Yang, J. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 2017, 68, 5191–5205. [Google Scholar] [CrossRef] [PubMed]
- Zait, Y.; Ferrero-Serrano, Á.; Assmann, S.M. The α subunit of the heterotrimeric G protein regulates mesophyll CO2 conductance and drought tolerance in rice. New Phytol. 2021, 232, 2324–2338. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhang, Z.; Huang, G.; Xiong, Z.; Peng, S.; Li, Y.; Buckley, T. High leaf mass per area Oryza genotypes invest more leaf mass to cell wall and show a low mesophyll conductance. AoB Plants 2020, 12, plaa028. [Google Scholar] [CrossRef] [PubMed]
- This, D.; Comstock, J.; Courtois, B.; Xu, Y.; Ahmadi, N.; Vonhof, W.M.; McCouch, S. Genetic analysis of water use efficiency in rice (Oryza sativa L.) at the leaf level. Rice Sci. 2010, 3, 72–86. [Google Scholar] [CrossRef]
- Baloch, A.; Soomro, A.; Javed, M.; Ahmed, M.; Bughio, H.; Bughio, M.; Mastoi, N. Optimum plant density for high yield in rice (Oryza sativa L.). Asian J. Plant Sci. 2002, 1, 25–27. [Google Scholar] [CrossRef]
- Hamaoka, N.; Yasui, H.; Yamagata, Y.; Inoue, Y.; Furuya, N.; Araki, T.; Ueno, O.; Yoshimura, A. A hairy-leaf gene, BLANKET LEAF, of wild Oryza nivara increases photosynthetic water use efficiency in rice. Rice 2017, 10, 20. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L.; Liu, X.; Liu, L.; Cao, W.; Zhu, Y. Modeling the leaf angle dynamics in rice plant. PLoS ONE 2017, 12, e0171890. [Google Scholar] [CrossRef]
- Luo, X.; Zheng, J.; Huang, R.; Huang, Y.; Wang, H.; Jiang, L.; Fang, X. Phytohormones signaling and crosstalk regulating leaf angle in rice. Plant Cell Rep. 2016, 35, 2423–2433. [Google Scholar] [CrossRef]
- Chang, T.-G.; Zhao, H.; Wang, N.; Song, Q.-F.; Xiao, Y.; Qu, M.; Zhu, X.-G. A three-dimensional canopy photosynthesis model in rice with a complete description of the canopy architecture, leaf physiology, and mechanical properties. J. Exp. Bot. 2019, 70, 2479–2490. [Google Scholar] [CrossRef]
- Burgess, A.J.; Retkute, R.; Herman, T.; Murchie, E.H. Exploring relationships between canopy architecture, light distribution, and photosynthesis in contrasting rice genotypes using 3D canopy reconstruction. Front. Plant Sci. 2017, 8, 734. [Google Scholar] [CrossRef]
- T, S.; Vijayalakshmi, D. Physiological and biotechnological approach to improve water use efficiency in rice (Oryza sativa L.): Review. J. Pharmacogn. Phytochem. 2020, 9, 1044–1048. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Breeding for high water-use efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.; Richards, R. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Funct. Plant Biol. 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Impa, S.M.; Nadaradjan, S.; Boominathan, P.; Shashidhar, G.; Bindumadhava, H.; Sheshshayee, M.S. Carbon Isotope Discrimination Accurately Reflects Variability in WUE Measured at a Whole Plant Level in Rice. Crop Sci. 2005, 45, 2517–2522. [Google Scholar] [CrossRef]
- Qu, M.; Hamdani, S.; Li, W.; Wang, S.; Tang, J.; Chen, Z.; Song, Q.; Li, M.; Zhao, H.; Chang, T. Rapid stomatal response to fluctuating light: An under-explored mechanism to improve drought tolerance in rice. Funct. Plant Biol. 2016, 43, 727–738. [Google Scholar] [CrossRef]
- Wu, A.; Hammer, G.L.; Doherty, A.; von Caemmerer, S.; Farquhar, G.D. Quantifying impacts of enhancing photosynthesis on crop yield. Nat. Plants 2019, 5, 380–388. [Google Scholar] [CrossRef]
