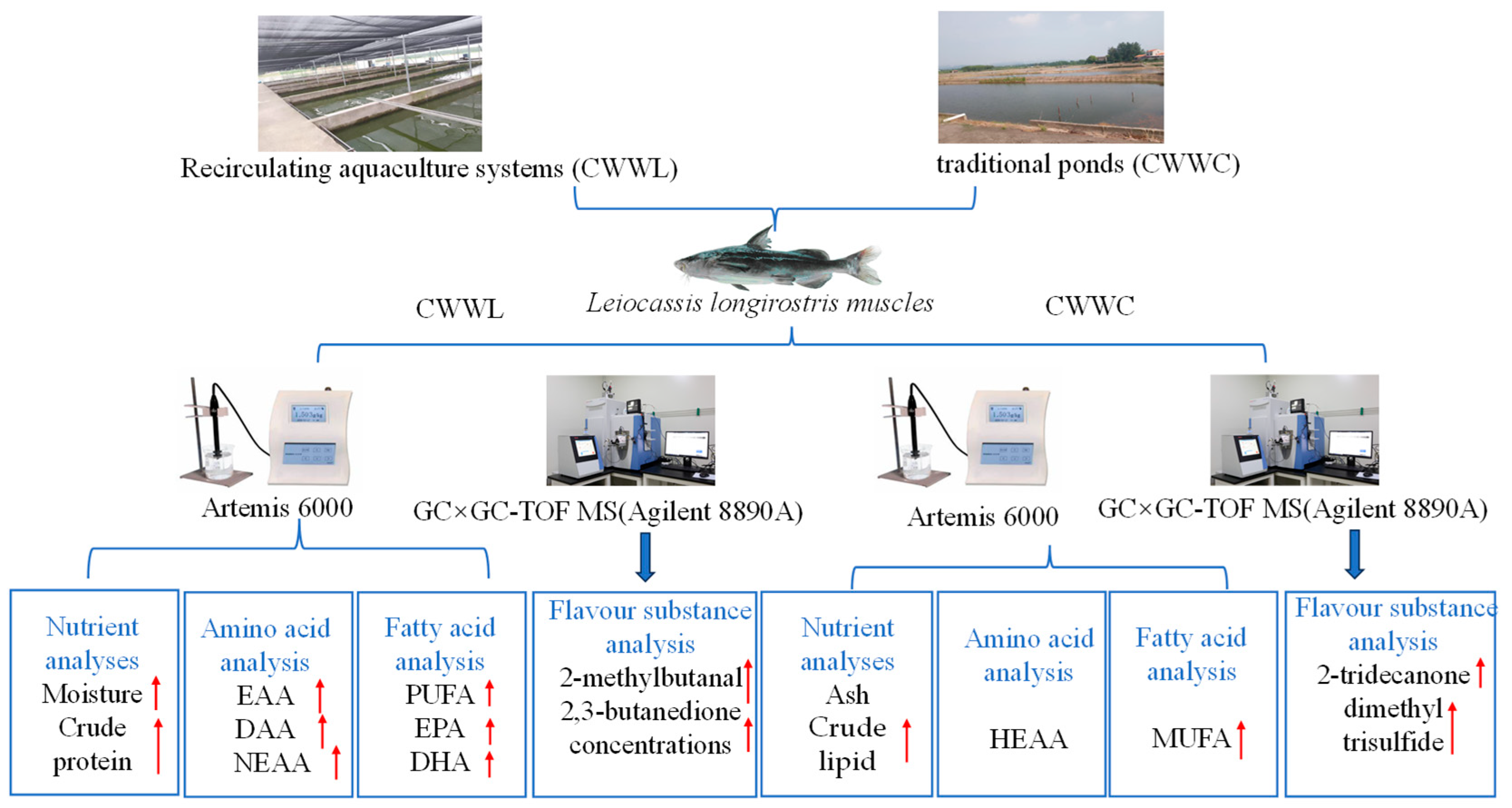
Effects of Two Culture Modes on Muscular Nutrition Content and Volatile Flavor in Chinese Longsnout Catfish (Leiocassis longirostris)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Sampling
2.2. Determination of Nutrient Composition
2.3. Determination of Dietary Flavor
2.4. Measurement of Major Volatile Compounds and Relative Contents
2.5. Data Processing
2.6. Statistical Analysis
3. Results
3.1. Summary of Basic Nutritional Content
3.2. Comparison of Amino Acid Composition
3.3. Differences in Fatty Acid Composition
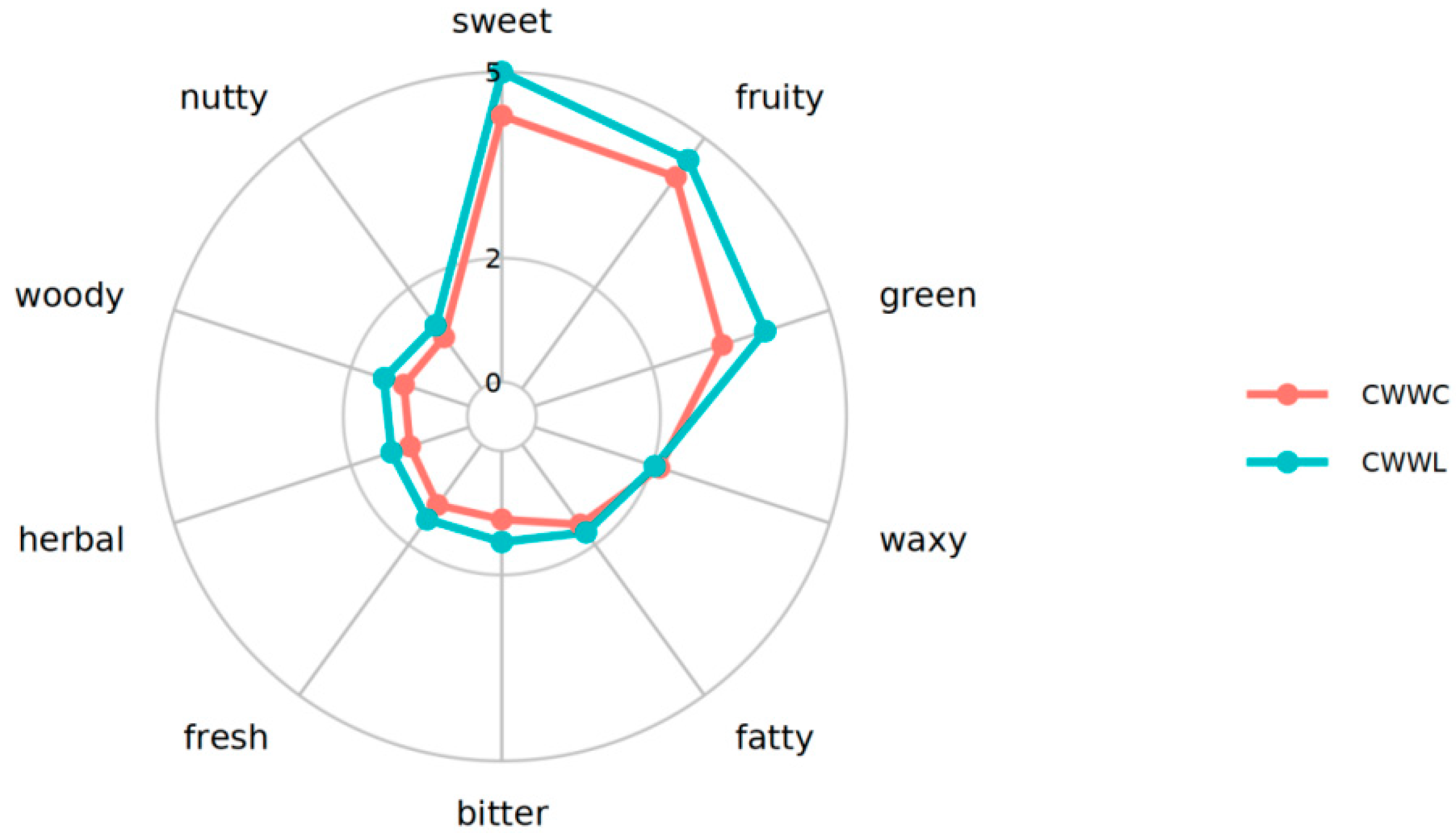
3.4. Volatile Flavor Compounds
3.4.1. Identification of Volatile Substances
3.4.2. Volatile Matter Threshold
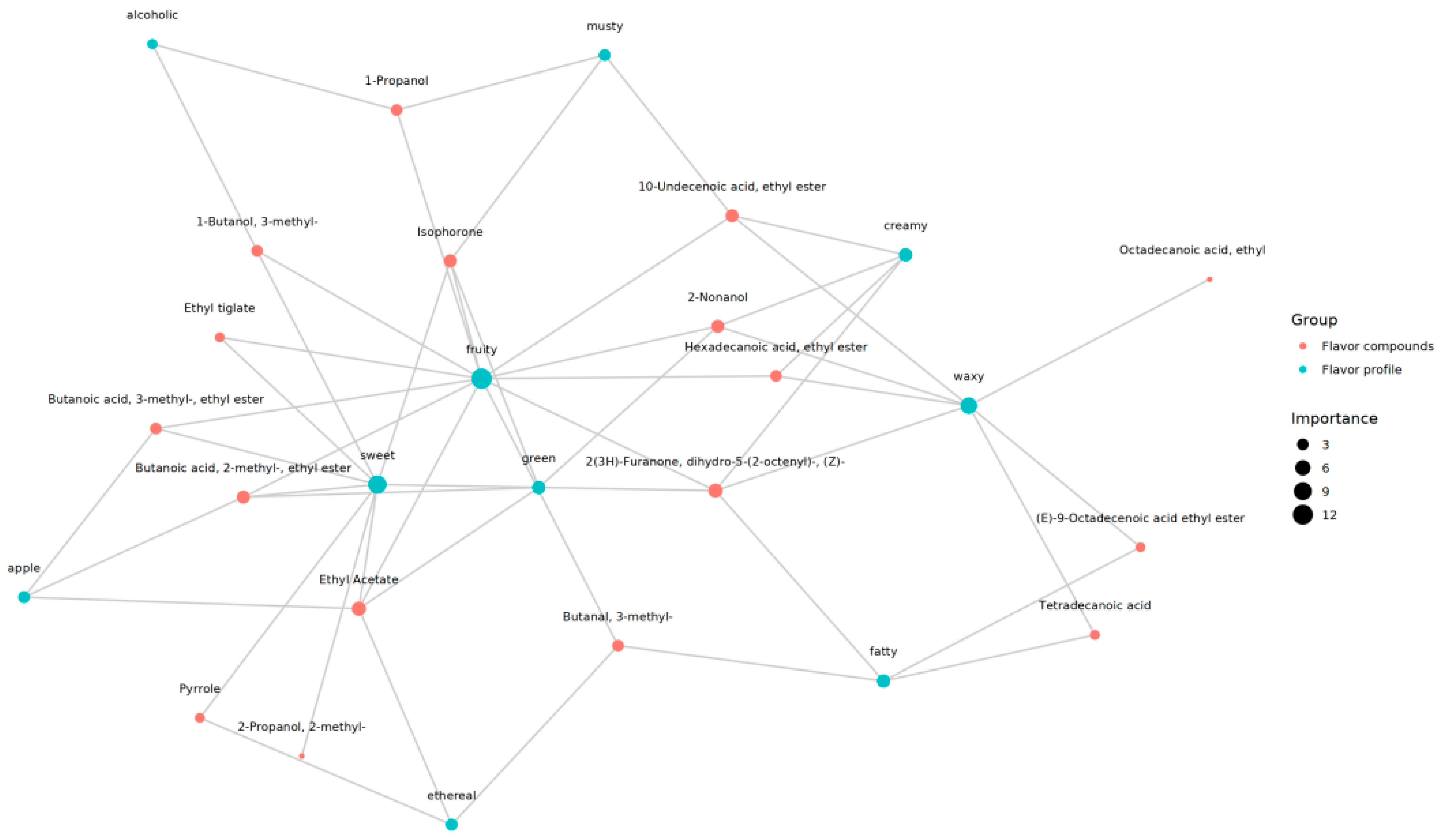
3.4.3. Data on Multivariate Statistics
3.4.4. Comparison of Differential Metabolites
4. Discussion
4.1. Effects of Culture Mode on Nutritional Composition of Chinese Longsnout Catfish
4.2. Effects of Culture Mode on Amino Acid Composition of Chinese Longsnout Catfish
4.3. Effects of Culture Mode on Fatty Acid Composition of Chinese Longsnout Catfish
4.4. Effects of Culture Mode on Volatile Flavor Compounds of Chinese Longsnout Catfish
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, Q.; Xie, S.; Zhu, X.; Lei, W.; Yang, Y. Effect of dietary carbohydrate to ipid ratios on growth and feed utilization in Chinese longsnout catfish (Leiocassis longirostris Günther). J. Appl. Ichthyol. 2007, 23, 605–610. [Google Scholar] [CrossRef]
