Species Diversity and Distribution of Amphibians in Tangjiahe National Nature Reserve, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Collection
2.3. Amphibian Conservation Status
2.4. Species Diversity
2.5. Statistical Analysis
3. Results
3.1. Species Composition Variation
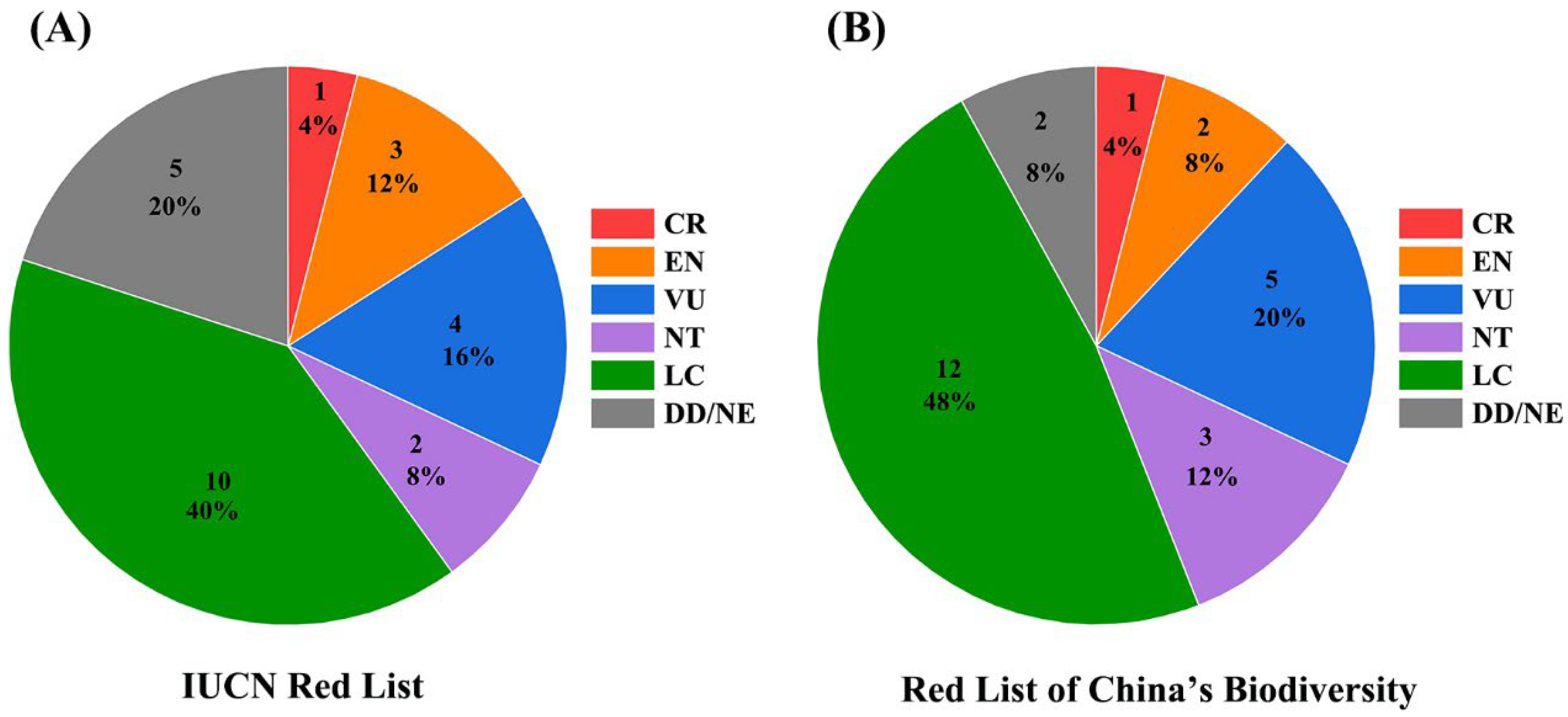
3.2. Conservation Status of Amphibian Species
3.3. Amphibian Distribution Across Habitat Types
3.4. Species Diversity Across Collected Sites
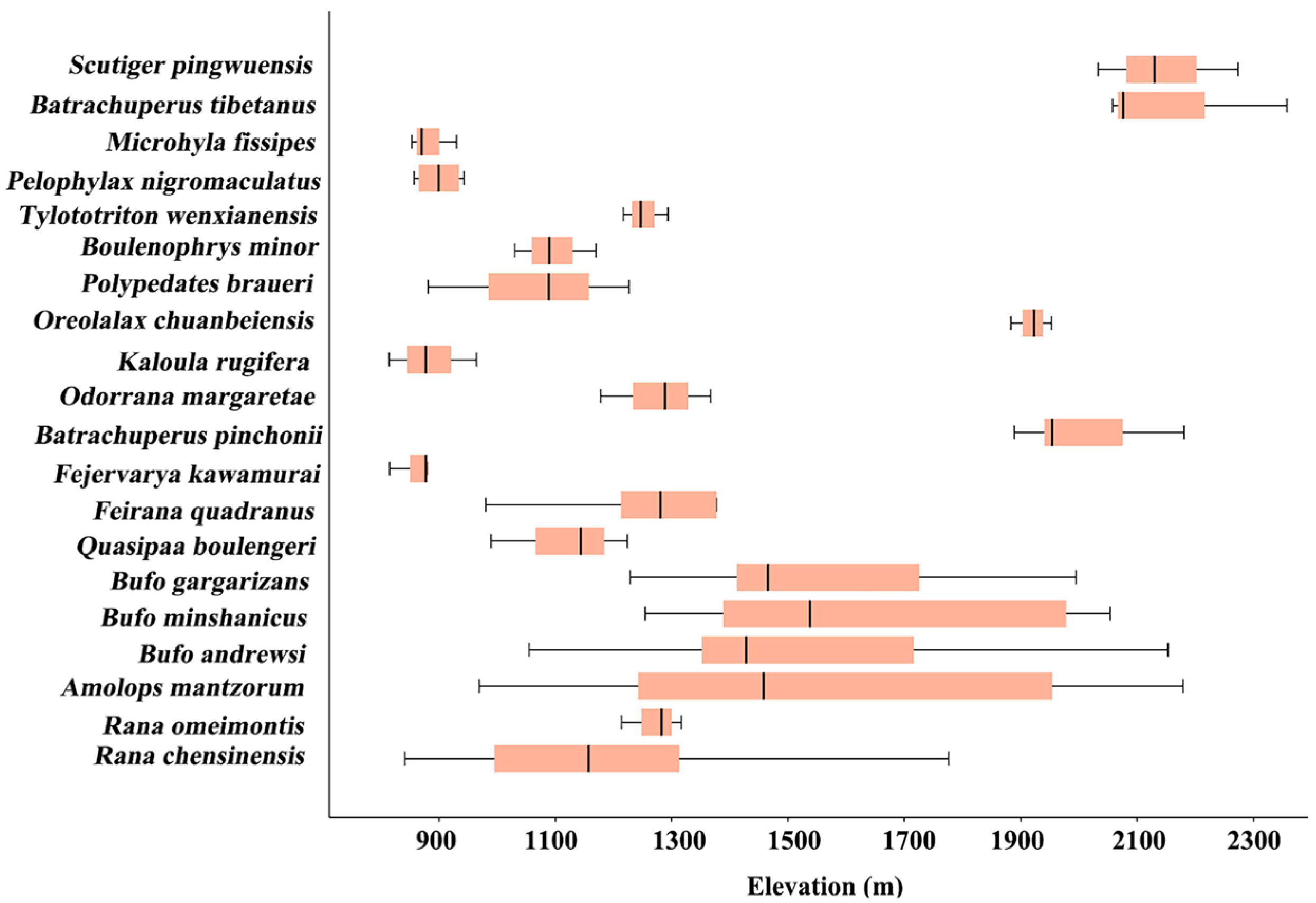
3.5. Species Richness Across Elevational Bands
4. Discussion
4.1. Species Composition
4.2. Conservation Status of Amphibian Species
4.3. Amphibian Distribution Across Habitat Types
4.4. Species Diversity Across Collection Sites
4.5. Species Richness Across Elevations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhong, M.J.; Zhang, J.; Xi, X.F.; Yang, S.N.; Jiang, J.P.; Hu, J.H. Multidimensional amphibian diversity and community structure along a 2 600 m elevational gradient on the eastern margin of the Qinghai-Tibetan Plateau. Zool. Res. 2022, 43, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.J.; Song, X.Q.; Chen, C.; Zhao, L.; Jin, L.; Liao, W.B. Diversity and Distribution Pattern of Amphibians and Reptiles in Yingjing Area of the Giant Panda National Park. Chin. J. Zool. 2022, 57, 707–721. [Google Scholar]
- Yu, F.; Zhang, L.J.; Wang, Y.; Yi, X.F.; Zhang, S.; Ma, J.M.; Dong, Z.M.; Chen, G.W.; Ma, K.M. High rodent abundance increases seed removal but decreases scatter-hoarding and seedling recruitment along an elevational gradient. Integr. Zool. 2023, 18, 843–858. [Google Scholar] [CrossRef]
- Voelker, G.; Wogan, G.O.; Huntley, J.W.; Kaliba, P.M.; De Swardt, D.H.; Bowie, R.C. Climate cycling did not affect haplotype distribution in an abundant Southern African avian habitat generalist species, the familiar chat (Oenanthe familiaris). Integr. Zool. 2024, 20, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cabello, I.; Franch, M.; Vilella, M.; Fernandez-Arrieta, N.; Rota, M.; Sanglas, A.; Baqué-Díaz, E.; Gallardet, M.; Federico, P.; Peris, A.; et al. Assessing the role of habitat, climate, and anthropization gradients on terrestrial mammal diversity in the western Mediterranean basin. Integr. Zool. 2024, 20, 485–503. [Google Scholar] [CrossRef]