- Raju, B.R.; Mohankumar, M.V.; Sumanth, K.K.; Rajanna, M.P.; Udayakumar, M.; Prasad, T.G.; Sheshshayee, M.S. Discovery of QTLs for water mining and water use efficiency traits in rice under water-limited condition through association mapping. Mol. Breed. 2016, 36, 35. [Google Scholar] [CrossRef]
- Vinarao, R.; Proud, C.; Snell, P.; Fukai, S.; Mitchell, J. QTL Validation and Development of SNP-Based High Throughput Molecular Markers Targeting a Genomic Region Conferring Narrow Root Cone Angle in Aerobic Rice Production Systems. Plants 2021, 10, 2099. [Google Scholar] [CrossRef]
- Yue, B.; Xue, W.-Y.; Luo, L.-J.; Xing, Y.-Z. QTL Analysis for Flag Leaf Characteristics and Their Relationships with Yield and Yield Traits in Rice. Acta Genet. Sin. 2006, 33, 824–832. [Google Scholar] [CrossRef]
- Hoang, G.T.; Gantet, P.; Nguyen, K.H.; Phung, N.T.P.; Ha, L.T.; Nguyen, T.T.; Lebrun, M.; Courtois, B.; Pham, X.H. Genome-wide association mapping of leaf mass traits in a Vietnamese rice landrace panel. PLoS ONE 2019, 14, e0219274. [Google Scholar] [CrossRef]
- Phetluan, W.; Wanchana, S.; Aesomnuk, W.; Adams, J.; Pitaloka, M.K.; Ruanjaichon, V.; Vanavichit, A.; Toojinda, T.; Gray, J.E.; Arikit, S. Candidate genes affecting stomatal density in rice (Oryza sativa L.) identified by genome-wide association. Plant Sci. 2023, 330, 111624. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Guo, Z.; Huang, C.; Wang, K.; Jiang, N.; Feng, H.; Chen, G.; Liu, Q.; Xiong, L. Genome-wide association study of rice (Oryza sativa L.) leaf traits with a high-throughput leaf scorer. J. Exp. Bot. 2015, 66, 5605–5615. [Google Scholar] [CrossRef]
- Liu, C.; Sack, L.; Li, Y.; Zhang, J.; Yu, K.; Zhang, Q.; He, N.; Yu, G. Relationships of stomatal morphology to the environment across plant communities. Nat. Commun. 2023, 14, 6629. [Google Scholar] [CrossRef]
- Xu, S. Estimation of Heritability. In Quantitative Genetics; Springer International Publishing: Cham, Switzerland, 2022; pp. 147–175. [Google Scholar]
- Narawatthana, S.; Phansenee, Y.; Thammasamisorn, B.-O.; Vejchasarn, P. Multi-model genome-wide association studies of leaf anatomical traits and vein architecture in rice. Front. Plant Sci. 2023, 14, 1107718. [Google Scholar] [CrossRef]
- Pavithra, S.; Senthil, A.; Djanaguiraman, M.; Raveendran, M.; Pushpam, R.; Boopathi, N.M. Estimating Genetic Variability and Diversity for Vein Density, Photosynthesis and Yield in Rice Genotypes. Int. J. Plant Soil Sci. 2022, 34, 48–56. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, W.; Chang, Y.; Ma, X.; Tu, H.; Xiong, F.; Jiang, N.; Feng, H.; Huang, C.; Yang, P.; et al. Genome-Wide Association Studies of Image Traits Reveal Genetic Architecture of Drought Resistance in Rice. Mol. Plant 2018, 11, 789–805. [Google Scholar] [CrossRef]
- Acevedo-Siaca, L.G.; Coe, R.; Quick, W.P.; Long, S.P. Evaluating natural variation, heritability, and genetic advance of photosynthetic traits in rice (Oryza sativa). Plant Breed. 2021, 140, 745–757. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Q.; Peng, S.; Xing, D.; Qin, J.; Laza, R.C.; Punzalan, B.R. Water use efficiency and physiological response of rice cultivars under alternate wetting and drying conditions. Sci. World J. 2012, 2012, 287907. [Google Scholar] [CrossRef]
- Dunn, B. Delaying Permanent Water on Drill Sown Rice, 4th ed.; Primefact 1238; NSW Department of Primary Industries: Orange, NS, USA; Leeton, NSW, Australia, 2023. Available online: https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0007/438955/Delaying-permanent-water-on-drill-sown-rice.pdf (accessed on 10 January 2025).