- Han, D.; Xie, S.; Lei, W.; Zhu, X.; Yang, Y.; Han, D.; Xie, S.; Lei, W.; Zhu, X.; Yang, Y. Effect of ration on the growth and energy budget of Chinese longsnout catfish, Leiocassis longirostris Gunther. Aquac. Res. 2015, 35, 866–873. [Google Scholar] [CrossRef]
- Wang, F.; Ma, X.Z.; Wang, W.; Liu, J.Y. Comparison of proximate composition, amino acid and fatty acid profiles in wild, pond- and cage-cultured longsnout catfish (Leiocassis longirostris). J. Food Sci. Technol. 2012, 47, 1772–1776. [Google Scholar] [CrossRef]
- Huang, G.; Wang, Q.; Yuan, T.; Xiong, M.; Liu, J.; Li, Z.; De Silva, S.S. Combined effects of fish cage culture and increased fishing pressure on wild fish assemblages in a large reservoir, Yangtze River basin, China. Aquaculture 2020, 526, 735373. [Google Scholar] [CrossRef]
- Jin, Y.; He, K.; Xiang, P.; Wang, X.; Tong, L.; Wei, Z.; Zhang, X.; Song, Z. Temporal genetic variation of the Chinese longsnout catfish (Leiocassis longirostris) in the upper Yangtze River with resource decline. Biol. Fishes 2022, 105, 1139–1151. [Google Scholar] [CrossRef]