- Rahbek, C.; Borregaard, M.K.; Colwell, R.K.; Dalsgaard, B.; Holt, B.G.; Morueta-Holme, N.; Nogues-Bravo, D.; Whittaker, R.J.; Fjeldsa, J. Humboldt’s enigma: What causes global patterns of mountain biodiversity? Science 2019, 365, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.B.; Che, X.L.; Ning, T.; Zou, F.S. Distribution of Birds in the High-Altitude Area of Mount Everest. Integr. Zool. 2023, 18, 199–204. [Google Scholar] [CrossRef]
- Pan, T.; Zhang, C.W.; Terwengel, P.O.; Wang, H.; Ding, L.; Yang, L.Y.; Hu, C.C.; Li, W.A.; Zhou, W.L.; Wu, X.B.; et al. Comparative phylogeography reveals dissimilar genetic differentiation patterns in two sympatric amphibian species. Integr. Zool. 2024, 19, 863–886. [Google Scholar] [CrossRef]
- Feoktistova, N.Y.; Meschersky, I.G.; Shenbrot, G.I.; Puzachenko, A.Y.; Meschersky, S.I.; Bogomolov, P.L.; Surov, A.V. Phylogeography of the Common Hamster (Cricetus cricetus): Paleoclimatic Reconstructions of Late Pleistocene Colonization. Integr. Zool. 2023, 18, 581–599. [Google Scholar] [CrossRef]
- Lei, B.Y.; Zheng, Z.F.; Cui, J.F.; Zhao, J.; Newman, C.; Zhou, Y.B. Ecotourist trail-use affects the taxonomic, functional and phylogenetic diversity of mammals in a protected area: Lessons for conservation management. Integr. Zool. 2023, 18, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Li, K.X.; Zheng, Y.X.; Xue, J.Y.; Wang, S.; Li, S.; Cao, P.; Liu, F.; Dai, Q.Y.; Feng, X.T.; et al. Mitogenomes of museum specimens provide new insight into species classification and recently reduced diversity of highly endangered Nomascus gibbons. Integr. Zool. 2024, 20, 674–684. [Google Scholar] [CrossRef]
- Allegrini, C.; Korine, C.; Krasnov, B.R. Climatic gradients and forest composition shape bat communities in Eastern Mediterranean pine plantations. Integr. Zool. 2024, 19, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Hereira-Pacheco, S.; Alberdi, A.; de la Vega-pérez, A.H.; Estrada-Torres, A.; Ancona, S.; Navarro-Noya, Y. DNA metabarcoding reveals seasonal changes in diet composition across four arthropod-eating lizard species (Phrynosomatidae: Sceloporus). Integr. Zool. 2024, 19, 480–495. [Google Scholar] [CrossRef]
- Perrigo, A.; Hoorn, C.; Antonelli, A. Why mountains matter for biodiversity. J. Biogeog. 2020, 47, 315–325. [Google Scholar] [CrossRef]
- McCain, C.M.; Grytnes, J.A. Elevational Gradients in Species Richness. In Encyclopedia of Life Sciences (ELS); Wiley: Chichester, UK, 2010. [Google Scholar]
- Körner, C. Why are there global gradients in species richness? Mountains might hold the answer. Trends Ecol. Evol. 2000, 15, 513–514. [Google Scholar] [CrossRef]
