Comparative Analysis of Letrozole and Estradiol Valerate PCOS Models: Reproductive and Metabolic Outcomes with and Without High-Fat Diet
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. PCOS Induction Models
2.2. Diets
2.3. Intraperitoneal Glucose Tolerance Test (IPGTT) and Insulin Tolerance Test (ITT)
2.4. Estrus Cycle Analysis
2.5. Tissue Extraction
2.6. Histological Analysis
2.7. Hormone Determination
2.8. Statistical Analysis
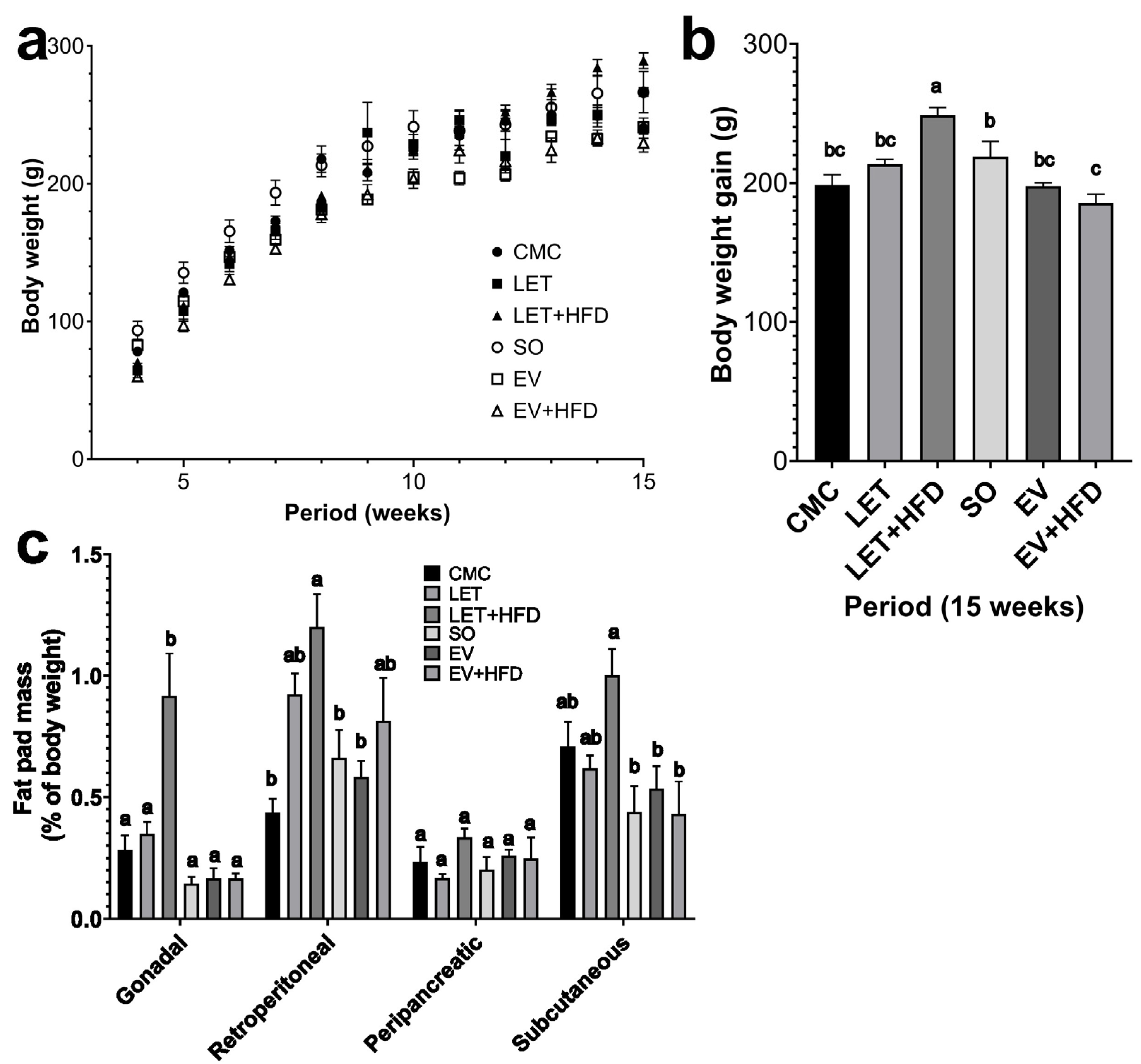
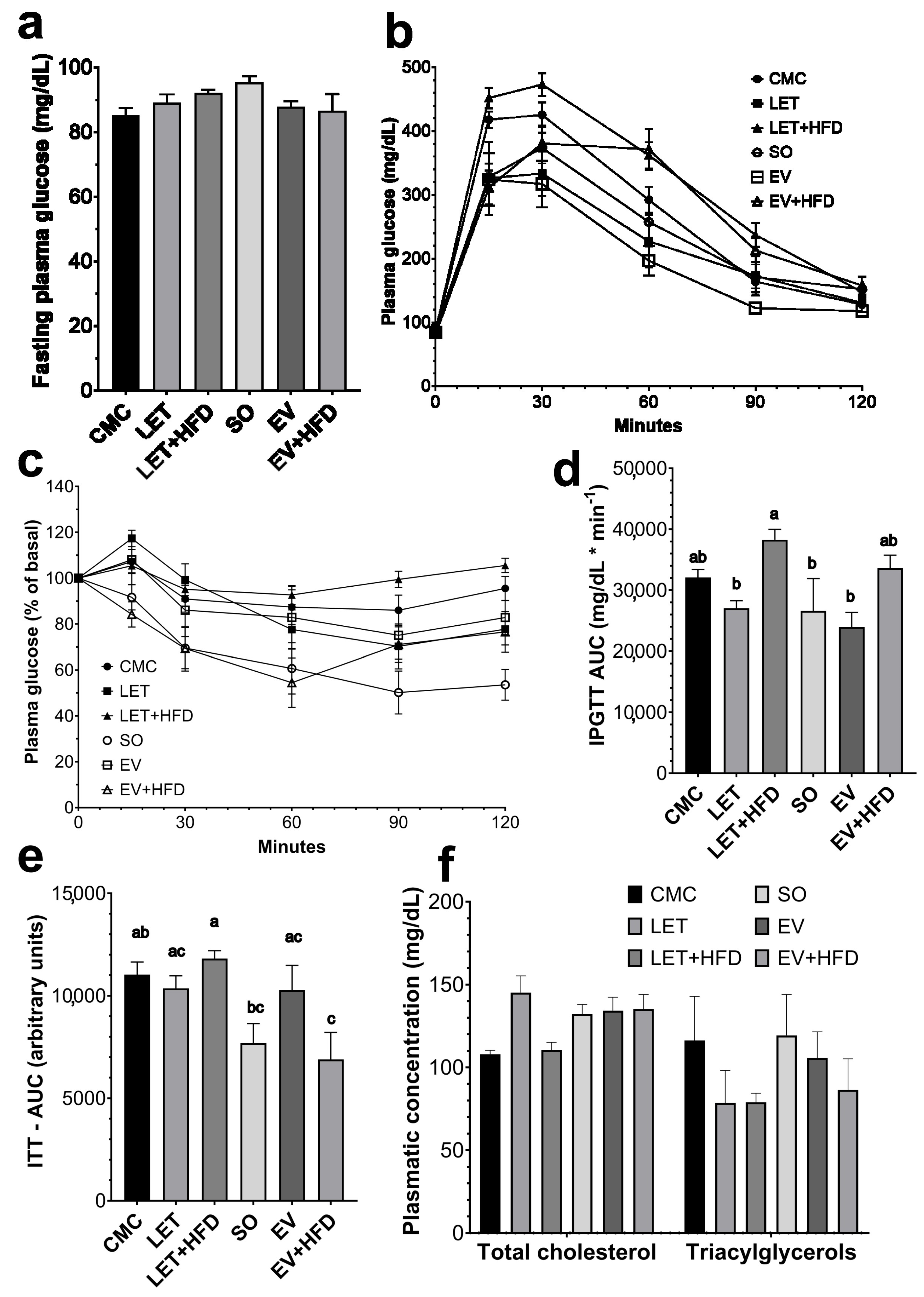
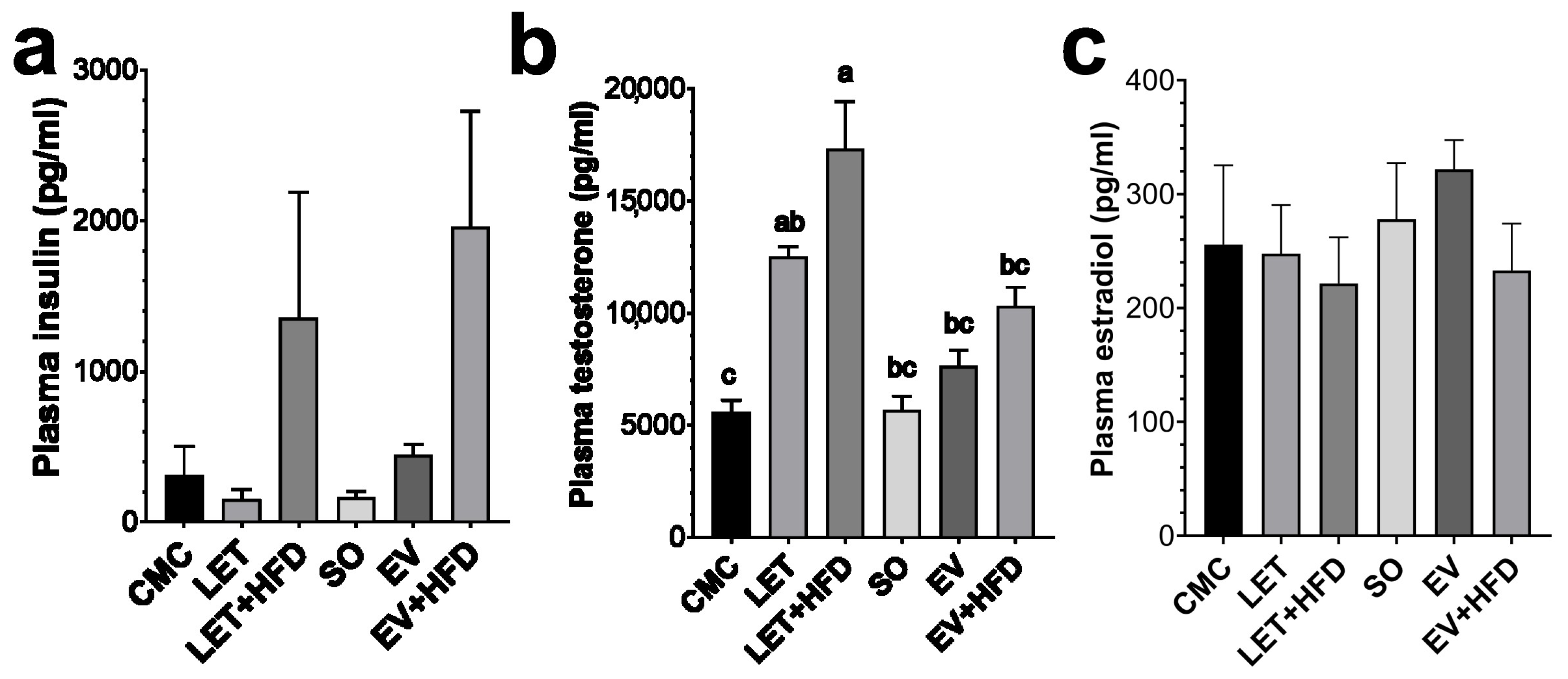
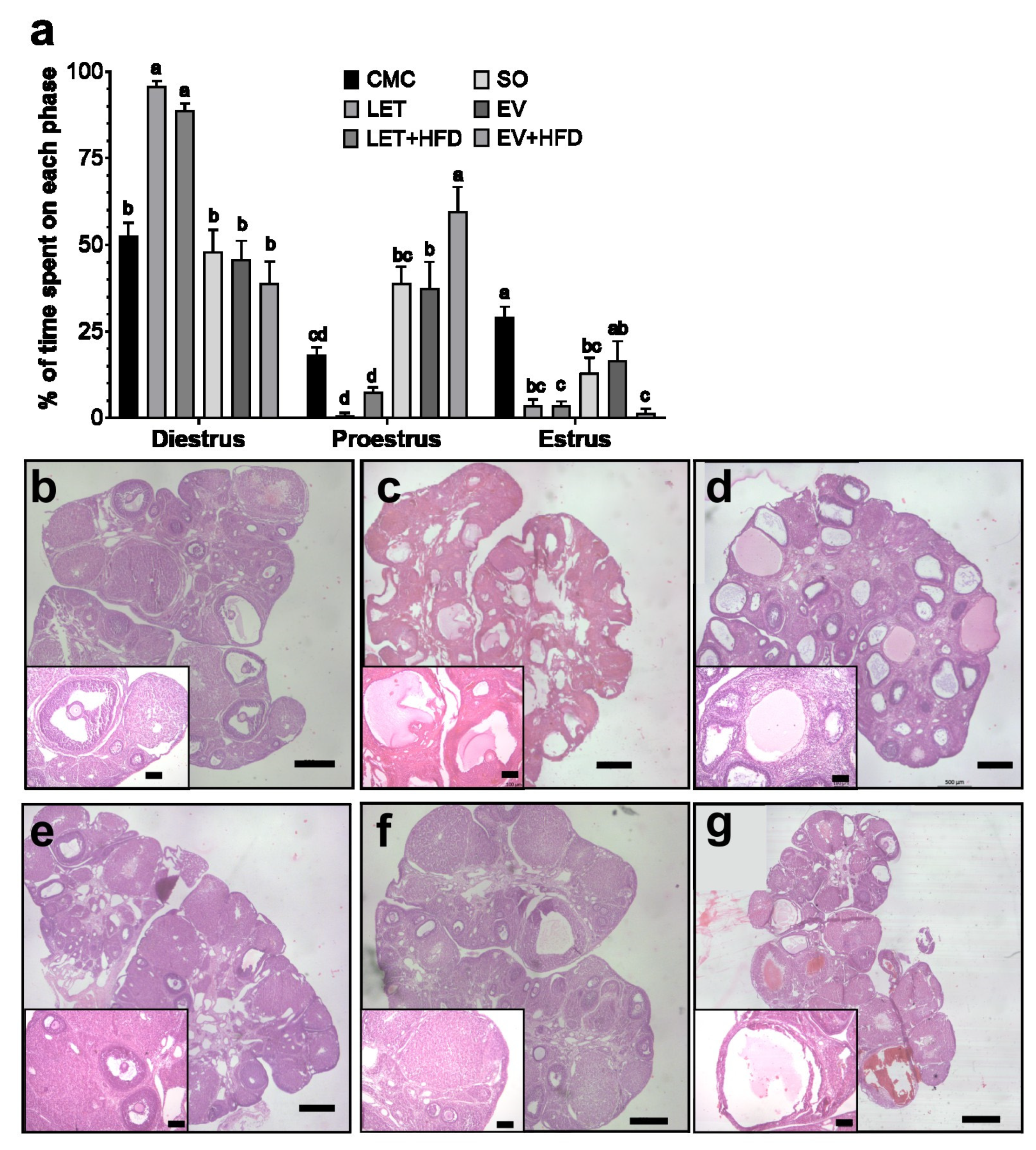
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R. Polycystic Ovary Syndrome. Obstet. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Joham, A.E.; Teede, H.J.; Ranasinha, S.; Zoungas, S.; Boyle, J. Prevalence of infertility and use of fertility treatment in women with polycystic ovary syndrome: Data from a large community-based cohort study. J. Women’s Health 2015, 24, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, W.; Xu, H.; Hu, M.; Guo, X.; Jia, W.; Liu, G.; Li, J.; Cui, P.; Lager, S.; et al. Hyperandrogenism and insulin resistance-induced fetal loss: Evidence for placental mitochondrial abnormalities and elevated reactive oxygen species production in pregnant rats that mimic the clinical features of polycystic ovary syndrome. J. Physiol. 2019, 597, 3927–3950. [Google Scholar] [CrossRef] [PubMed]
- McAllister, J.M.; Legro, R.S.; Modi, B.P.; Strauss, J.F., 3rd. Functional genomics of PCOS: From GWAS to molecular mechanisms. Trends Endocrinol. Metab. TEM 2015, 26, 118–124. [Google Scholar] [CrossRef]
- Yang, J.; Chen, C. Hormonal changes in PCOS. J. Endocrinol. 2024, 261, e230342. [Google Scholar] [CrossRef]
