Anti-Diabetic and Antioxidant Effect Evaluation of Thai Shallot and Cha-Miang in Diabetic Rats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Shallot and Cha-Miang Extracts
2.2. Experimental Animals
2.3. Experimental Design
- -
- Group I (control: N-DW): rats in this group were orally administered 1 mL/day of distilled water.
- -
- Group II (Diabetic control: D-DW): rats were orally administered 1 mL/day of distilled water.
- -
- Group III (Positive control: D-M): rats were orally administered metformin (Met) at doses 100 mg/kg BW.
- -
- Group IV (Diabetic with SHE: D-S): rats in this group were orally administered SHE 1000 mg/kg BW.
- -
- Group V (Diabetic with CME: D-C): rats in this group were orally administered CME 300 mg/kg BW.
- -
- Group VI (Diabetic with FCME: D-F): rats in this group were orally administered FCME 300 mg/kg BW.
- -
- Group VII (Diabetic with SHE + CME: D-SC): rats in this group were orally administered SHE and CME 300 mg/kg BW.
- -
- Group VIII (Diabetic with SHE + FCME: D-SF): rats in this group were orally administered SHE 1000 mg/kg BW and FCME 300 mg/kg BW.
- -
- Group IX (Diabetic with SHE + Met: D-SM): rats in this group were orally administered SHE 1000 mg/kg BW and Met 100 mg/kg BW.
- -
- Group X (Diabetic with CME + Met: D-CM): rats in this group were orally administered CME 300 mg/kg BW and Met 100 mg/kg BW.
- -
- Group XI (Diabetic with FCME + Met: D-FM): rats in this group were orally administered FCME 300 mg/kg BW and Met 100 mg/kg BW.
- -
- Group XII (Diabetic with SHE + CME + Met: D-SCM): rats in this group were orally administered SHE 1000 mg/kg BW, CME 300 mg/kg BW, and Met 100 mg/kg BW.
- -
- Group XIII (Diabetic with SHE + FCME + Met: D-SFM): rats in this group were orally administered SHE 1000 mg/kg BW, FCME 300 mg/kg BW, and Met 100 mg/kg BW.
2.4. Measurement of Body Weight and Organ Weight
2.5. Oral Glucose Tolerance Test (OGTT)
2.6. Determination of Fasting Blood Glucose, Plasma Insulin, and Glycated Hemoglobin
2.7. Biochemical Analysis
2.8. Antioxidant Assessment
2.9. Histological Evaluation
2.10. Statistical Analysis
3. Results
3.1. Effect of Plant Supplementation Consumption on Body Weight and Relative Organ Weight
3.2. Effect of Plant Supplementation Consumption on OGTT
3.3. Effect of Plant Supplementation Consumption on Fasting Blood Glucose, Plasma Insulin, and Glycated Hemoglobin