- Wei, X.Y.; Wang, J.; Guo, S.T.; Lv, Y.Y.; Li, Y.P.; Qin, C.J.; Zou, Y.C.; Shi, Q.C.; Hu, P.; Xiong, X.Q.; et al. Molecular characterization of a teleost-specific toll-like receptor 22 (tlr22) gene from yellow catfish (Pelteobagrus fulvidraco) and its transcriptional change in response to poly I:C and Aeromonas hydrophila stimuli. Fish Shellfish Immunol. 2023, 134, 108579. [Google Scholar] [CrossRef]
- Wang, L.Y.; Feng, L.; Jiang, W.D.; Kuang, S.Y.; Jiang, J.; Li, S.H.; Tang, L.; Zhou, X.Q. Effects of dietary arginine supplementation on growth performance, flesh quality, muscle antioxidant capacity and antioxidant-related signalling molecule expression in young grass carp (Ctenopharyngodon idella). Food Chem. 2015, 167, 91–99. [Google Scholar] [CrossRef]
- Brown, T.W.; Tucker, C.S.; Rutland, B.L. Performance evaluation of four different methods for circulating water in commercial-scale, split-pond aquaculture systems. Aquac. Eng. 2016, 70, 33–41. [Google Scholar] [CrossRef]
- Bao, W.; Zhu, S.; Guo, S.; Wang, L.; Ye, Z. Assessment of water quality and fish production in an intensive pond aquaculture system. Trans. ASABE 2018, 61, 1425–1433. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Li, G.; Wu, H.B.; Liu, X.G.; Yao, Y.H.; Tao, L.; Liu, H. An integrated recirculating aquaculture system (RAS) for land-based fish farming: The effects on water quality and fish production. Aquac. Eng. 2011, 45, 93–102. [Google Scholar] [CrossRef]