- Haonan, Q.; Jie, W.; Shihong, Y.; Zewei, J.; Yi, X. Current status of global rice water use efficiency and water-saving irrigation technology recommendations. J. Agron. Crop Sci. 2023, 209, 734–746. [Google Scholar] [CrossRef]
- Farmonaut. Revolutionizing Australian Rice Farming: Sustainable Water-Saving Technologies for Enhanced Productivity. 2024. Available online: https://farmonaut.com/australia/sustainable-rice-farming-5-water-saving-techs-boosting-australia (accessed on 20 October 2024).
- Smarter Irrigation for Profit Phase 2. Smart Irrigation Control in Rice Growing Systems: Application in Bankless Channel Irrigation. Smarter Irrigation for Profit Phase 2. 2021. Available online: https://smarterirrigation.com.au/wp-content/uploads/2021/08/Smart-Irrigation-control-in-rice-growing-systems_Case-Study_July-2021.pdf (accessed on 20 October 2024).
- Authority, M.D.B. New Technology Helping Rice Growers Use Less Water. 27 June 2022. Available online: https://www.mdba.gov.au/news-and-events/newsroom/new-technology-helping-rice-growers-use-less-water (accessed on 20 October 2024).
- Thakur, A.; Roychowdhury, S.; Kundu, D.; Singh, R. Evaluation of planting methods in irrigated rice. Arch. Agron. Soil Sci. 2004, 50, 631–640. [Google Scholar] [CrossRef]
- Sangavi, S.; Porpavai, S. Impact of irrigation scheduling and weed management on water use efficiency and yield of direct dry seeded rice. Madras Agric. J. 2018, 105, 1. [Google Scholar] [CrossRef]
- Cabangon, R.J.; Tuong, T.P.; Castillo, E.G.; Bao, L.X.; Lu, G.; Wang, G.; Cui, Y.; Bouman, B.A.M.; Li, Y.; Chen, C.; et al. Effect of irrigation method and N-fertilizer management on rice yield, water productivity and nutrient-use efficiencies in typical lowland rice conditions in China. Paddy Water Environ. 2004, 2, 195–206. [Google Scholar] [CrossRef]
- He, Q.; Di, D.; Yang, R.; Yuan, W.; Xiao, J.; Yao, Y.; Chen, Q.; Shi, W. Released control of vapor pressure deficit on rainfed rice evapotranspiration responses to extreme droughts in the subtropical zone. Plant Soil 2024, 1–19. [Google Scholar] [CrossRef]
- Haque, M.M.; Mackill, D.J.; Ingram, K.T. Inheritance of Leaf Epicuticular Wax Content in Rice. Crop Sci. 1992, 32, 865–868. [Google Scholar] [CrossRef]
- Wang, C.; Fa, X.; Meng, Q.; Zhang, Y.; Wang, W.; Zhu, K.; Zhang, W.; Gu, J.; Liu, L.; Zhang, J.; et al. Comparison of Agronomic and Physiological Characteristics for Rice Varieties Differing in Water Use Efficiency under Alternate Wetting and Drying Irrigation. Agronomy 2024, 14, 1986. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Q.; Gong, D.; Liu, H.; Luo, L.; Cui, J.; Qi, H.; Ma, F.; He, W.; Mancl, K. Biodegradable film mulching reduces the climate cost of saving water without yield penalty in dryland rice production. Resour. Conserv. Recycl. 2023, 197, 107071. [Google Scholar] [CrossRef]
- Malumpong, C.; Ruensuk, N.; Rossopa, B.; Channu, C.; Intarasathit, W.; Wongboon, W.; Poathong, K.; Kunket, K. Alternate Wetting and Drying (AWD) in broadcast rice (Oryza sativa L.) management to maintain yield, conserve water, and reduce gas emissions in Thailand. Agric. Res. 2021, 10, 116–130. [Google Scholar] [CrossRef]