3.4. Effect of Plant Supplementation Consumption on Biochemical Parameters
3.5. Effect of Plant Supplementation Consumption on Antioxidant Activity
3.6. Effect of Plant Supplementation Consumption on Organs Histological Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alrefaei, A.F.; Elbeeh, M.E. Hepatoprotective effects of Glycyrrhiza glabra in diabetic male rats: Addressing liver function, oxidative stress, and histopathological changes. Biology 2025, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Rattanapan, Y.; Duangchan, T.; Sai-Ong, T.; Chareonsirisuthigul, T. miR-4428 and miR-185-5p as key modulators of insulin sensitivity and glucose homeostasis: Insights into pathways and therapeutic potential in type 2 diabetes mellitus. Biology 2025, 14, 424. [Google Scholar] [CrossRef]
- Chen, R.; Ovbiagele, B.; Feng, W. Diabetes and stroke: Epidemiology, pathophysiology, pharmaceuticals and outcomes. Am. J. Med. Sci. 2016, 351, 380–386. [Google Scholar] [CrossRef]
- Lovic, D.; Piperidou, A.; Zografou, I.; Grassos, H.; Pittaras, A.; Manolis, A. The growing epidemic of diabetes mellitus. Curr. Vasc. Pharmacol. 2020, 18, 104–109. [Google Scholar] [CrossRef]
- Riewpaiboon, A.; Pornlertwadee, P.; Pongsawat, K. Diabetes cost model of a hospital in Thailand. Value Health 2007, 10, 223–230. [Google Scholar] [CrossRef] [PubMed]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef]
- Lee, J.; Noh, S.; Lim, S.; Kim, B. Plant extracts for type 2 diabetes: From traditional medicine to modern drug discovery. Antioxidants 2021, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Yaqub, A.; Sanda, K.; Nicholas, A.; Arastus, W.; Muhammad, M.; Abdullahi, S. Review on diabetes, synthetic drugs and glycemic effects of medicinal plants. J. Med. Plants Res. 2013, 7, 2628–2637. [Google Scholar]
- Ounjaijean, S.; Chachiyo, S.; Kulprachakarn, K.; Boonyapranai, K.; Srichairatanakool, S.; Rerkasem, K. Antioxidant and anti-inflammatory protective properties of Thai shallot (Allium ascalonicum cv. Chiangmai) juice on human vascular endothelial cell lines (EA. hy926). Walailak J. Sci. Tech. 2019, 16, 175–184. [Google Scholar] [CrossRef]
- Kaurinovic, B.; Vastag, D. Flavonoids and phenolic acids as potential natural antioxidants. Antioxidants 2019, 2, 1–14. [Google Scholar]
- Unban, K.; Khatthongngam, N.; Shetty, K.; Khanongnuch, C. Nutritional biotransformation in traditional fermented tea (Miang) from north Thailand and its impact on antioxidant and antimicrobial activities. J. Food Sci. Technol. 2019, 56, 2687–2699. [Google Scholar] [CrossRef]