- Du, X.; Zhang, W.; He, J.; Zhao, M.; Wang, J.; Dong, X.; Fu, Y.; Xie, X.; Miao, S. The impact of rearing salinity on flesh texture, taste, and fatty acid composition in largemouth bass Micropterus salmoides. Foods 2022, 11, 203261. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wang, L.; Huang, J.; Chen, Y.; Zhang, L.; Zhang, M.; Yu, M.; Jiang, H.; Qiao, Z. Comparative study on nutritional quality and serum biochemical indices of common carp (Cyprinus carpio) aged 11 to 13 months aged cultured in traditional ponds and land-based container aquaculture systems. Food Res. 2023, 169, 112869. [Google Scholar] [CrossRef]
- Hu, B.; Zhou, J.; Qiu, H.; Lai, X.; Li, J.; Wu, D.; Sheng, J.; Hong, Y. Comparison of nutritional quality and volatile flavor compounds among bighead carp from three aquaculture systems. Saudi J. Biol. Sci. 2021, 28, 4291–4299. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Q.; Wang, Y.; Tang, R.; Li, D. The growth performance, antioxidative status and muscle quality of grass carp (Ctenopharyngodon idellus) cultured in the recirculating pond aquaculture system (RPAS). Aquaculture 2023, 562, 738829. [Google Scholar] [CrossRef]
- Smedt, J.M.D. AOAC validation of qualitative and quantitative methods for microbiology in foods. Association of Official Agricultural Chemists. Int. J. Food Microbiol. 1998, 45, 25–28. [Google Scholar] [CrossRef]
- GB/T 6435-2014; Determination of Moisture in Feedstuffs. China Standard Press: Beijing, China, 2014.
