Reproductive Strategies of the Swelled Vent Frog (Nanorana quadranus): Testicular Size, Sperm Traits, and Fecundity Responses to Geographical Gradients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
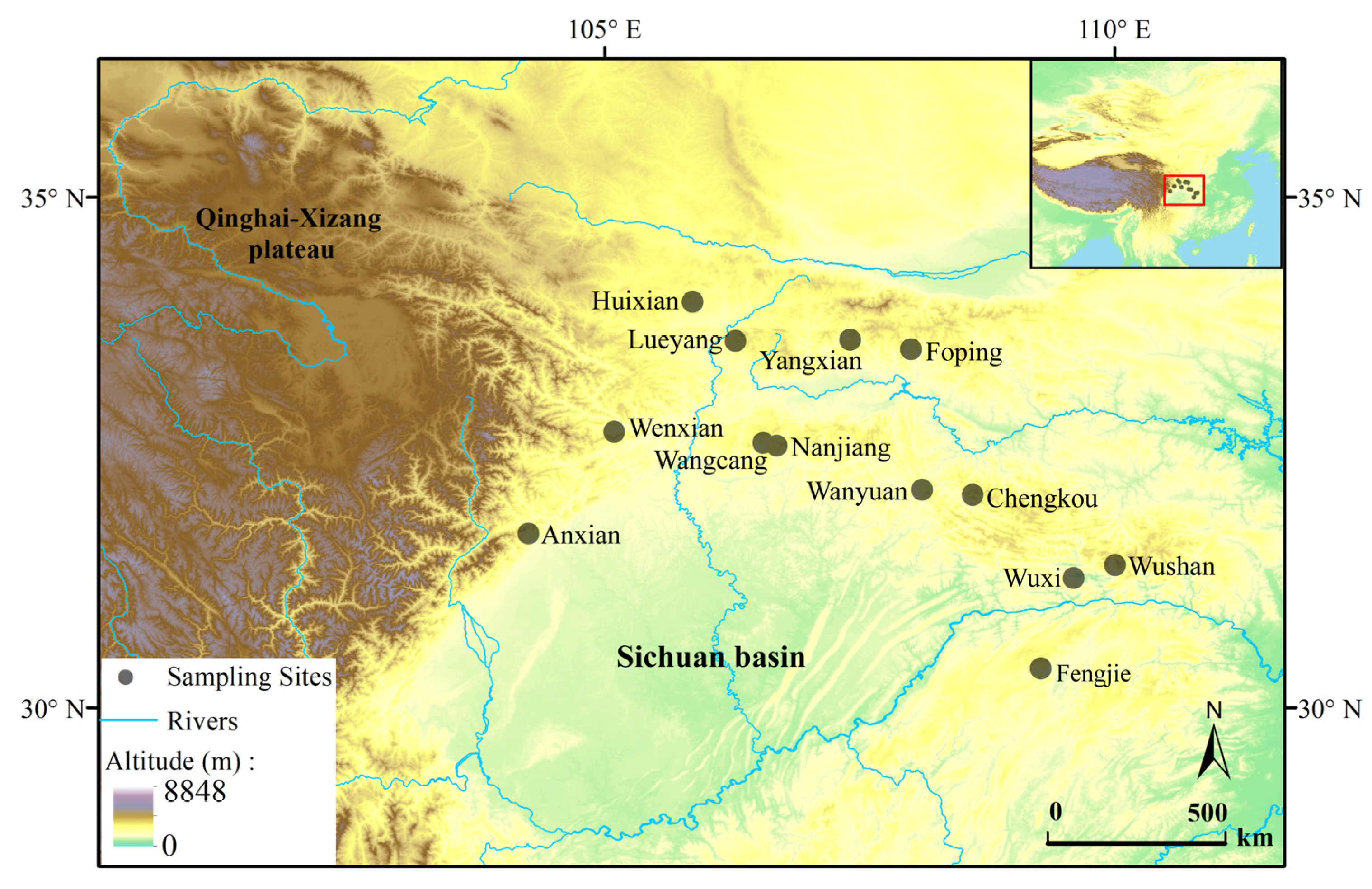
2.1. Fieldwork and Sampling
2.2. Age Determination
2.3. Testicular Morphology and Sperm Analysis in Males
2.4. Fecundity Estimation in Females
2.5. Environment Variables
2.6. Statistical Analyses
3. Results
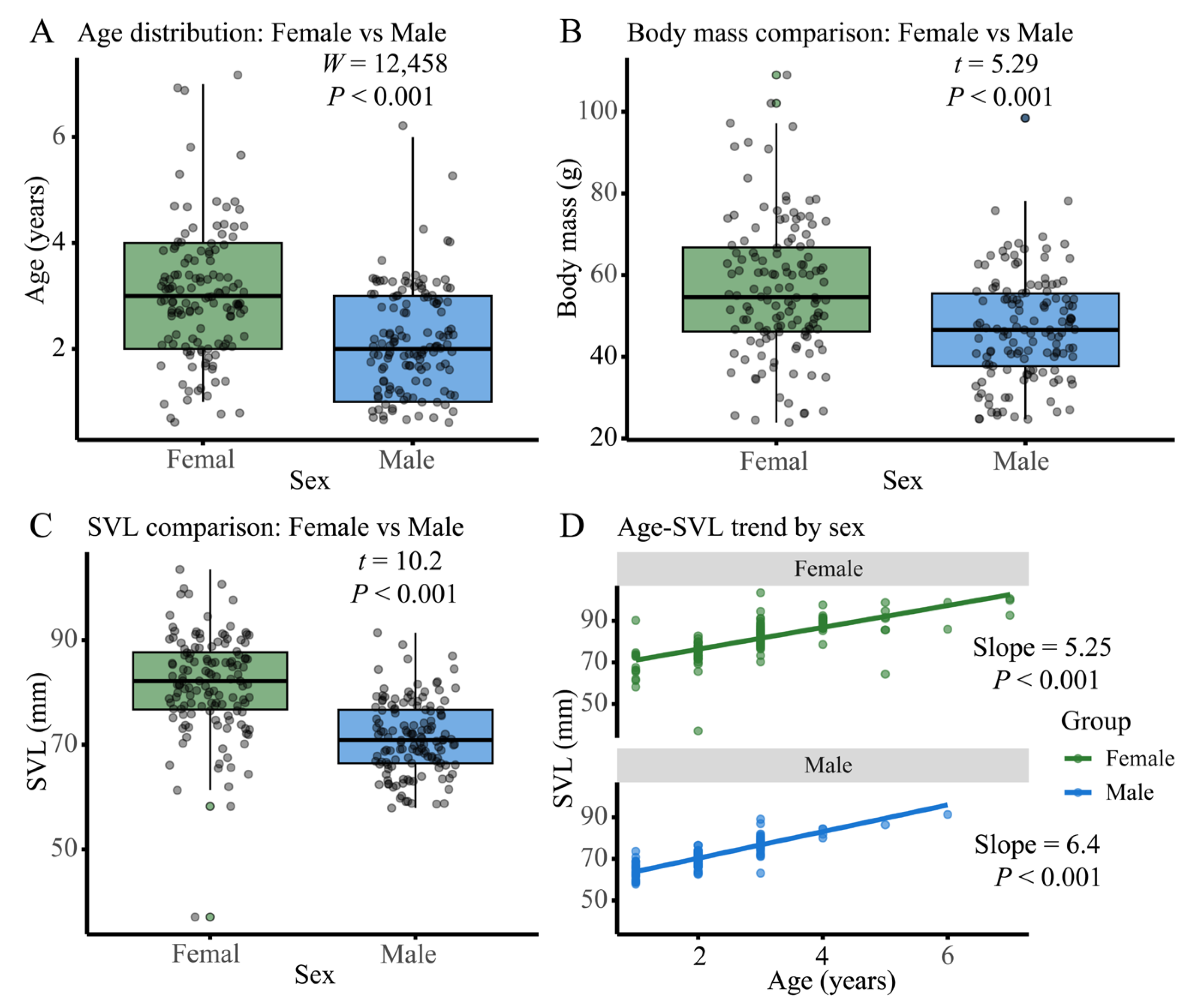
3.1. Sexual Dimorphism in Age Distribution, Body Size, and Growth Patterns of N. quadranus
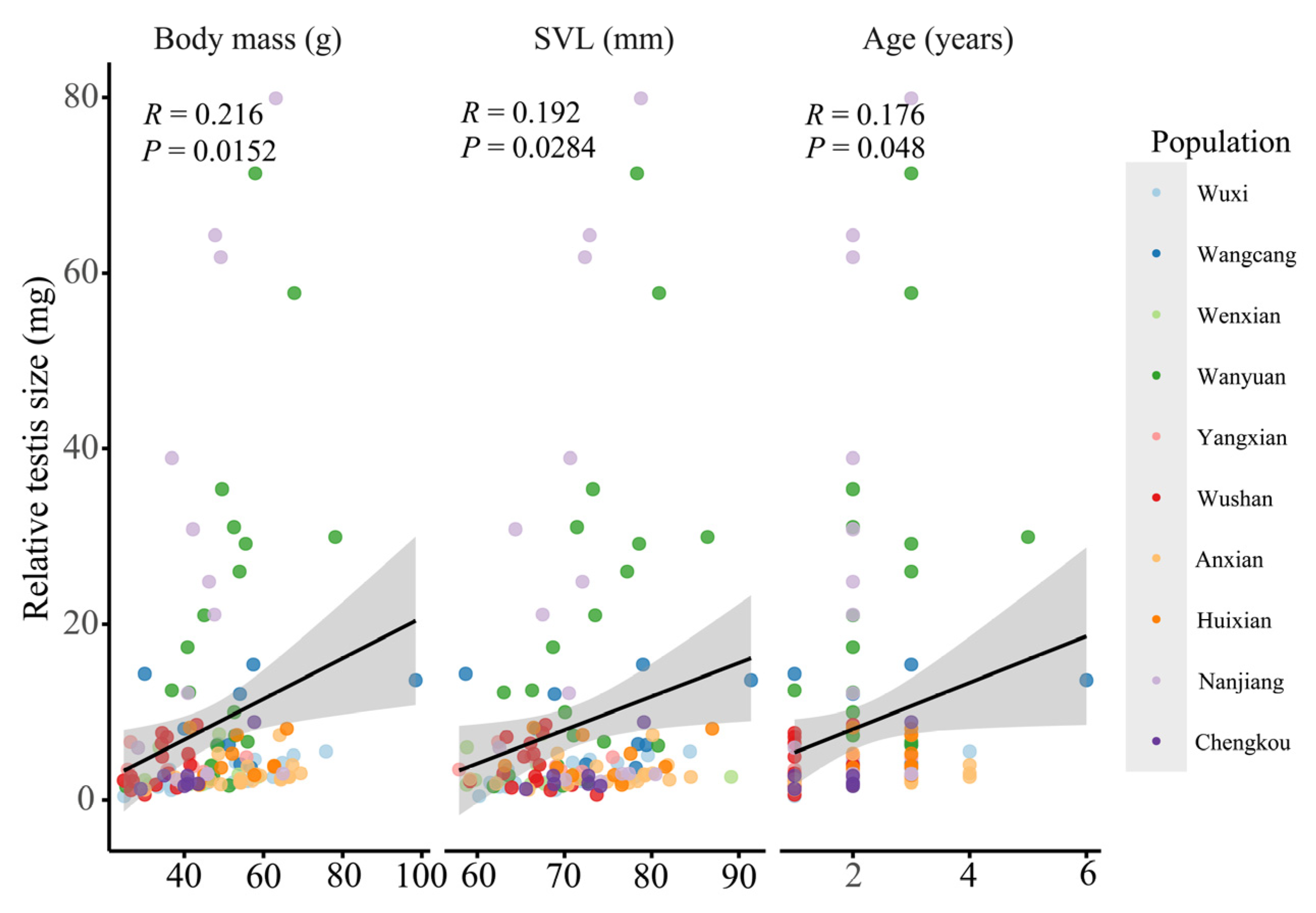
3.2. Geographical Variation in Testis Asymmetry and Relative Size
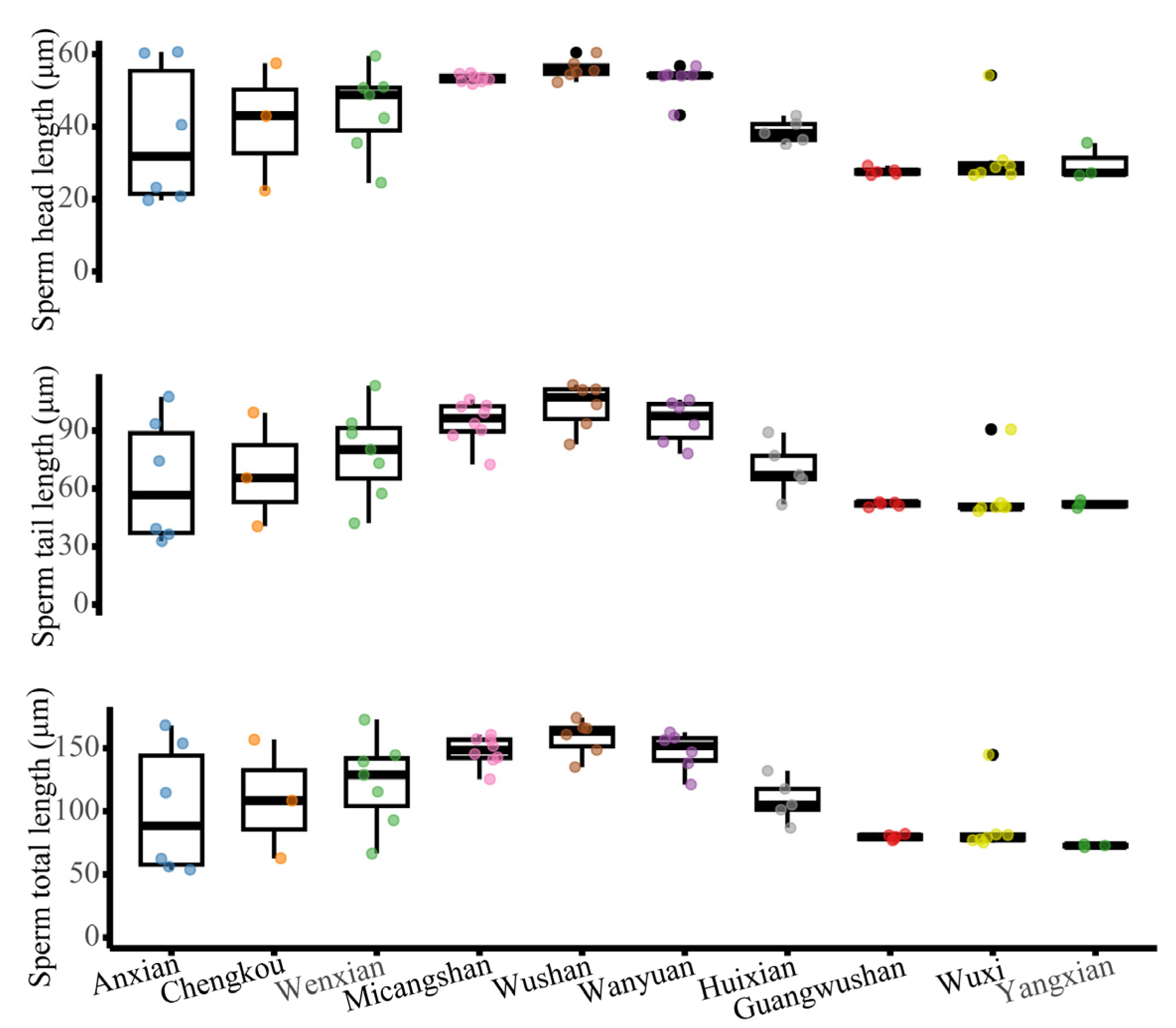
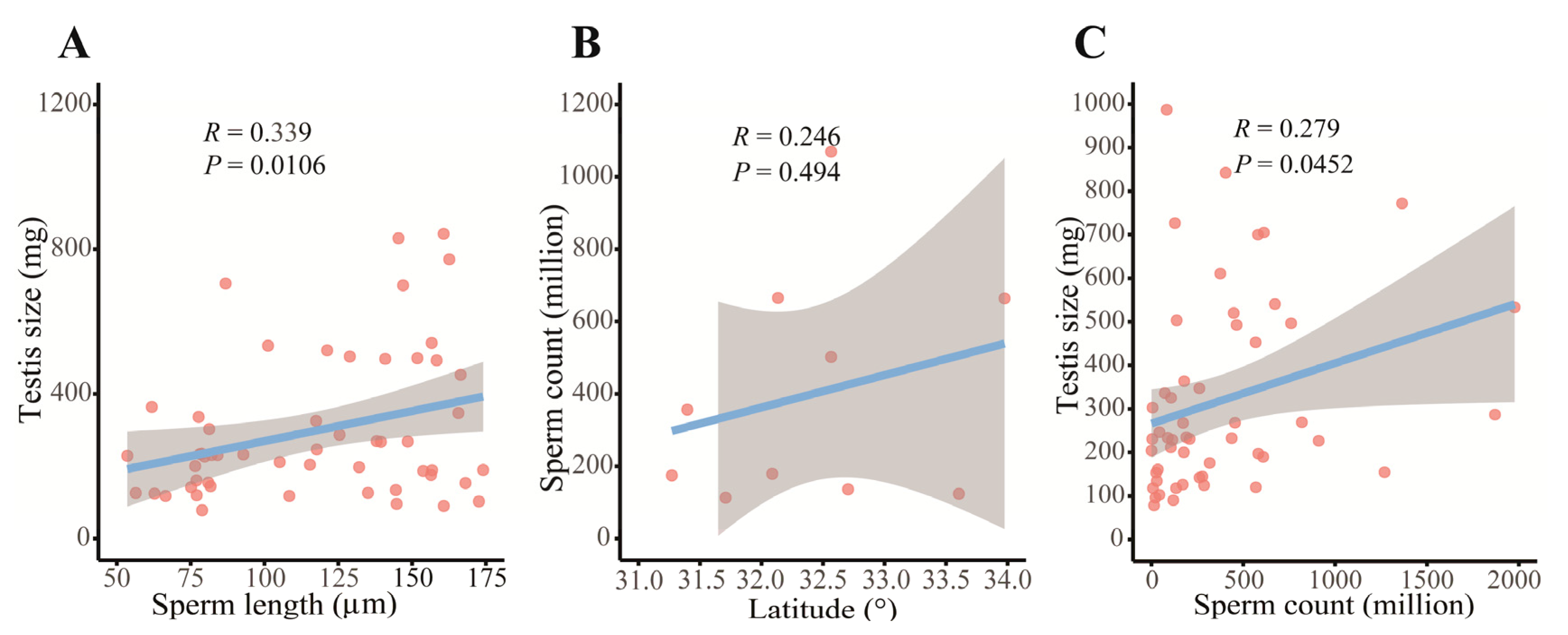
3.3. Geographical Patterns in Sperm Morphology and Count
3.4. Altitudinal Variation in Female Fecundity
3.5. Multivariate Environmental Predictors of Reproductive Traits
4. Discussion
4.1. Geographic Variation in Male Reproductive Investment: Testicular and Sperm Traits
4.2. Geographic Patterns in Female Fecundity and Life-History Traits