- Anandh Babu, P.V.; Liu, D. Green tea catechins and cardiovascular health: An update. Curr. Med. Chem. 2008, 15, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef]
- Unban, K.; Khatthongngam, N.; Pattananandecha, T.; Saenjum, C.; Shetty, K.; Khanongnuch, C. Microbial community dynamics during the non-filamentous fungi growth-based fermentation process of Miang, a traditional fermented tea of north Thailand and their product characterizations. Front. Microbiol. 2020, 11, 1515. [Google Scholar] [CrossRef] [PubMed]
- Chachiyo, S.; Kulprachakarn, K.; Saenjum, C.; Rerkasem, K.; Srichairatakool, S.; Boonyapranai, K.; Parklak, W.; Somsak, V.; Ounjaijean, S. Toxicity evaluation of Camellia sinensis var. assamica and its fermented miang product. Pharmacogn. Res. 2020, 12, 430–436. [Google Scholar]
- Hosker, J.; Matthews, D.; Rudenski, A.; Burnett, M.; Darling, P.; Bown, E.; Turner, R. Continuous infusion of glucose with model assessment: Measurement of insulin resistance and β-cell function in man. Diabetologia 1985, 28, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Rafiullah, M.; Hossain, M.A.; Ali, M. Antidiabetic activity of Cichorium intybus L. water extract against streptozotocin-induced diabetic rats. J. Umm Al-qura Univ. Appl. Sci. 2023, 9, 565–571. [Google Scholar] [CrossRef]
- Patias, N.S.; Maia, S.V.; Ferreira, Y.G.; de Oliveira, N.L.F.; Ferrarini, S.R.; Bomfim, G.F.; Sinhorin, A.P.; Aguiar, D.H.; de Queiroz, E.A.I.F.; Sinhorin, V.D.G. Effects of extended treatment with Protium heptaphyllum liposomes on metabolic parameters of obese rats. Biology 2024, 13, 771. [Google Scholar] [CrossRef]
- Satyanarayana, N.; Chinni, S.V.; Gobinath, R.; Sunitha, P.; Uma Sankar, A.; Muthuvenkatachalam, B.S. Antidiabetic activity of Solanum torvum fruit extract in streptozotocin-induced diabetic rats. Front. Nutr. 2022, 9, 987552. [Google Scholar] [CrossRef]
- Harries, A.; Kumar, A.; Satyanarayana, S.; Lin, Y.; Zachariah, R.; Lönnroth, K.; Kapur, A. Diabetes mellitus and tuberculosis: Programmatic management issues. Int. J. Tuberc. Lung Dis. 2015, 19, 879–886. [Google Scholar] [CrossRef]
- Kumar, S.; Behl, T.; Sachdeva, M.; Sehgal, A.; Kumari, S.; Kumar, A.; Kaur, G.; Yadav, H.N.; Bungau, S. Implicating the effect of ketogenic diet as a preventive measure to obesity and diabetes mellitus. Life Sci. 2021, 264, 118661. [Google Scholar] [CrossRef] [PubMed]