- GB/T 6438-2007; Animal Feeding Stuffs—Determination of crude ash. China Standard Press: Beijing, China, 2007.
- GB 5009.6-2016; National Food Safety Standard—Determination of fat in foods. China Standard Press: Beijing, China, 2016.
- GB 5009.5-2016; National Food Safety Standard—Determination of protein in foods. China Standard Press: Beijing, China, 2016.
- GB/T 18246-2019; Determination of Amino Acids in Feeds. China Standard Press: Beijing, China, 2019.
- GB 5009.168-2016; National Food Safety Standard—Determination of Fatty Acids in Foods. China Standard Press: Beijing, China, 2016.
- Iglesias, J.; Gallardo, R.M.; Medina, R. Determination of carbonyl compounds in fish species samples with solid-phase microextraction with on-fibre derivatization. Food Chem. 2010, 123, 771–778. [Google Scholar] [CrossRef]
- Xiao, N.; Huang, H.; Liu, J.; Jiang, X.; Chen, Q.; Chen, Q.; Shi, W. Comparison of different edible parts of bighead carp (Aristichthys nobilis) flavor. J. Food Biochem. 2021, 45, e13946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, L.; Zhang, Y.; Song, H.; Raza, A.; Pan, W.; Gong, L.; Jiang, C. Comparison of different volatile extraction methods for the identification of fishy off-odor in fish by-products. Molecules 2022, 27, 6177. [Google Scholar] [CrossRef]
- Eisner, R.; Djoumbou Feunang, Y.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated chemical classification with a comprehensive, computable taxonomy. J. Cheminform. 2016, 8, 1–20. [Google Scholar]
- Soons, J.; Zheng, X.; Thompson, R.; Singh, S.; Constantin, C. NIST Ballistics Toolmark Research Database. J. Res. Natl. Inst. Stand. Technol. 2020, 125, 125004. [Google Scholar]
- Suzek, T.O.; Wang, Y.; Xiao, J.; Zhang, J.; Wang, J.; Zhou, Z.; Han, L.; Karapetyan, K.; Dracheva, S.; Shoemaker, B.A.; et al. PubChem’s BioAssay Database. Nucleic Acids Res. 2012, 40, D400–D412. [Google Scholar]
- Yu, P. Applications of hierarchical cluster analysis (CLA) and principal component analysis (PCA) in feed structure and feed molecular chemistry research, using synchrotron-based Fourier transform infrared (FTIR) microspectroscopy. J. Agric. Food Chem. 2005, 53, 7115–7127. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xu, G.; Dai, H.; Xu, P.; Zhang, C.; Gu, R. Differences in muscle cellularity and flesh quality between wild and farmed Coilia nasus (Engraulidae). J. Sci. Food Agric. 2012, 92, 1504–1510. [Google Scholar] [CrossRef]
- Hua, K.; Bureau, D.P. Estimating changes in essential amino acid requirements of rainbow trout and Atlantic salmon as a function of body weight or diet composition using a novel factorial requirement model. Aquaculture 2019, 513, 734440. [Google Scholar] [CrossRef]
- Contreras, A.V.; Torres, N.; Tovar, A.R. PPAR-α as a key nutritional and environmental sensor for metabolic adaptation. Adv. Nutr. 2013, 4, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shi, W.; Zhou, S.; Qu, Y.; Wang, Z. Research on the changes of water-soluble flavor substances in grass carp during steaming. Food Biochem. 2019, 43, e12993. [Google Scholar] [CrossRef]