4.3. Environmental Determinants of Reproductive Strategy Divergence
4.4. Conservation Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vance, R.R. On reproductive strategies in marine benthic invertebrates. Am. Nat. 1973, 107, 339–352. [Google Scholar] [CrossRef]
- Fan, Y.Z.; Wang, X.Y.; Tang, K.C.; Chen, Q.F.; Li, S.; Sun, L.; Hu, J.H. Breeding in mountain tops: Water velocity determines oviposition site selection of the Chinting alpine toad in streams. Asian Herpetol. Res. 2024, 15, 195–205. [Google Scholar] [CrossRef]
- Chen, H.; Bu, R.; Ning, M.; Yang, B.; Wu, Z.; Huang, H. Sexual dimorphism in the Chinese endemic species Hynobius maoershanensis (Urodela: Hynobiidae). Animals 2022, 12, 1712. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Feng, H.; Jin, L.; Mi, Z.P.; Zhou, Z.M.; Liao, W.B. Latitudinal variation in body size in Fejervarya limnocharis supports the inverse of Bergmann’s rule. Anim. Biol. 2018, 68, 113–128. [Google Scholar] [CrossRef]
- Krasnov, B.R.; Surkova, E.N.; Shenbrot, G.I.; Khokhlova, I.S. Latitudinal gradients in body size and sexual size dimorphism in fleas: Males drive Bergmann’s pattern. Integr. Zool. 2023, 18, 414–426. [Google Scholar] [CrossRef]