- Mihailović, M.; Dinić, S.; Arambašić Jovanović, J.; Uskoković, A.; Grdović, N.; Vidaković, M. The influence of plant extracts and phytoconstituents on antioxidant enzymes activity and gene expression in the prevention and treatment of impaired glucose homeostasis and diabetes complications. Antioxidants 2021, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.F.; Carpentier, A.C.; Pereira, S.; Hahn, M.; Giacca, A. Direct and indirect control of hepatic glucose production by insulin. Cell Metab. 2021, 33, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Akhtar, M.A.; Khan, M.; Hossain, M.S.; Alam, A.; Ibne-Wahed, M.I.; Amran, M.S.; Rahman, B.M.; Ahmed, M. Oral glucose tolerance test (OGTT) in normal control and glucose induced hyperglycemic rats with Coccinia cordifolia L. and Catharanthus roseus L. Pak. J. Pharm. Sci. 2009, 22, 402–404. [Google Scholar]
- Ejike, C.E.; Awazie, S.O.; Nwangozi, P.A.; Godwin, C.D. Synergistic postprandial blood glucose modulatory properties of Vernonia amygdalina (Del.), Gongronema latifolium (Benth.) and Occimum gratissimum (Linn.) aqueous decoctions. J. Ethnopharmacol. 2013, 149, 111–116. [Google Scholar] [CrossRef]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- Henning, R.J. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 2018, 14, 491–509. [Google Scholar] [CrossRef]
- Janssen, J.A. Hyperinsulinemia and its pivotal role in aging, obesity, type 2 diabetes, cardiovascular disease and cancer. Int. J. Mol. Sci. 2021, 22, 7797. [Google Scholar] [CrossRef]
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Khan, S.A. Antidiabetic phytochemicals from medicinal plants: Prospective candidates for new drug discovery and development. Front. Endocrinol. 2022, 13, 800714. [Google Scholar] [CrossRef]
- Praparatana, R.; Maliyam, P.; Barrows, L.R.; Puttarak, P. Flavonoids and phenols, the potential anti-diabetic compounds from Bauhinia strychnifolia Craib. stem. Molecules 2022, 27, 2393. [Google Scholar] [CrossRef]
- Semwal, D.K.; Kumar, A.; Aswal, S.; Chauhan, A.; Semwal, R.B. Protective and therapeutic effects of natural products against diabetes mellitus via regenerating pancreatic β-cells and restoring their dysfunction. Phytother. Res 2021, 35, 1218–1229. [Google Scholar] [CrossRef]