- Jia, S.-P.; Wang, L.; Zhang, J.-M.; Zhang, L.; Ma, F.-R.; Huang, M.-L.; Liu, S.-S.; Gong, J.-H.; Zhang, M.; Yu, M.; et al. Comparative study on the morphological characteristics and nutritional quality of largemouth bass (Micropterus salmoides) cultured in an aquaculture system using land-based container with recycling water and a traditional pond system. Aquaculture 2022, 549, 73772. [Google Scholar] [CrossRef]
- Heissenberger, M.; Watzke, J.; Kainz, M.J. Effect of nutrition on fatty acid profiles of riverine, lacustrine, and aquaculture-raised salmonids of pre-alpine habitats. Hydrobiologia 2010, 650, 243–254. [Google Scholar] [CrossRef]
- Li, X.-M.; Yuan, J.-M.; Fu, S.-J.; Zhang, Y.-G. The effect of sustained swimming exercise on the growth performance, muscle cellularity and flesh quality of juvenile qingbo (Spinibarbus sinensis). Aquaculture 2016, 465, 287–295. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, A.; Wang, W.; Bao, L.; Wang, J.; Sun, R. Changes of protein-bound and free amino acids in the muscle of the freshwater prawn Macrobrachium nipponense in different salinities. Aquaculture 2004, 233, 561–571. [Google Scholar]
- Rebolé, A.; Velasco, S.; Rodríguez, M.L.; Treviño, J.; Alzueta, C.; Tejedor, J.L.; Ortiz, L.T. Nutrient content in the muscle and skin of fillets from farmed rainbow trout (Oncorhynchus mykiss). Food Chem. 2015, 174, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Sakurai, Y.; Honryo, T.; Kawahara, M.; Ishibashi, Y. Improving rearing performance in sea-cage culture of Pacific bluefin tuna Thunnus orientalis juveniles (Temminck and Schlegel) using small sea cages. Aquac. Res. 2020, 51, 3017–3302. [Google Scholar] [CrossRef]
- Trushenski, J.T.; Crouse, C.C.; Rombenso, A.N. Effects of fish oil sparing on fillet fatty acid composition in hybrid Striped Bass are influenced by dietary levels of saturated and unsaturated fatty acids. N. Am. J. Aquac. 2015, 77, 160–169. [Google Scholar] [CrossRef]
- Zeng, W.-H.; Wei, X.-Y.; Qin, W.; Qin, C.-J.; Shi, Q.; Guo, S.-T.; Prathomya, P.; Zhang, S.-Y.; Fu, P.; Hu, W. Molecular characterization, spatiotemporal expression patterns of fatty acid elongase (elovl8) gene, and its transcription changes in response to different diet stimuli in yellow catfish (Pelteobagrus fulvidraco). Front. Mar. Sci. 2023, 10, 1270776. [Google Scholar] [CrossRef]
- Wen, Z.; Li, Y.; Bian, C.; Shi, Q.; Li, Y. Genome-wide identification of a novel elovl4 gene and its transcription in response to nutritional and osmotic regulations in rabbitfish (Siganus canaliculatus). Aquaculture 2020, 529, 735666. [Google Scholar] [CrossRef]
- Neill, B.; Roux, A.L.; Hoffman, L.C. Comparative study of the nutritional composition of wild versus farmed yellowtail (Seriola lalandi). Aquaculture 2015, 448, 169–175. [Google Scholar] [CrossRef]
- Parma, L.; Badiani, A.; Bonaldo, A.; Viroli, C.; Farabegoli, F.; Silvi, M.; Bonvini, E.; Pirini, M.; Gatta, P.P. Farmed and wild common sole (Solea solea L.): Comparative assessment of morphometric parameters, processing yields, selected nutritional traits and sensory profile. Aquaculture 2019, 502, 63–71. [Google Scholar] [CrossRef]