- Mahabeer, S.; Jialal, I.; Norman, R.J.; Naidoo, C.; Reddi, K.; Joubert, S.M. Insulin and C-peptide secretion in non-obese patients with polycystic ovarian disease. Horm. Metab. Res. 1989, 21, 502–506. [Google Scholar] [CrossRef]
- O’Meara, N.M.; Blackman, J.D.; Ehrmann, D.A.; Barnes, R.B.; Jaspan, J.B.; Rosenfield, R.L.; Polonsky, K.S. Defects in beta-cell function in functional ovarian hyperandrogenism. J. Clin. Endocrinol. Metab. 1993, 76, 1241–1247. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Padmanabhan, V.; Walters, K.A.; Campbell, R.E.; Benrick, A.; Giacobini, P.; Dumesic, D.A.; Abbott, D.H. Animal Models to Understand the Etiology and Pathophysiology of Polycystic Ovary Syndrome. Endocr. Rev. 2020, 41, bnaa010. [Google Scholar] [CrossRef]
- Osuka, S.; Nakanishi, N.; Murase, T.; Nakamura, T.; Goto, M.; Iwase, A.; Kikkawa, F. Animal models of polycystic ovary syndrome: A review of hormone-induced rodent models focused on hypothalamus-pituitary-ovary axis and neuropeptides. Reprod. Med. Biol. 2018, 18, 151–160. [Google Scholar] [CrossRef]
- Begum, N.; Manipriya, K.; Veeresh, B. Role of high-fat diet on letrozole-induced polycystic ovarian syndrome in rats. Eur. J. Pharmacol. 2022, 917, 174746. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pinto, J.; Piquer, B.; Tiszavari, M.; Lara, H.E. Neonatal exposure to estradiol valerate reprograms the rat ovary androgen receptor and anti-Müllerian hormone to a polycystic ovary phenotype. Reprod. Toxicol. 2018, 75, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Brawer, J.R.; Munoz, M.; Farookhi, R. Development of the polycystic ovarian condition (PCO) in the estradiol valerate-treated rat. Biol. Reprod. 1986, 35, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.X.; Yin, Q.; Xu, X. A Rat Model of Polycystic Ovary Syndrome with Insulin Resistance Induced by Letrozole Combined with High Fat Diet. Med. Sci. Monit. 2020, 26, e922136. [Google Scholar] [CrossRef]