- Lozano-Paniagua, D.; Parrón, T.; Alarcón, R.; Requena, M.; López-Guarnido, O.; Lacasaña, M.; Hernández, A.F. Evaluation of conventional and non-conventional biomarkers of liver toxicity in greenhouse workers occupationally exposed to pesticides. Food Chem. Toxicol. 2021, 151, 112127. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.C. Medicinal and theraptic properties of minor fruits-a review. Int. J. Minor Fruits Med. Aromat. Plants 2021, 7, 2021. [Google Scholar] [CrossRef]
- Kilari, B.P.; Mudgil, P.; Azimullah, S.; Bansal, N.; Ojha, S.; Maqsood, S. Effect of camel milk protein hydrolysates against hyperglycemia, hyperlipidemia, and associated oxidative stress in streptozotocin (STZ)-induced diabetic rats. J. Dairy Sci. 2021, 104, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Heeren, J.; Scheja, L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef]
- Laoung-On, J.; Anuduang, A.; Saenjum, C.; Srichairatanakool, S.; Boonyapranai, K.; Ounjaijean, S. Pharmacological activity of cha-miang (Camellia sinensis var. assamica) in high fat diet-induced insulin-resistant rats. Life 2024, 14, 1515. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic insight into oxidative stress-triggered signaling pathways and type 2 diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef]
- Laoung-On, J.; Nuchniyom, P.; Intui, K.; Jaikang, C.; Saenphet, K.; Boonyapranai, K.; Konguthaithip, G.; Outaitaveep, N.; Phankhieo, S.; Sudwan, P. The potential effect of bualuang (White Nelumbo nucifera Gaertn.) extract on sperm quality and metabolomic profiles in mancozeb-induced oxidative stress in male rats. Life 2024, 15, 6. [Google Scholar] [CrossRef]
- Intui, K.; Nuchniyom, P.; Laoung-On, J.; Jaikang, C.; Quiggins, R.; Sudwan, P. Neuroprotective effect of white Nelumbo nucifera Gaertn. petal tea in rats poisoned with mancozeb. Foods 2023, 12, 2175. [Google Scholar] [CrossRef]
- Nuchniyom, P.; Intui, K.; Laoung-On, J.; Jaikang, C.; Quiggins, R.; Photichai, K.; Sudwan, P. Effects of Nelumbo nucifera Gaertn. petal tea extract on hepatotoxicity and oxidative stress induced by mancozeb in rat model. Toxics 2023, 11, 480. [Google Scholar] [CrossRef]