- Tuttle, J.T.; Smith, M.A.; Roy, L.A.; Jones, M.; Lochmann, R.; Kelly, A.M. Effects of different feeding regimes on growth rates and fatty acid composition of largemouth bass Micropterus nigricans at high water temperatures. Animals 2022, 12, 2797. [Google Scholar] [CrossRef]
- Burnette, J.A.; Flick, G.J.; Ward, D.R.; Young, R.W. Comparison of composition and selected enzyme activities in Crassostrea virginica and Crassostrea gigas, Eastern and Korean oysters. J. Food Prot. 1979, 42, 251–255. [Google Scholar] [CrossRef]
- Menegazzo, M.L.; Petenuci, M.E.; Fonseca, G.G. Production and characterization of crude and refined oils obtained from the co-products of Nile tilapia and hybrid sorubim processing. Food Chem. 2014, 157, 100–110. [Google Scholar] [CrossRef]
- Zhuang, K.; Wu, N.; Wang, X.; Wu, X.; Wang, S.; Long, X.; Wei, X. Effects of 3 Feeding Modes on the Volatile and Nonvolatile Compounds in the Edible Tissues of Female Chinese Mitten Crab (Eriocheir sinensis). J. Food Sci. 2016, 81, S968–S981. [Google Scholar] [CrossRef] [PubMed]
- Bonacic, K.; Conde-Sieira, M.; Velasco, C.; Valente, L.M.; Morais, S.; Soengas, J.L. Hypothalamic fatty acid sensing in Senegalese sole (Solea senegalensis): Response to long-chain saturated, monounsaturated, and polyunsaturated (n-3) fatty acids. Intgr. Comp. Physiol. 2015, 309, R1521–R1531. [Google Scholar]
- Lu, C.X.; Weng, L.P.; Wang, H.H. Investigation on the key odor compounds of three cage-farming fishes. Food Ferment. Ind. 2010, 36, 163–169. [Google Scholar]
- Huo, J.; Yao, H.; Li, J.; Wang, J.; Benjakul, S.; Zhang, B. Comparison of physicochemical and volatile flavor properties of neon flying squid (Ommastrephes bartramii), jumbo squid (Dosidicus gigas), and Argentine shortfin squid (Illex argentinus) during chilled storage. Front. Nutr. 2023, 10, 195944. [Google Scholar] [CrossRef]
- Theron, L.; Tournayre, P.; Kondjoyan, N.; Abouelkaram, S.; Sante-Lhoutellier, V.; Berdague, J.L. Analysis of the volatile profile and identification of odour-active compounds in Bayonne ham. Meat Sci. 2010, 85, 453–460. [Google Scholar] [CrossRef]
- Chen, H.; Kang, X.; Wang, X.; Chen, X.; Nie, X.; Xiang, L.; Liu, D.; Zhao, Z. Potential correlation between microbial diversity and volatile flavor substances in a novel Chinese-style sausage during storage. Foods 2023, 12, 3190. [Google Scholar] [CrossRef]
- Shahidi, F. Headspace volatile aldehydes as indicators of lipid oxidation in foods. Adv. Exp. Med. Biol. 2001, 488, 113–123. [Google Scholar]
- Zheng, Z.; Nie, Z.; Zheng, Y.; Tang, X.; Sun, Y.; Zhu, H.; Gao, J.; Xu, P.; Xu, G. Effects of Submerged Macrophytes on the Growth, Morphology, Nutritional Value, and Flavor of Cultured Largemouth Bass (Micropterus salmoides). Molecules 2022, 27, 4927. [Google Scholar] [CrossRef]
- Zhang, K.; Zheng, X.; He, Z.; Yang, T.; Shu, L.; Xiao, F.; Yan, Q. Fish growth enhances microbial sulfur cycling in aquaculture pond sediments. Microb. Biotechnol. 2020, 13, 1597–1610. [Google Scholar] [CrossRef]





| Items | CWWC | CWWL |
|---|---|---|
| Ash (%) | 1.0 ± 0.09 | 1.10 ± 0.1 |
| Moisture (%) | 78.6 ± 0.08 a | 79.4 ± 0.12 b |
| Crude protein (%) | 16.99 ± 0.13 a | 17.7 ± 0.05 b |
| Crude lipid (%) | 4.40 ± 0.05 a | 1.60 ± 0.03 b |
| Amino Acid | Content g/100 g | |
|---|---|---|