- Amezquita, A.; Lima, A.P.; Jehle, R.; Castellanos, L.; Ramos, O.; Crawford, A.J.; Gasser, H.; Hoedl, W. Calls, colours, shape, and genes: A multi-trait approach to the study of geographic variation in the Amazonian frog Allobates femoralis. Biol. J. Linn. Soc. 2009, 98, 826–838. [Google Scholar] [CrossRef]
- Morrison, C.; Hero, J.M. Geographic variation in life-history characteristics of amphibians: A review. J. Anim. Ecol. 2003, 72, 270–279. [Google Scholar] [CrossRef]
- Feria-Ortiz, M.; Salgado-Ugarte, I.h. Reproductive Biology of a Blue-tail Skinks (Plestiodon) Population from a Temperate Forest, East-central Puebla, México. Asian Herpetol. Res. 2024, 15, 183–193. [Google Scholar] [CrossRef]
- Bose, A.P.; Hodgson, R.Y.; Brown, N.; Cogliati, K.M.; Balshine, S. Lunar synchrony and reproductive strategies of intertidal-breeding fishes. Mar. Biol. 2025, 172, 75. [Google Scholar] [CrossRef]
- Darmis, F.; Vezyrakis, A.; Guenther, A. Male reproductive tactics in house mice: Consistent individual differences, intrinsic factors and density effects. J. Anim. Ecol. 2025, 94, 1244–1258. [Google Scholar] [CrossRef]