- Kafali, H.; Iriadam, M.; Ozardali, I.; Demir, N. Letrozole-induced polycystic ovaries in the rat: A new model for cystic ovarian disease. Arch. Med. Res. 2004, 35, 103–108. [Google Scholar] [CrossRef]
- Noorafshan, A.; Ahmadi, M.; Mesbah, S.F.; Karbalay-Doust, S. Stereological study of the effects of letrozole and estradiol valerate treatment on the ovary of rats. Clin. Exp. Reprod. Med. 2013, 40, 115–121. [Google Scholar] [CrossRef]
- Cruz, G.; Barra, R.; González, D.; Sotomayor-Zárate, R.; Lara, H.E. Temporal window in which exposure to estradiol permanently modifies ovarian function causing polycystic ovary morphology in rats. Fertil. Steril. 2012, 98, 1283–1290. [Google Scholar] [CrossRef]
- Larqué, C.; Lugo-Martínez, H.; Mendoza, X.; Nochebuena, M.; Novo, L.; Vilchis, R.; Sánchez-Bringas, G.; Ubaldo, L.; Velasco, M.; Escalona, R. Paternal Obesity Induced by High-Fat Diet Impairs the Metabolic and Reproductive Health of Progeny in Rats. Metabolites 2023, 13, 1098. [Google Scholar] [CrossRef]
- Hubscher, C.H.; Brooks, D.L.; Johnson, J.R. A quantitative method for assessing stages of the rat estrous cycle. Biotech. Histochem. 2005, 80, 79–87. [Google Scholar] [CrossRef]
- Helvaci, N.; Yildiz, B.O. Polycystic ovary syndrome as a metabolic disease. Nat. Rev. Endocrinol. 2025, 21, 230–244. [Google Scholar] [CrossRef]
- Walters, K.A.; Allan, C.M.; Handelsman, D.J. Rodent models for human polycystic ovary syndrome. Biol. Reprod. 2012, 86, 1–12, 149. [Google Scholar] [CrossRef] [PubMed]