- Peters, M.K.; Hemp, A.; Appelhans, T.; Behler, C.; Classen, A.; Detsch, F.; Ensslin, A.; Ferger, W.S.; Frederiksen, B.S.; Gebert, F.; et al. Predictors of elevational biodiversity gradients change from single taxa to the multi-taxa community level. Nat. Commun. 2016, 7, 13736. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, D.; Brambilla, M.; Caprio, E.; Pedrini, P.; Rolando, A. Alpine bird distributions along elevation gradients: The consistency of climate and habitat effects across geographic regions. Oecologia 2016, 181, 1139–1150. [Google Scholar] [CrossRef]
- Wells, K.D. The Ecology and Behavior of Amphibians; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Coates, M.I.; Ruta, M.; Friedman, M. Ever since owen: Changing perspectives on the early evolution of tetrapods. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 571–592. [Google Scholar] [CrossRef]
- Green, D.M.; Lannoo, M.J.; Lesbarrères, D.; Muths, E. Amphibian population declines: 30 years of progress in confronting a complex problem. Herpetologica 2020, 76, 97–100. [Google Scholar] [CrossRef]
- Tan, W.C.; Herrel, A.; Rödder, D. A global analysis of habitat fragmentation research in reptiles and amphibians: What have we done so far? Biodivers. Conserv. 2023, 32, 439–468. [Google Scholar] [CrossRef]
- Hocking, D.J.; Babbitt, K.J. Amphibian contributions to ecosystem services. Herpetol. Conserv. Biol. 2014, 9, 1–17. [Google Scholar]
- Xu, W.; Wu, Y.H.; Zhou, W.W.; Chen, H.M.; Zhang, B.L.; Chen, J.M.; Xu, W.; Rao, D.Q.; Zhao, H.; Yan, F.; et al. Hidden hotspots of amphibian biodiversity in China. Proc. Natl. Acad. Sci. USA 2024, 121, e2320674121. [Google Scholar] [CrossRef] [PubMed]
- Campbell Grant, E.H.; Miller, D.A.W.; Muths, E. A synthesis of evidence of drivers of amphibian declines. Herpetologica 2020, 76, 101–107. [Google Scholar] [CrossRef]
- Hu, J.C. A Report of the Comprehensive Survey on Tangjiahe Nature Reserve in Sichuan; Sichuan Science and Technical Press: Chengdu, China, 2005. [Google Scholar]
- Wei, J.; Zheng, W.C.; Yang, C.; Zhang, X.Y.; Lan, Y.X.; Shen, L.M.; Hao, J.F. Analysis on medicinal plant resources and diversity characteristics in the Tangjiahe National Nature Reserve. Acta Bot. Boreal. Occident. Sin. 2019, 39, 1307–1315. [Google Scholar]
- Shen, L.; Gao, Z.; Ou, W.; Chen, W.; Ma, W. Survey Report on Amphibians and Reptiles in Tangjiahe Nature Reserve, Sichuan. Sichuan J. Zool. 1999, 18, 132–134. [Google Scholar]