- Goodman, Z.D.; Pan, X.; Ge, C.; Guo, Y.; Zhang, P.; Yan, T.; Zhou, J.; He, Q.; Goodman, Z. The impact of obesity on liver histology. Clin. Liver Dis. 2014, 18, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.M.; Abdel Fattah, A.A.; Abdul-Hamid, M.; Abdel-Aziz, A.M.; Sakr, H.I.; Damanhory, A.A.; Abdel-Kawi, S.H.; Ghaboura, N.; Awad, M.M. Antidiabetic and liver histological and ultrastructural effects of Cynara scolymus leaf and flower head hydroethanolic extracts in Nicotinamide/Streptozotocin-induced diabetic rats. J. Evid. Based Complement. Altern. Med. 2023, 2023, 4223026. [Google Scholar] [CrossRef]
- Attah, M.O.; Jacks, T.W.; Garba, S.H. Evaluation of the effect of n-hexane extract of Leptadenia hastata on the histomorphology of the liver in streptozotocin induced diabetic Wistar rat. Eur. J. Anat. 2021, 25, 135–143. [Google Scholar]


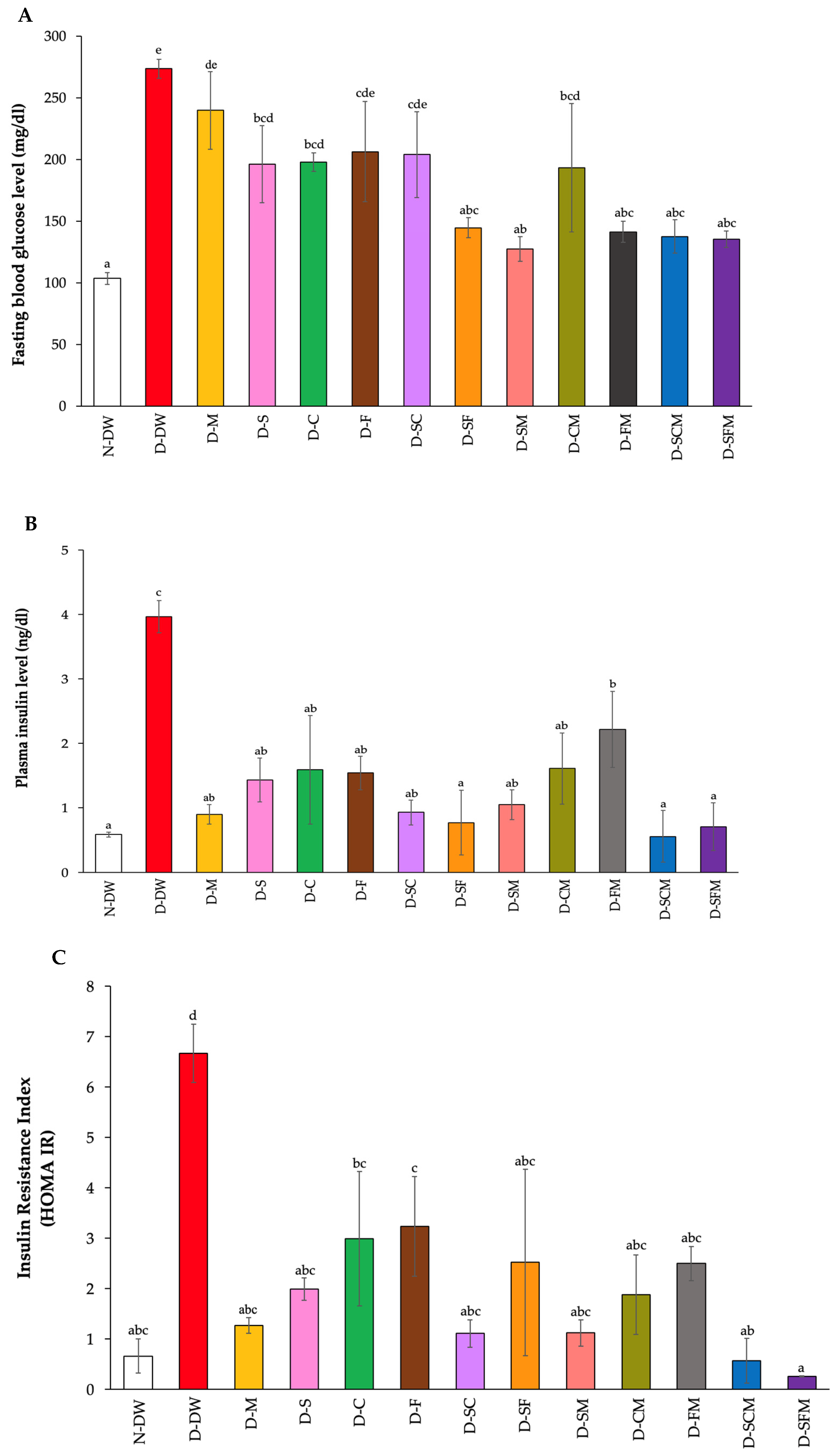

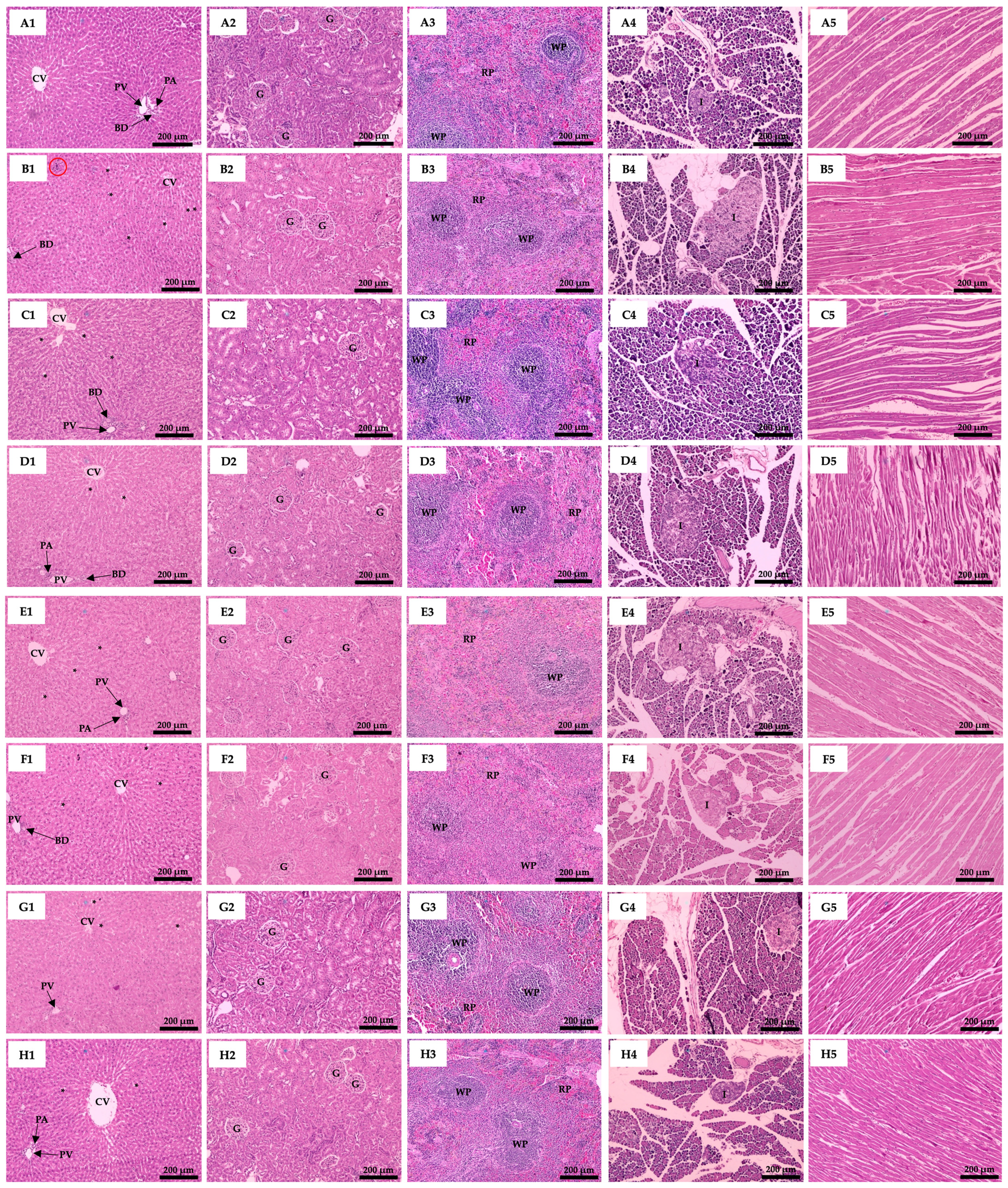
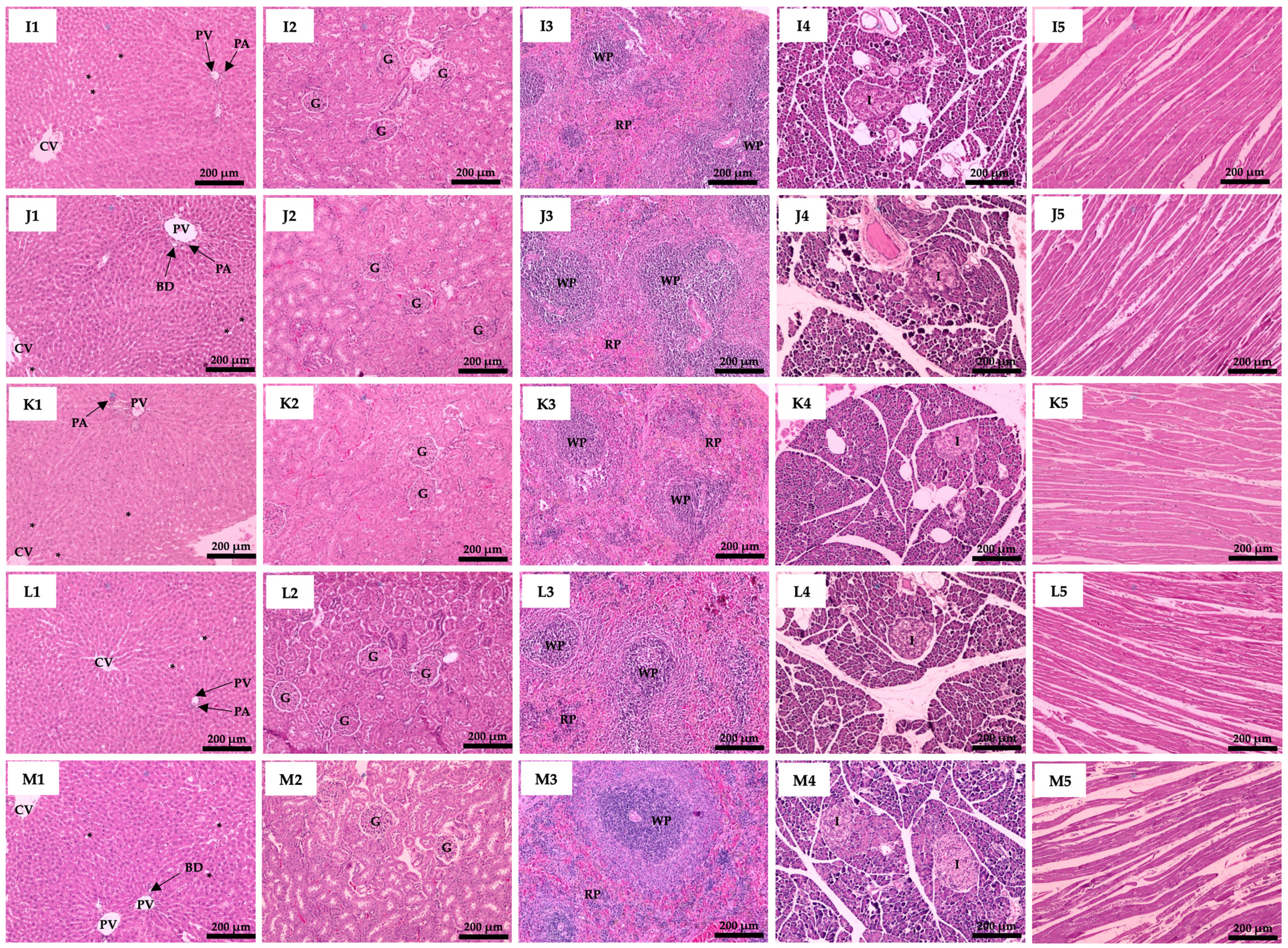
| Groups | Body Weight (g) | Relative Organs Weight (g/100 g BW) | |||||
|---|---|---|---|---|---|---|---|
| Livers | Kidneys | Spleen | Heart | Pancreas | WAT | ||
| N-DW | 554.00 ± 8.72 bc | 2.00 ± 0.02 a | 0.19 ± 0.01 a | 0.16 ± 0.01 | 0.26 ± 0.01 ab | 0.24 ± 0.02 | 6.12 ± 0.24 |
| D-DW | 446.25 ± 21.35 a | 3.02 ± 0.17 f | 0.27 ± 0.02 c | 0.19 ± 0.02 | 0.34 ± 0.02 c | 0.31 ± 0.03 | 7.91 ± 0.69 |
| D-M | 497.50 ± 31.46 ab | 2.79 ± 0.15 ef | 0.23 ± 0.01 b | 0.16 ± 0.01 | 0.28 ± 0.02 b | 0.30 ± 0.02 | 7.68 ± 0.69 |
| D-S | 547.50 ± 12.50 bc | 2.69 ± 0.10 def | 0.22 ± 0.02 ab | 0.17 ± 0.01 | 0.26 ± 0.02 ab | 0.30 ± 0.01 | 7.33 ± 0.92 |
| D-C | 557.50 ± 11.09 bc | 2.63 ± 0.12 cdef | 0.20 ± 0.01 ab | 0.15 ± 0.01 | 0.24 ± 0.01 ab | 0.26 ± 0.01 | 8.28 ± 1.00 |
| D-F | 530.00 ± 17.32 bc | 2.63 ± 0.03 cdef | 0.22 ± 0.01 ab | 0.15 ± 0.01 | 0.27 ± 0.01 ab | 0.26 ± 0.02 | 8.39 ± 0.80 |
| D-SC | 522.50 ± 32.24 b | 2.22 ± 0.26 abc | 0.22 ± 0.01 ab | 0.16 ± 0.01 | 0.27 ± 0.01 ab | 0.29 ± 0.02 | 8.29 ± 1.25 |
| D-SF | 591.25 ± 29.18 c | 2.49 ± 0.24 bcde | 0.20 ± 0.01 ab | 0.15 ± 0.02 | 0.24 ± 0.02 ab | 0.28 ± 0.03 | 10.37 ± 1.29 |
| D-SM | 537.50 ± 10.31 bc | 2.46 ± 0.07 bcde | 0.22 ± 0.01 ab | 0.18 ± 0.01 | 0.26 ± 0.01 ab | 0.28 ± 0.03 | 7.39 ± 0.39 |
| D-CM | 551.25 ± 22.95 bc | 2.80 ± 0.18 ef | 0.23 ± 0.01 b | 0.17 ± 0.01 | 0.25 ± 0.01 ab | 0.31 ± 0.02 | 7.87 ± 0.30 |
| D-FM | 537.00 ± 15.46 bc | 2.52 ± 0.11 bcde | 0.21 ± 0.01 ab | 0.15 ± 0.01 | 0.24 ± 0.01 ab | 0.31 ± 0.03 | 7.89 ± 0.62 |
| D-SCM | 550.00 ± 17.80 bc | 2.32 ± 0.08 abcd | 0.21 ± 0.01 ab | 0.17 ± 0.01 | 0.26 ± 0.02 ab | 0.29 ± 0.02 | 8.58 ± 0.68 |