- Mannerås, L.; Cajander, S.; Holmäng, A.; Seleskovic, Z.; Lystig, T.; Lönn, M.; Stener-Victorin, E. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology 2007, 148, 3781–3791. [Google Scholar] [CrossRef] [PubMed]
- Poojary, P.S.; Nayak, G.; Panchanan, G.; Rao, A.; Kundapur, S.D.; Kalthur, S.G.; Mutalik, S.; Adiga, S.K.; Zhao, Y.; Bakkum-Gamez, J.; et al. Distinctions in PCOS Induced by Letrozole vs Dehydroepiandrosterone with High-fat Diet in Mouse Model. Endocrinology 2022, 163, bqac097. [Google Scholar] [CrossRef]
- Sotomayor-Zárate, R.; Dorfman, M.; Paredes, A.; Lara, H.E. Neonatal exposure to estradiol valerate programs ovarian sympathetic innervation and follicular development in the adult rat. Biol. Reprod. 2008, 78, 673–680. [Google Scholar] [CrossRef]
- Komal, F.; Khan, M.K.; Imran, M.; Ahmad, M.H.; Anwar, H.; Ashfaq, U.A.; Ahmad, N.; Masroor, A.; Ahmad, R.S.; Nadeem, M.; et al. Impact of different omega-3 fatty acid sources on lipid, hormonal, blood glucose, weight gain and histopathological damages profile in PCOS rat model. J. Transl. Med. 2020, 18, 349. [Google Scholar] [CrossRef] [PubMed]
- Mehraban, M.; Jelodar, G.; Rahmanifar, F. A combination of spearmint and flaxseed extract improved endocrine and histomorphology of ovary in experimental PCOS. J. Ovarian Res. 2020, 13, 32. [Google Scholar] [CrossRef]
- Manni, L.; Holmäng, A.; Cajander, S.; Lundeberg, T.; Aloe, L.; Stener-Victorin, E. Effect of anti-NGF on ovarian expression of alpha1- and beta2-adrenoceptors, TrkA, p75NTR, and tyrosine hydroxylase in rats with steroid-induced polycystic ovaries. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R826–R835. [Google Scholar] [CrossRef]