| CWWC | CWWL | |
| Thr | 0.76 ± 0.01 a | 0.84 ± 0.02 b |
| Val | 0.73 ± 0.01 | 0.79 ± 0.04 |
| Met | 0.47 ± 0.04 | 0.51 ± 0.02 |
| Phe | 0.67 ± 0.03 | 0.73 ± 0.04 |
| Ile | 0.72 ± 0.03 | 0.77 ± 0.01 |
| Leu | 1.32 ± 0.01 a | 1.44 ± 0.01 b |
| Lys | 1.58 ± 0.03 a | 1.70 ± 0.01 b |
| Asp | 1.70 ± 0.05 a | 1.86 ± 0.03 b |
| Glu | 2.49 ± 0.04 a | 2.72 ± 0.02 b |
| Gly | 0.74 ± 0.01 | 0.75 ± 0.04 |
| Ala | 0.91 ± 0.02 | 0.97 ± 0.04 |
| Ser | 0.68 ± 0.03 a | 0.74 ± 0.01b |
| Tyr | 0.61 ± 0.04 | 0.66 ± 0.01 |
| Cys | 0.13 ± 0.02 | 0.13 ± 0.03 |
| Pro | 0.50 ± 0.05 | 0.53 ± 0.01 |
| His | 0.42 ± 0.04 | 0.45 ± 0.04 |
| Arg | 1.0 ± 0.04 | 1.07 ± 0.03 |
| Total ∑TAA | 15.43 ± 0.23 a | 16.66 ± 0.10 b |
| Total ∑EAA | 6.25 ± 0.05 a | 6.78 ± 0.10 b |
| Total ∑NEAA | 7.76 ± 0.19 a | 8.36 ± 0.08 b |
| Total ∑HEAA | 1.42 ± 0.04 | 1.52 ± 0.07 |
| Total ∑DAA | 5.84 ± 0.10 a | 6.3 ± 0.08 b |
| Fatty Acid | Content 100% | |
|---|---|---|
| CWWC | CWWL | |
| C12:0 | 0.04 ± 0.01 | 0.03 ± 0.01 |
| C14:0 | 1.46 ± 0.06 a | 2.46 ± 0.05 b |
| C15:0 | 0.18 ± 0.04 a | 0.29 ± 0.04 b |
| C16:0 | 19.3 ± 0.14 a | 20.30 ± 0.07 b |
| C17:0 | 0.32 ± 0.04 a | 0.56 ± 0.06 b |
| C18:0 | 5.54 ± 0.05 | 5.58 ± 0.07 |
| C20:0 | 0.27 ± 0.02 | 0.23 ± 0.04 |
| C14:1n5 | 0.03 ± 0.02 a | 0.07 ± 0.02 b |
| C16:1n7 | 3.49 ± 0.07 a | 5.01 ± 0.10 b |
| C17:1n7 | 0.31 ± 0.02 a | 0.47 ± 0.03 |
| C18:1n9c | 39.00 ± 0.14 a | 31.10 ± 0.17 b |
| C22:1n9 | 0.17 ± 0.03 | 0.14 ± 0.03 |
| C18:2n6c | 16.70 ± 0.07 a | 14.10 ± 0.03 b |
| C18:3n6 | 0.09 ± 0.03 | 0.12 ± 0.03 |
| C18:3n3 | 1.39 ± 0.04 a | 1.70 ± 0.03 b |
| C20:2 | 1.06 ± 0.07 | 0.98 ± 0.10 |
| C20:3n6 | 0.50 ± 0.05 a | 0.75 ± 0.12 b |
| C20:3n3 | 0.19 ± 0.02 | 0.24 ± 0.09 |
| C20:4n6 (AA) | 0.71 ± 0.02 a | 1.44 ± 0.06 b |
| C22:2n6 | 0.03 ± 0.01 a | 0.16 ± 0.03 b |
| C20:5n3 (EPA) | 1.40 ± 0.07 a | 2.50 ± 0.03 b |
| C22:6n3 (DHA) | 4.74 ± 0.05 a | 9.09 ± 0.05 b |
| ΣSFA | 27.11 ± 0.08 a | 32.25 ± 0.06 b |
| ΣMUFA | 43.00 ± 0.13 a | 36.79 ± 0.12 b |
| ΣPUFA | 26.81 ± 0.04 a | 31.07 ± 0.15 b |
| EPA + DHA | 6.14 ± 0.06 a | 11.59 ± 0.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Su, Y.; Yang, D.; Shi, Q.; Yi, T.; Wen, Z. Effects of Two Culture Modes on Muscular Nutrition Content and Volatile Flavor in Chinese Longsnout Catfish (Leiocassis longirostris). Biology 2025, 14, 694. https://doi.org/10.3390/biology14060694
Zhou L, Su Y, Yang D, Shi Q, Yi T, Wen Z. Effects of Two Culture Modes on Muscular Nutrition Content and Volatile Flavor in Chinese Longsnout Catfish (Leiocassis longirostris). Biology. 2025; 14(6):694. https://doi.org/10.3390/biology14060694
Chicago/Turabian StyleZhou, Luo, Yingbing Su, Daiqin Yang, Qiong Shi, Tilin Yi, and Zhengyong Wen. 2025. "Effects of Two Culture Modes on Muscular Nutrition Content and Volatile Flavor in Chinese Longsnout Catfish (Leiocassis longirostris)" Biology 14, no. 6: 694. https://doi.org/10.3390/biology14060694
APA StyleZhou, L., Su, Y., Yang, D., Shi, Q., Yi, T., & Wen, Z. (2025). Effects of Two Culture Modes on Muscular Nutrition Content and Volatile Flavor in Chinese Longsnout Catfish (Leiocassis longirostris). Biology, 14(6), 694. https://doi.org/10.3390/biology14060694