| D-SFM | 531.25 ± 9.66 bc | 2.18 ± 0.03 ab | 0.21 ± 0.01 ab | 0.18 ± 0.01 | 0.24 ± 0.01 a | 0.29 ± 0.01 | 8.26 ± 0.61 |
| Groups | AST (U/L) | ALT (U/L) | BUN (mg/dL) | Creatinine (mg/dL) | TC (mg/dL) | TG (mg/dL) | LDL (mg/dL) |
|---|---|---|---|---|---|---|---|
| N-DW | 99.01 ± 7.81 a | 38.85 ± 3.04 a | 15.25 ± 0.81 ab | 1.12 ± 0.05 | 75.67 ± 2.51 a | 110.83 ± 8.42 abc | 26.26 ± 3.22 a |
| D-DW | 156.92 ± 14.69 b | 92.86 ± 1.32 b | 20.79 ± 2.64 c | 1.41 ± 0.30 | 144.00 ± 5.05 d | 248.50 ± 33.07 d | 61.06 ± 9.89 d |
| D-M | 97.92 ± 18.72 a | 56.02 ± 4.53 a | 14.65 ± 1.01 ab | 0.99 ± 0.06 | 72.00 ± 1.78 a | 103.25 ± 3.82 ab | 8.52 ± 0.39 b |
| D-S | 81.12 ± 3.97 a | 55.24 ± 1.44 a | 14.96 ± 0.79 ab | 1.12 ± 0.07 | 103.17 ± 8.30 bc | 120.80 ± 9.78 abc | 35.50 ± 7.00 ac |
| D-C | 93.70 ± 7.37 a | 56.35 ± 8.82 a | 13.07 ± 1.29 a | 0.96 ± 0.06 | 107.20 ± 1.93 bc | 107.60 ± 5.13 abc | 52.06 ± 3.21 cd |
| D-F | 77.23 ± 3.86 a | 49.20 ± 4.17 a | 10.74 ± 1.55 a | 1.06 ± 0.09 | 105.80 ± 6.02 bc | 100.75 ± 16.42 a | 42.47 ± 8.53 ac |
| D-SC | 92.38 ± 6.41 a | 47.17 ± 4.39 a | 14.13 ± 3.78 ab | 1.10 ± 0.14 | 92.40 ± 4.88 b | 105.80 ± 10.39 ab | 40.29 ± 2.59 ac |
| D-SF | 81.79 ± 4.57 a | 45.37 ± 1.92 a | 14.30 ± 2.10 ab | 1.05 ± 0.12 | 102.25 ± 2.95 bc | 151.25 ± 18.23 c | 39.65 ± 4.94 ac |
| D-SM | 85.36 ± 12.86 a | 38.70 ± 4.75 a | 14.04 ± 0.69 ab | 1.11 ± 0.10 | 108.60 ± 3.16 bc | 133.60 ± 7.13 abc | 46.41 ± 4.59 cd |
| D-CM | 82.02 ± 12.92 a | 56.34 ± 11.22 a | 14.20 ± 1.00 ab | 0.87 ± 0.30 | 110.50 ± 4.72 c | 117.00 ± 12.23 abc | 49.21 ± 3.48 cd |
| D-FM | 103.74 ± 17.66 a | 51.62 ± 5.82 a | 14.99 ± 1.46 ab | 1.11 ± 0.13 | 109.33 ± 4.54 c | 131.80 ± 14.08 abc | 42.71 ± 5.22 ac |
| D-SCM | 102.46 ± 8.86 a | 55.02 ± 6.97 a | 18.53 ± 1.85 bc | 1.04 ± 0.11 | 113.25 ± 2.50 c | 146.50 ± 11.42 bc | 46.15 ± 3.04 cd |
| D-SFM | 108.61 ± 18.71 a | 108.61 ± 18.71 a | 14.68 ± 1.87 ab | 1.20 ± 0.23 | 104.75 ± 7.30 bc | 134.50 ± 7.24 abc | 39.86 ± 5.97 ac |
| Groups | Plasma MDA (µM) | Liver MDA (µM/mg protein) | RBC-GHS (µM) | Liver-GHS (×102 µM/mg protein) | RBC-GPx (µM) | RBC-SOD (µM) |
|---|---|---|---|---|---|---|
| N-DW | 1.27 ± 0.15 a | 14.75 ± 2.50 ab | 85.27 ± 6.76 ad | 36.12 ± 11.12 a | 705.44 ± 31.24 abc | 5.78 ± 0.45 a |
| D-DW | 8.64 ± 0.19 d | 46.58 ± 1.64 e | 41.45 ± 17.39 b | 14.62 ± 4.70 bc | 627.18 ± 45.86 ab | 1.79 ± 0.16 b |
| D-M | 6.34 ± 0.65 c | 21.75 ± 1.23 abc | 88.18 ± 7.50 d | 25.20 ± 3.32 abcd | 646.37 ± 19.63 abc | 5.54 ± 0.57 a |