- Piermaría, J.; Cónsole, G.; Perelló, M.; Moreno, G.; Gaillard, R.C.; Spinedi, E. Impact of estradiol on parametrial adipose tissue function: Evidence for establishment of a new set point of leptin sensitivity in control of energy metabolism in female rat. Endocrine 2003, 20, 239–245. [Google Scholar] [CrossRef]
- Reid, L.D.; Marinelli, P.W.; Bennett, S.M.; Fiscale, L.T.; Narciso, S.P.; Oparowski, C.J.; Reid, M.L.; Merrigan, B.A.; Moricone, J.; Hubbell, C.L.; et al. One injection of estradiol valerate induces dramatic changes in rats’ intake of alcoholic beverages. Pharmacol. Biochem. Behav. 2002, 72, 601–616. [Google Scholar] [CrossRef]
- Simpkins, J.W.; Anderson, W.R.; Dawson, R., Jr.; Bodor, N. Effects of a brain-enhanced chemical delivery system for estradiol on body weight and food intake in intact and ovariectomized rats. Pharm. Res. 1989, 6, 592–600. [Google Scholar] [CrossRef]
- Quirarte, G.L.; Reid, L.D.; de la Teja, I.S.; Reid, M.L.; Sánchez, M.A.; Díaz-Trujillo, A.; Aguilar-Vazquez, A.; Prado-Alcalá, R.A. Estradiol valerate and alcohol intake: Dose-response assessments. BMC Pharmacol. 2007, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Akopians, A.L.; Madrigal, V.K.; Ramirez, E.; Margolis, D.J.; Sarma, M.K.; Thomas, A.M.; Grogan, T.R.; Haykal, R.; Schooler, T.A.; et al. Hyperandrogenism Accompanies Increased Intra-Abdominal Fat Storage in Normal Weight Polycystic Ovary Syndrome Women. J. Clin. Endocrinol. Metab. 2016, 101, 4178–4188. [Google Scholar] [CrossRef] [PubMed]
- Alexanderson, C.; Stener-Victorin, E.; Kullberg, J.; Nilsson, S.; Levin, M.; Cajander, S.; Lönn, L.; Lönn, M.; Holmäng, A. A single early postnatal estradiol injection affects morphology and gene expression of the ovary and parametrial adipose tissue in adult female rats. J. Steroid Biochem. Mol. Biol. 2010, 122, 82–90. [Google Scholar] [CrossRef]