- Hovestadt, T.; Kohl, P.; Mitesser, O. Optimal fissioning strategies of social insects with respect to colony dynamics and nest founding probability. Insect. Soc. 2024, 71, 171–183. [Google Scholar] [CrossRef]
- Moura, M.R.; Dixo, M.; Feio, R.N. Integrating life-history traits and amphibian upland habitat use in a Neotropical hotspot. Acta. Oecol. 2015, 69, 87–95. [Google Scholar] [CrossRef]
- Sadik, A.S.; Datta, A.K. Nesting on Cell Phone Towers: An Inexplicable Breeding Strategy by Asian Woollynecks Ciconia episcopus in Bangladesh. Ecol. Evol. 2025, 15, e71353. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.N.; Santacruz, A.; Wu, D.; Bertho, S.; Fritz, E.; Morales-Sosa, P.; McKinney, S.; Nowotarski, S.H.; Rohner, N. Reproductive adaptation of Astyanax mexicanus under nutrient limitation. Dev. Biol. 2025, 523, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Roff, D.A.; Mostowy, S.; Fairbairn, D.J. The evolution of trade-offs: Testing predictions on response to selection and environmental variation. Evolution 2002, 56, 84–95. [Google Scholar] [PubMed]
- Xu, F.; Yang, W.; Li, Y. Enlarged Egg Size Increases Offspring Fitness of a Frog Species on the Zhoushan Archipelago of China. Sci. Rep. 2019, 9, 11653. [Google Scholar] [CrossRef]
- Wang, G.; Gong, Y.; Han, J.; Cheng, L.I.; Xie, F. Oviposition Site Selection of the Plateau Frog (Nanorana pleskei)in the Zoige Wetland, China. Asian Herpetol. Res. 2017, 8, 269–274. [Google Scholar]
- Hatle, J.D.; Crowley, M.C.; Andrews, A.L.; Juliano, S.A. Geographic variation of reproductive tactics in lubber grasshoppers. Oecologia 2002, 132, 517–523. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, X.; Sheng, Y.; Zhong, X.; Liao, J.; Liu, Z.; Chen, W. No Male Preference for Large Females in the Asian Common Toad (Duttaphrynus melanostictus): Effect of the Sex Ratio and Breeding System. Asian Herpetol. Res. 2020, 11, 328–334. [Google Scholar]
- Wells, K.D. The social behaviour of anuran amphibians. Anim. Behav. 1977, 25, 666–693. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, R.; Ai, S.; Yang, Y.; Ding, J.; Zhang, Y. Long-term heavy metal pollution varied female reproduction investment in free-living anura, Bufo raddei. Ecotoxicol. Environ. Saf. 2018, 159, 136–142. [Google Scholar] [CrossRef]