| D-S | 2.46 ± 1.00 ab | 40.48 ± 8.22 e | 58.95 ± 1.20 bc | 27.64 ± 1.66 acd | 595.88 ± 26.96 a | 5.52 ± 0.32 a |
| D-C | 1.37 ± 0.28 a | 33.20 ± 5.75 cde | 62.55 ± 2.56 c | 30.72 ± 3.71 ad | 676.86 ± 55.94 abc | 5.34 ± 0.37 a |
| D-F | 1.36 ± 0.09 a | 23.97 ± 2.98 abc | 65.09 ± 2.30 ac | 27.07 ± 2.84 abcd | 720.19 ± 44.35 bc | 4.63 ± 0.22 ae |
| D-SC | 1.75 ± 0.24 a | 39.25 ± 12.68 de | 59.18 ± 1.91 bc | 23.01 ± 5.06 abcd | 673.77 ± 50.36 abc | 5.33 ± 0.14 a |
| D-SF | 2.26 ± 0.44 a | 26.89 ± 2.18 bcd | 62.59 ± 2.30 c | 22.60 ± 0.51 abcd | 691.46 ± 31.42 abc | 2.45 ± 0.38 bc |
| D-SM | 1.56 ± 0.08 a | 18.11 ± 0.53 ab | 65.09 ± 2.80 ac | 17.29 ± 1.67 bcd | 759.19 ± 17.11 c | 4.12 ± 0.71 de |
| D-CM | 4.00 ± 1.25 b | 12.88 ± 1.67 ab | 65.41 ± 6.55 acd | 13.86 ± 1.81 bc | 707.46 ± 32.42 abc | 4.02 ± 0.59 de |
| D-FM | 2.71 ± 0.57 ab | 11.36 ± 0.74 a | 75.27 ± 2.66 acd | 14.96 ± 0.53 bc | 751.28 ± 30.41 c | 2.24 ± 0.19 bc |
| D-SCM | 2.22 ± 0.16 a | 13.70 ± 2.03 ab | 73.09 ± 10.19 acd | 12.72 ± 1.68 b | 723.40 ± 8.89 bc | 3.05 ± 0.05 cd |
| D-SFM | 1.07 ± 0.13 a | 10.82 ± 1.00 a | 77.45 ± 2.94 acd | 12.55 ± 0.61 b | 757.64 ± 25.52 c | 2.32 ± 0.24 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laoung-on, J.; Anuduang, A.; Saenjum, C.; Rerkasem, K.; Srichairatanakool, S.; Boonyapranai, K.; Ounjaijean, S. Anti-Diabetic and Antioxidant Effect Evaluation of Thai Shallot and Cha-Miang in Diabetic Rats. Biology 2025, 14, 627. https://doi.org/10.3390/biology14060627
Laoung-on J, Anuduang A, Saenjum C, Rerkasem K, Srichairatanakool S, Boonyapranai K, Ounjaijean S. Anti-Diabetic and Antioxidant Effect Evaluation of Thai Shallot and Cha-Miang in Diabetic Rats. Biology. 2025; 14(6):627. https://doi.org/10.3390/biology14060627
Chicago/Turabian StyleLaoung-on, Jiraporn, Artorn Anuduang, Chalermpong Saenjum, Kittipan Rerkasem, Somdet Srichairatanakool, Kongsak Boonyapranai, and Sakaewan Ounjaijean. 2025. "Anti-Diabetic and Antioxidant Effect Evaluation of Thai Shallot and Cha-Miang in Diabetic Rats" Biology 14, no. 6: 627. https://doi.org/10.3390/biology14060627
APA StyleLaoung-on, J., Anuduang, A., Saenjum, C., Rerkasem, K., Srichairatanakool, S., Boonyapranai, K., & Ounjaijean, S. (2025). Anti-Diabetic and Antioxidant Effect Evaluation of Thai Shallot and Cha-Miang in Diabetic Rats. Biology, 14(6), 627. https://doi.org/10.3390/biology14060627