- Xu, J.; Dun, J.; Yang, J.; Zhang, J.; Lin, Q.; Huang, M.; Ji, F.; Huang, L.; You, X.; Lin, Y. Letrozole Rat Model Mimics Human Polycystic Ovarian Syndrome and Changes in Insulin Signal Pathways. Med. Sci. Monit. 2020, 26, e923073. [Google Scholar] [CrossRef] [PubMed]
- Dăneasă, A.; Cucolaş, C.; Lenghel, L.M.; Olteanu, D.; Orăsan, R.; Filip, G.A. Letrozole vs estradiol valerate induced PCOS in rats: Glycemic, oxidative and inflammatory status assessment. Reproduction 2016, 151, 401–409. [Google Scholar] [CrossRef]
- Azin, F.; Khazali, H. Neuropeptide galanin and its effects on metabolic and reproductive disturbances in female rats with estradiol valerate (EV)-Induced polycystic ovary syndrome (PCOS). Neuropeptides 2020, 80, 102026. [Google Scholar] [CrossRef]
- Ghafurniyan, H.; Azarnia, M.; Nabiuni, M.; Karimzadeh, L. The Effect of Green Tea Extract on Reproductive Improvement in Estradiol Valerate-Induced Polycystic Ovarian Syndrome in Rat. Iran. J. Pharm. Res. IJPR 2015, 14, 1215–1233. [Google Scholar]
- Barath, B.; Varga, A.; Matrai, A.A.; Deak-Pocsai, K.; Nemeth, N.; Deak, A. Estradiol Valerate Affects Hematological and Hemorheological Parameters in Rats. Metabolites 2022, 12, 602. [Google Scholar] [CrossRef]
- Rakic, D.; Joksimovic Jovic, J.; Jakovljevic, V.; Zivkovic, V.; Nikolic, M.; Sretenovic, J.; Nikolic, M.; Jovic, N.; Bicanin Ilic, M.; Arsenijevic, P.; et al. High Fat Diet Exaggerate Metabolic and Reproductive PCOS Features by Promoting Oxidative Stress: An Improved EV Model in Rats. Medicina 2023, 59, 1104. [Google Scholar] [CrossRef]
- Patel, R.; Shah, G. High-fat diet exposure from pre-pubertal age induces polycystic ovary syndrome (PCOS) in rats. Reproduction 2018, 155, 141–151. [Google Scholar] [CrossRef]