- Zhang, Z. Research on Biodiversity in Tangjiahe National Nature Reserve, China; Science Press: Beijing, China, 2016. [Google Scholar]
- Yao, G.; Fan, Y.; Li, D.; Hull, V.; Shen, L.; Li, Y.; Hu, J. The influence of environmental variables on home range size and use in the golden snub-nosed monkey (Rhinopithecus roxellana) in Tangjiahe National Nature Reserve, China. Animals 2022, 12, 2338. [Google Scholar] [CrossRef]
- He, X.; Wang, X.; Shen, L.; Zhuang, Y. A Framework for Adaptive Collaborative Governance of National Park Communities and its Effectiveness in Practice: A Case Study of Community a in Tangjiahe Area of Giant Panda National Park. Landsc. Architect. 2023, 30, 42–48. [Google Scholar]
- Díaz-García, J.M.; Pineda, E.; López-Barrera, F.; Moreno, C.E. Amphibian species and functional diversity as indicators of restoration success in tropical montane forest. Biodivers. Conserv. 2017, 26, 2569–2589. [Google Scholar] [CrossRef]
- Sumanasekara, V.; Dissanayake, D.; Seneviratne, H. Review on use of Amphibian Taxa as a Bio-Indicator for Watershed Health and Stresses. In Proceedings of the NBRO Symposium Proceedings, Colombo, Sri Lanka, 22 December 2015. [Google Scholar]
- Pigot, A.L.; Merow, C.; Wilson, A.; Trisos, C.H. Abrupt expansion of climate change risks for species globally. Nat. Ecol. Evol. 2023, 7, 1060–1071. [Google Scholar] [CrossRef]
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzee, A.; Hamidy, A.; Aowphol, A.; Jean, A.; et al. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 2023, 622, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.R.; Grabowski, J.H.; Leslie, H.M.; Scyphers, S.; Williams, S.L. Inclusion of biodiversity in habitat restoration policy to facilitate ecosystem recovery. Conserv. Lett. 2018, 11, e12419. [Google Scholar] [CrossRef]
- De Zoysa, M. Ecotourism development and biodiversity conservation in Sri Lanka: Objectives, conflicts and resolutions. Open J. Ecol. 2022, 12, 638–666. [Google Scholar] [CrossRef]
- Cunningham, A.A.; Turvey, S.T.; Zhou, F.; Meredith, H.M.; Guan, W.; Liu, X.L.; Sun, C.M.; Wang, Z.Q.; Wu, M.Y. Development of the Chinese Giant Salamander Andrias davidianus Farming Industry in Shaanxi Province, China: Conservation threats and opportunities. Oryx 2016, 50, 265–273. [Google Scholar] [CrossRef]
- Turvey, S.T.; Chen, S.; Tapley, B.; Wei, G.; Xie, F.; Yan, F.; Yang, J.; Liang, Z.; Tian, H.; Wu, M.; et al. Imminent extinction in the wild of the world’s largest amphibian. Curr. Biol. 2018, 28, R592–R594. [Google Scholar] [CrossRef]