- Omasa, K.; Konishi, A.; Tamura, H.; Hosoi, F. 3D Confocal laser scanning microscopy for the analysis of chlorophyll fluorescence parameters of chloroplasts in intact leaf tissues. Plant Cell Physiol. 2009, 50, 90–105. [Google Scholar] [CrossRef]
- Luan, Y.; Xu, J.; Lv, Y.; Liu, X.; Wang, H.; Liu, S. Improving the performance in crop water deficit diagnosis with canopy temperature spatial distribution information measured by thermal imaging. Agric. Water Manag. 2021, 246, 106699. [Google Scholar] [CrossRef]
- Jones, H.G.; Serraj, R.; Loveys, B.R.; Xiong, L.; Wheaton, A.; Price, A.H. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 2009, 36, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Mathers, A.W.; Hepworth, C.; Baillie, A.L.; Sloan, J.; Jones, H.; Lundgren, M.; Fleming, A.J.; Mooney, S.J.; Sturrock, C.J. Investigating the microstructure of plant leaves in 3D with lab-based X-ray computed tomography. Plant Methods 2018, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Van As, H. Intact plant MRI for the study of cell water relations, membrane permeability, cell-to-cell and long distance water transport. J. Exp. Bot. 2007, 58, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Gotarkar, D.; Doran, L.; Burns, M.; Hinkle, A.; Kromdijk, J.; Burgess, S.J. High-Throughput Analysis of Non-Photochemical Quenching in Crops Using Pulse Amplitude Modulated Chlorophyll Fluorometry. J. Vis. Exp. 2022, 2022, e63485. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Thorp, K.R.; Thompson, A.L.; Harders, S.J.; French, A.N.; Ward, R.W. High-throughput phenotyping of crop water use efficiency via multispectral drone imagery and a daily soil water balance model. Remote Sens. 2018, 10, 1682. [Google Scholar] [CrossRef]
- Langstroff, A.; Heuermann, M.C.; Stahl, A.; Junker, A. Opportunities and limits of controlled-environment plant phenotyping for climate response traits. Theor. Appl. Genet. 2022, 135, 1–16. [Google Scholar] [CrossRef]
- Fullana-Pericàs, M.; Conesa, M.À.; Gago, J.; Ribas-Carbó, M.; Galmés, J. High-throughput phenotyping of a large tomato collection under water deficit: Combining UAVs’ remote sensing with conventional leaf-level physiologic and agronomic measurements. Agric. Water Manag. 2022, 260, 107283. [Google Scholar] [CrossRef]
- Berni, J.A.; Zarco-Tejada, P.J.; Suárez, L.; Fereres, E. Thermal and narrowband multispectral remote sensing for vegetation monitoring from an unmanned aerial vehicle. IEEE Trans. Geosci. Remote Sens. 2009, 47, 722–738. [Google Scholar] [CrossRef]
- Asaari, M.S.M.; Mertens, S.; Verbraeken, L.; Dhondt, S.; Inzé, D.; Bikram, K.; Scheunders, P. Non-destructive analysis of plant physiological traits using hyperspectral imaging: A case study on drought stress. Comput. Electron. Agric. 2022, 195, 106806. [Google Scholar] [CrossRef]
- Omasa, K.; Hosoi, F.; Konishi, A. 3D lidar imaging for detecting and understanding plant responses and canopy structure. J. Exp. Bot. 2007, 58, 881–898. [Google Scholar] [CrossRef]