- Ressler, I.B.; Grayson, B.E.; Ulrich-Lai, Y.M.; Seeley, R.J. Diet-induced obesity exacerbates metabolic and behavioral effects of polycystic ovary syndrome in a rodent model. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1076–E1084. [Google Scholar] [CrossRef] [PubMed]
- Wild, R.A. Dyslipidemia in PCOS. Steroids 2012, 77, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Maliqueo, M.; Sun, M.; Johansson, J.; Benrick, A.; Labrie, F.; Svensson, H.; Lönn, M.; Duleba, A.J.; Stener-Victorin, E. Continuous administration of a P450 aromatase inhibitor induces polycystic ovary syndrome with a metabolic and endocrine phenotype in female rats at adult age. Endocrinology 2013, 154, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.H.; Xu, Y.; Ma, H.X.; Liang, C.J.; Yang, T. Effect of High-Fat Diet on the Intestinal Flora in Letrozole-Induced Polycystic Ovary Syndrome Rats. Evid.-Based Complement. Altern. Med. 2021, 2021, 6674965. [Google Scholar] [CrossRef]
- Cai, T.; Al-Jubairi, N.N.; Santos, H.O.; de Souza IG, O.; Chen, Y. Does letrozole treatment have favorable effects on the lipid profile? A systematic review and meta-analysis of randomized clinical trials. Steroids 2021, 172, 108875. [Google Scholar] [CrossRef]
- Lalithamma, A.; Changamma, C. Effect of Estradiol Valerate on Serum Biochemical Profiles in Aged Female Albino Rats. Annu. Res. Rev. Biol. 2014, 6, 115–120. [Google Scholar] [CrossRef]
- Moghetti, P.; Tosi, F. Insulin resistance and PCOS: Chicken or egg? J. Endocrinol. Investig. 2021, 44, 233–244. [Google Scholar] [CrossRef]
- Sinha, S.; Kaseta, J.; Santner, S.J.; Demers, L.M.; Bremmer, W.J.; Santen, R.J. Effect of CGS 20267 on ovarian aromatase and gonadotropin levels in the rat. Breast Cancer Res. Treat. 1998, 48, 45–51. [Google Scholar] [CrossRef]