- Chai, J.; Lu, C.Q.; Yi, M.R.; Dai, N.H.; Weng, X.D.; Di, M.X.; Peng, Y.; Tang, Y.; Shan, Q.H.; Wang, K.; et al. Discovery of a wild, genetically pure Chinese giant salamander creates new conservation opportunities. Zool. Res. 2022, 43, 469–480. [Google Scholar] [CrossRef]
- Yan, F.; Lu, J.; Zhang, B.; Yuan, Z.; Zhao, H.; Huang, S.; Wei, G.; Mi, X.; Zou, D.; Xu, W.; et al. The Chinese giant salamander exemplifies the hidden extinction of cryptic species. Curr. Biol. 2018, 28, R590–R592. [Google Scholar] [CrossRef]
- Ma, Q.; Wan, L.; Shi, S.; Wang, Z. Impact of climate change on the distribution of three rare salamanders (Liua shihi, Pseudohynobius jinfo and Tylototriton wenxianensis) in Chongqing, China, and their conservation implications. Animals 2024, 14, 672. [Google Scholar] [CrossRef]
- Gong, D.; Mu, M. Behavioral observations and descriptions of the endangered knobby newt Tylototriton wenxianensis and their application in conservation. Asian Herpetol. Res. 2008, 1, 31–38. [Google Scholar]
- Hoffmann, M. Threatened Amphibians of the World; Lynx Edicions: Barcelona, Spain, 2008. [Google Scholar]
- Ficetola, G.F.; Rondinini, C.; Bonardi, A.; Baisero, D.; Padoa-Schioppa, E. Habitat availability for amphibians and extinction threat: A global analysis. Divers. Distrib. 2015, 21, 302–311. [Google Scholar] [CrossRef]
- Burrow, A.; Maerz, J. How plants affect amphibian populations. Biol. Rev. 2022, 97, 1749–1767. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.F.B.; Maltchik, L. Does organic agriculture benefit anuran diversity in rice fields? Wetlands 2014, 34, 725–733. [Google Scholar] [CrossRef]
- Harper, E.B. The Role of Terrrestrial Habitat in the Population Dynamics and Conservation of Pond-Breeding Amphibians; University of Missouri: Columbia, MO, USA, 2007. [Google Scholar]
- Khatiwada, J.R.; Zhao, T.; Chen, Y.; Wang, B.; Xie, F.; Cannatella, D.C.; Jiang, J. Amphibian community structure along elevation gradients in eastern Nepal Himalaya. BMC Ecol. 2019, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Smalling, K.L.; Rowe, J.C.; Pearl, C.A.; Iwanowicz, L.R.; Givens, C.E.; Anderson, C.W.; McCreary, B.; Adams, M.J. Monitoring wetland water quality related to livestock grazing in amphibian habitats. Environ. Monit. Assess. 2021, 193, 58. [Google Scholar] [CrossRef]
| Habitat Types | Habitat Type | Species | ||||||
|---|---|---|---|---|---|---|---|---|