- Zhang, M.; Li, C.; Liu, Y.; Zhang, Y.; Nie, J.; Shao, S.; Mei, H.; Rogers, K.M.; Zhang, W.; Yuan, Y. Effects of Water Isotope Composition on Stable Isotope Distribution and Fractionation of Rice and Plant Tissues. J. Agric. Food Chem. 2024, 72, 8955–8962. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ellsworth, P.Z.; Zhou, J.; Cousins, A.B.; Sankaran, S. Evaluation of water-use efficiency in foxtail millet (Setaria italica) using visible-near infrared and thermal spectral sensing techniques. Talanta 2016, 152, 531–539. [Google Scholar] [CrossRef]
- Pérez-Bueno, M.L.; Pineda, M.; Barón, M. Phenotyping plant responses to biotic stress by chlorophyll fluorescence imaging. Front. Plant Sci. 2019, 10, 477268. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Rathnasamy, S.A.; Kambale, R.; Elangovan, A.; Mohanavel, W.; Shanmugavel, P.; Ramasamy, G.; Alagarsamy, S.; Marimuthu, R.; Rajagopalan, V.R.; Manickam, S. Altering Stomatal Density for Manipulating Transpiration and Photosynthetic Traits in Rice through CRISPR/Cas9 Mutagenesis. Curr. Issues Mol. Biol. 2023, 45, 3801–3814. [Google Scholar] [CrossRef] [PubMed]
- Kosová, K.; Urban, M.O.; Vítámvás, P.; Prášil, I.T. Drought stress response in common wheat, durum wheat, and barley: Transcriptomics, proteomics, metabolomics, physiology, and breeding for an enhanced drought tolerance. In Drought Stress Tolerance in Plants, Vol 2: Molecular and Genetic Perspectives; Springer: Berlin/Heidelberg, Germany, 2016; pp. 277–314. [Google Scholar]
- Sharma, N.; Raman, H.; Wheeler, D.; Kalenahalli, Y.; Sharma, R. Data-driven approaches to improve water-use efficiency and drought resistance in crop plants. Plant Sci. 2023, 336, 111852. [Google Scholar] [CrossRef]
- Mo, X.; Liu, S.; Lin, Z.; Xu, Y.; Xiang, Y.; McVicar, T. Prediction of crop yield, water consumption and water use efficiency with a SVAT-crop growth model using remotely sensed data on the North China Plain. Ecol. Model. 2005, 183, 301–322. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, B.; Cao, Y.; Zhang, Y.; Zhan, X.; Shen, X.; Cheng, S.; Lou, X.; Cao, L. Detection of epistatic and gene-environment interactions underlying three quality traits in rice using high-throughput genome-wide data. BioMed Res. Int. 2015, 2015, 135782. [Google Scholar] [CrossRef]
- Bouman, B.A.M.; Barker, R.; Humphreys, E.; Tuong, T.P.; Atlin, G.; Bennett, J.; Dawe, D.; Dittert, K.; Dobermann, A.; Facon, T.; et al. Rice: Feeding the billions. In Water for Food, Water for Life: A Comprehensive Assessment of Water Management in Agriculture; Molden, D., Ed.; Earthscan: London, UK, 2007; pp. 515–550. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, F.; Zhang, F.; Wang, W.; Zhou, Y.; Fu, B.; Li, Z. Comparative transcriptome sequencing of tolerant rice introgression line and its parents in response to drought stress. BMC Genom. 2014, 15, 1026. [Google Scholar] [CrossRef] [PubMed]
- Lenka, S.K.; Katiyar, A.; Chinnusamy, V.; Bansal, K.C. Comparative analysis of drought-responsive transcriptome in Indica rice genotypes with contrasting drought tolerance. Plant Biotechnol. J. 2011, 9, 315–327. [Google Scholar] [CrossRef]
- Chung, P.J.; Jung, H.; Jeong, D.-H.; Ha, S.-H.; Choi, Y.D.; Kim, J.-K. Transcriptome profiling of drought responsive noncoding RNAs and their target genes in rice. BMC Genom. 2016, 17, 563. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gong, F.; Cao, D.; Hu, X.; Wang, W. Advances in crop proteomics: PTMs of proteins under abiotic stress. Proteomics 2016, 16, 847–865. [Google Scholar] [CrossRef]