- Pournaderi, P.S.; Yaghmaei, P.; Khodaei, H.; Noormohammadi, Z.; Hejazi, S.H. The effects of 6-Gingerol on reproductive improvement, liver functioning and Cyclooxygenase-2 gene expression in estradiol valerate—Induced polycystic ovary syndrome in Wistar rats. Biochem. Biophys. Res. Commun. 2017, 484, 461–466. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escalona, X.A.; Ayala, R.S.; Cortez, K.; Sánchez, S.F.; Tomé-Dehesa, T.; Díaz-Hernández, V.; Larqué, C.; Escalona, R. Comparative Analysis of Letrozole and Estradiol Valerate PCOS Models: Reproductive and Metabolic Outcomes with and Without High-Fat Diet. Biology 2025, 14, 592. https://doi.org/10.3390/biology14060592
Escalona XA, Ayala RS, Cortez K, Sánchez SF, Tomé-Dehesa T, Díaz-Hernández V, Larqué C, Escalona R. Comparative Analysis of Letrozole and Estradiol Valerate PCOS Models: Reproductive and Metabolic Outcomes with and Without High-Fat Diet. Biology. 2025; 14(6):592. https://doi.org/10.3390/biology14060592
Chicago/Turabian StyleEscalona, Xóchitl Acuña, Rocio Sarahy Ayala, Karla Cortez, Sophie Fernández Sánchez, Teresa Tomé-Dehesa, Verónica Díaz-Hernández, Carlos Larqué, and Rene Escalona. 2025. "Comparative Analysis of Letrozole and Estradiol Valerate PCOS Models: Reproductive and Metabolic Outcomes with and Without High-Fat Diet" Biology 14, no. 6: 592. https://doi.org/10.3390/biology14060592
APA StyleEscalona, X. A., Ayala, R. S., Cortez, K., Sánchez, S. F., Tomé-Dehesa, T., Díaz-Hernández, V., Larqué, C., & Escalona, R. (2025). Comparative Analysis of Letrozole and Estradiol Valerate PCOS Models: Reproductive and Metabolic Outcomes with and Without High-Fat Diet. Biology, 14(6), 592. https://doi.org/10.3390/biology14060592