| Arbo-Real | Terrestrial-Soil Cave | Terrestrial-Farmland | Terrestrial-Highland | Aquatic-Turbulence | Aquatic-Running Water | Aquatic-Quite Water | ||
| 5 | √ | √ | √ | √ | √ | Bufo gargarizans | ||
| 5 | √ | √ | √ | √ | √ | Bufo andrewsi | ||
| 5 | √ | √ | √ | √ | √ | Bufo minshanicus | ||
| 2 | √ | √ | Fejervarya kawamurai | |||||
| 2 | √ | √ | Rana chensinensis | |||||
| 3 | √ | √ | √ | Pelophylax nigromaculatus | ||||
| 2 | √ | √ | Rana omeimontis | |||||
| 1 | √ | Amolops mantzorum | ||||||
| 1 | √ | Amolops lifanensis | ||||||
| 2 | √ | √ | Odorrana margaretae | |||||
| 3 | √ | √ | √ | Quasipaa boulengeri | ||||
| 3 | √ | √ | √ | Feirana quadranus | ||||
| 2 | √ | √ | Kaloula rugifera | |||||
| 4 | √ | √ | √ | √ | Polypedates braueri | |||
| 2 | √ | √ | Hylarana guentheri | |||||
| 2 | √ | √ | Boulenophrys minor | |||||
| 2 | √ | √ | Microhyla fissipes | |||||
| 3 | √ | √ | √ | Oreolalax chuanbeiensis | ||||
| 1 | √ | Oreolalax nanjiangensis | ||||||
| 3 | √ | √ | √ | Scutiger pingwuensis | ||||
| 2 | √ | √ | Megophrys omeimontis | |||||
| 2 | √ | √ | Tylototriton wenxianensis | |||||
| 1 | √ | Batrachuperus pinchonii | ||||||
| 1 | √ | Batrachuperus tibetanus | ||||||
| 1 | √ | Andrias davidianus | ||||||
| Sites | Shannon–Wiener Diversity Index (H’) | Pielou’s Evenness Index (E) | Ecological Dominance Index (D) |
|---|---|---|---|
| Baiguoping | 1.69 | 0.71 | 0.22 |
| Baixiongping | 1.69 | 0.87 | 0.23 |
| Caijiaba | 1.76 | 0.85 | 0.22 |
| Motianling | 1.67 | 0.86 | 0.23 |
| Shuichiping | 1.64 | 0.92 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Xiao, M.; Zhao, L.; Wu, Y.; Jin, L.; Yan, C.; Liao, W. Species Diversity and Distribution of Amphibians in Tangjiahe National Nature Reserve, China. Biology 2025, 14, 614. https://doi.org/10.3390/biology14060614
Li M, Xiao M, Zhao L, Wu Y, Jin L, Yan C, Liao W. Species Diversity and Distribution of Amphibians in Tangjiahe National Nature Reserve, China. Biology. 2025; 14(6):614. https://doi.org/10.3390/biology14060614
Chicago/Turabian StyleLi, Mingfu, Mei Xiao, Li Zhao, Yiming Wu, Long Jin, Chengzhi Yan, and Wenbo Liao. 2025. "Species Diversity and Distribution of Amphibians in Tangjiahe National Nature Reserve, China" Biology 14, no. 6: 614. https://doi.org/10.3390/biology14060614
APA StyleLi, M., Xiao, M., Zhao, L., Wu, Y., Jin, L., Yan, C., & Liao, W. (2025). Species Diversity and Distribution of Amphibians in Tangjiahe National Nature Reserve, China. Biology, 14(6), 614. https://doi.org/10.3390/biology14060614