- Lawas, L.M.F.; Li, X.; Erban, A.; Kopka, J.; Jagadish, S.V.K.; Zuther, E.; Hincha, D.K. Metabolic responses of rice cultivars with different tolerance to combined drought and heat stress under field conditions. GigaScience 2019, 8, giz050. [Google Scholar] [CrossRef]
- Liang, Y.; Tabien, R.E.; Tarpley, L.; Mohammed, A.R.; Septiningsih, E.M. Transcriptome profiling of two rice genotypes under mild field drought stress during grain-filling stage. AoB Plants 2021, 13, plab043. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.M.; Mir, R.A.; Ebinezer, L.B.; Masi, A.; Hami, A.; Manzoor, M.; Salgotra, R.K.; Sofi, N.R.; Mushtaq, R.; Rohila, J.S. Physiological and multi-omics approaches for explaining drought stress tolerance and supporting sustainable production of rice. Front. Plant Sci. 2022, 12, 803603. [Google Scholar] [CrossRef]
- Roy, N.; Debnath, P.; Gaur, H.S. Adoption of Multi-omics Approaches to Address Drought Stress Tolerance in Rice and Mitigation Strategies for Sustainable Production. Mol. Biotechnol. 2025, 1–13. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Z.; Tao, F.; Zhang, L.; Luo, Y.; Zhang, J.; Han, J.; Xie, J. Integrating multi-source data for rice yield prediction across China using machine learning and deep learning approaches. Agric. For. Meteorol. 2021, 297, 108275. [Google Scholar] [CrossRef]
- Singh, N.; Patel, D.; Khalekar, G. Methanogenesis and methane emission in rice/paddy fields. In Sustainable Agriculture Reviews 33. Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2018; Volume 33, pp. 135–170. [Google Scholar] [CrossRef]
- International Rice Research Institute (IRRI). Rice to Zero Hunger. 2019. Available online: https://www.irri.org/world-food-day-2019-rice-zero-hunger (accessed on 12 October 2024).
- Siopongco, J.D.; Sekiya, K.; Yamauchi, A.; Egdane, J.; Ismail, A.M.; Wade, L.J. Stomatal responses in rainfed lowland rice to partial soil drying; evidence for root signals. Plant Prod. Sci. 2008, 11, 28–41. [Google Scholar] [CrossRef]
- International Rice Research Institute (IRRI), SNP-Seek Database. Available online: https://snp-seek.irri.org/ (accessed on 25 January 2024).
- Yu, H.; Murchie, E.H.; González-Carranza, Z.H.; Pyke, K.A.; Roberts, J.A. Decreased photosynthesis in the erect panicle 3 (ep3) mutant of rice is associated with reduced stomatal conductance and attenuated guard cell development. J. Exp. Bot. 2015, 66, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Yoon, J.; Kim, H.Y.; Min, M.K.; Kim, J.-A.; Choi, E.-H.; Lan, W.; Bae, Y.-M.; Luan, S.; Cho, H.; et al. Unique Features of Two Potassium Channels, OsKAT2 and OsKAT3, Expressed in Rice Guard Cells. PLoS ONE 2013, 8, e72541. [Google Scholar] [CrossRef]
- Moon, S.-J.; Kim, H.Y.; Hwang, H.; Kim, J.-A.; Lee, Y.; Min, M.K.; Yoon, I.S.; Kwon, T.-R.; Kim, B.-G. A Dominant Negative OsKAT2 Mutant Delays Light-Induced Stomatal Opening and Improves Drought Tolerance without Yield Penalty in Rice. Front. Plant Sci. 2017, 8, 772. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, L.; Yu, Q.; Zhou, W.; Gou, X.; Li, J.; Hou, S. Multiple transcriptional factors control stomata development in rice. New Phytol. 2019, 223, 220–232. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.; Zhai, L.; Shao, K.; Jiang, K.; Shen, C.; Chen, K.; Wang, S.; Wang, Y.; Xu, J. Genetic Bases of the Stomata-Related Traits Revealed by a Genome-Wide Association Analysis in Rice (Oryza sativa L.). Front. Genet. 2020, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.N.; Lee, S.B.; Suh, M.C.; An, G.; Jung, K.-H. OsABCG9 Is an Important ABC Transporter of Cuticular Wax Deposition in Rice. Front. Plant Sci. 2018, 9, 960. [Google Scholar] [CrossRef]
- Islam, M.A.; Du, H.; Ning, J.; Ye, H.; Xiong, L. Characterization of Glossy1-homologous genes in rice involved in leaf wax accumulation and drought resistance. Plant Mol. Biol. 2009, 70, 443–456. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, L.; Zhang, L.; Zhang, Z.; Zhang, H.; Quan, R.; Zhou, S.; Huang, R. An ethylene response factor OsWR1 responsive to drought stress transcriptionally activates wax synthesis related genes and increases wax production in rice. Plant Mol. Biol. 2011, 78, 275–288. [Google Scholar] [CrossRef]
- Sheng, Z.; Lv, Y.; Li, W.; Luo, R.; Wei, X.; Xie, L.; Jiao, G.; Shao, G.; Wang, J.; Tang, S.; et al. Yellow-Leaf 1 encodes a magnesium-protoporphyrin IX monomethyl ester cyclase, involved in chlorophyll biosynthesis in rice (Oryza sativa L.). PLoS ONE 2017, 12, e0177989. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B. MicroRNAs in Control of Plant Development. J. Cell. Physiol. 2015, 231, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, Y.; Guo, Z.; Ding, Y.; Ding, C. RICE CENTRORADIALIS 1, a TFL1-like Gene, Responses to Drought Stress and Regulates Rice Flowering Transition. Rice 2020, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, D.; Wang, K.; Wu, X.; Lu, L.; Yu, H.; Gu, M.; Yan, C.; Cheng, Z. Mutations in the F-box gene LARGER PANICLE improve the panicle architecture and enhance the grain yield in rice. Plant Biotechnol. J. 2011, 9, 1002–1013. [Google Scholar] [CrossRef]
- Sakamoto, T. Phytohormones and rice crop yield: Strategies and opportunities for genetic improvement. Transgenic Res. 2006, 15, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, A.; Ozawa, M.; Nagasaki, H.; Kato, M.; Noda, Y.; Yamaguchi, T.; Nosaka, M.; Shimizu-Sato, S.; Nagasaki, A.; Maekawa, M.; et al. Two WUSCHEL-related homeobox Genes, narrow leaf2 and narrow leaf3, Control Leaf Width in Rice. Plant Cell Physiol. 2013, 54, 779–792. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, L.; Zeng, D.; Gao, Z.; Guo, L.; Fang, Y.; Zhang, G.; Dong, G.; Yan, M.; Liu, J.; et al. Identification and characterization of NARROW AND ROLLED LEAF 1, a novel gene regulating leaf morphology and plant architecture in rice. Plant Mol. Biol. 2010, 73, 283–292. [Google Scholar] [CrossRef]
- Sonah, H.; Deshmukh, R.; Chand, S.; Srinivasprasad, M.; Rao, G.; Upreti, H.; Singh, A.; Singh, N.; Sharma, T. Molecular mapping of quantitative trait loci for flag leaf length and other agronomic traits in rice (Oryza sativa). Cereal Res. Commun. 2012, 40, 362–372. [Google Scholar] [CrossRef]
- Miura, K.; Ikeda, M.; Matsubara, A.; Song, X.-J.; Ito, M.; Asano, K.; Matsuoka, M.; Kitano, H.; Ashikari, M. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat. Genet. 2010, 42, 545–549. [Google Scholar] [CrossRef]
- Hu, L.; Chen, W.; Yang, W.; Li, X.; Zhang, C.; Zhang, X.; Zheng, L.; Zhu, X.; Yin, J.; Qin, P.; et al. OsSPL9 Regulates Grain Number and Grain Yield in Rice. Front. Plant Sci. 2021, 12, 682018. [Google Scholar] [CrossRef]
- Yu, D.; Ranathunge, K.; Huang, H.; Pei, Z.; Franke, R.; Schreiber, L.; He, C. Wax Crystal-Sparse Leaf1 encodes a β–ketoacyl CoA synthase involved in biosynthesis of cuticular waxes on rice leaf. Planta 2008, 228, 675–685. [Google Scholar] [CrossRef]
- Tang, Y.; Bao, X.; Zhi, Y.; Wu, Q.; Guo, Y.; Yin, X.; Zeng, L.; Li, J.; Zhang, J.; He, W.; et al. Overexpression of a MYB Family Gene, OsMYB6, Increases Drought and Salinity Stress Tolerance in Transgenic Rice. Front. Plant Sci. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, J.; Ishikawa, F.; Yamaguchi, T.; Uemura, M.; Maeshima, M. Identification of 33 Rice Aquaporin Genes and Analysis of Their Expression and Function. Plant Cell Physiol. 2005, 46, 1568–1577. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, W.; Liu, L.; Chen, T.; Zhou, F.; Lin, Y. Identification and functional characterization of a rice NAC gene involved in the regulation of leaf senescence. BMC Plant Biol. 2013, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Matsukura, S.; Mizoi, J.; Yoshida, T.; Todaka, D.; Ito, Y.; Maruyama, K.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Comprehensive analysis of rice DREB2-type genes that encode transcription factors involved in the expression of abiotic stress-responsive genes. Mol. Genet. Genom. 2010, 283, 185–196. [Google Scholar] [CrossRef]
- Xiang, Y.; Tang, N.; Du, H.; Ye, H.; Xiong, L. Characterization of OsbZIP23 as a Key Player of the Basic Leucine Zipper Transcription Factor Family for Conferring Abscisic Acid Sensitivity and Salinity and Drought Tolerance in Rice. Plant Physiol. 2008, 148, 1938–1952. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Li, S.; He, S.; Waßmann, F.; Yu, C.; Qin, G.; Schreiber, L.; Qu, L.-J.; Gu, H. CFL1, a WW Domain Protein, Regulates Cuticle Development by Modulating the Function of HDG1, a Class IV Homeodomain Transcription Factor, in Rice and Arabidopsis. Plant Cell 2011, 23, 3392–3411. [Google Scholar] [CrossRef]
- Guo, T.; Wang, D.; Fang, J.; Zhao, J.; Yuan, S.; Xiao, L.; Li, X. Mutations in the Rice OsCHR4 Gene, Encoding a CHD3 Family Chromatin Remodeler, Induce Narrow and Rolled Leaves with Increased Cuticular Wax. Int. J. Mol. Sci. 2019, 20, 2567. [Google Scholar] [CrossRef]
- Cai, S.; Jiang, G.; Ye, N.; Chu, Z.; Xu, X.; Zhang, J.; Zhu, G.; Nguyen, H.T. A Key ABA Catabolic Gene, OsABA8ox3, Is Involved in Drought Stress Resistance in Rice. PLoS ONE 2015, 10, e0116646. [Google Scholar] [CrossRef]
- Jardim-Messeder, D.; Caverzan, A.; Balbinott, N.; Menguer, P.K.; Paiva, A.L.S.; Lemos, M.; Cunha, J.R.; Gaeta, M.L.; Costa, M.; Zamocky, M.; et al. Stromal Ascorbate Peroxidase (OsAPX7) Modulates Drought Stress Tolerance in Rice (Oryza sativa). Antioxidants 2023, 12, 387. [Google Scholar] [CrossRef]
- Li, H.-W.; Zang, B.-S.; Deng, X.-W.; Wang, X.-P. Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 2011, 234, 1007–1018. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).