- Zheng, P.; Gong, Y.; Wang, B.; Yu, H.; Huang, S.; Liao, X.; Jiang, J.; Ran, J.; Xie, F. Love Hug—Functional Validation of Nuptial Pad-Secreted Pheromone in Anurans. Animals 2024, 14, 1550. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.L.; Liu, Y.H.; Jin, L.; Mi, Z.P.; Liao, W.B. Altitudinal variation in somatic condition and reproductive investment of male Yunnan pond frogs (Dianrana pleuraden). Zool. Anz. 2017, 266, 189–195. [Google Scholar] [CrossRef]
- Lüpold, S.; Jin, L.; Liao, W.B. Population density and structure drive differential investment in pre-and postmating sexual traits in frogs. Evolution 2017, 71, 1686–1699. [Google Scholar] [CrossRef] [PubMed]
- Li, J. Research Progress on Evolutionary Mechanism of Monogyny and Polygyny in Tailless Amphibians. Shengwu Guocheng 2024, 14, 23–33. [Google Scholar]
- Joseph, P.N.; Emberts, Z.; Sasson, D.A.; Miller, C.W. Males that drop a sexually selected weapon grow larger testes. Evolution 2018, 72, 113–122. [Google Scholar] [CrossRef]
- Zhong, M.J.; Yu, X.; Liao, W.B. A review for life-history traits variation in frogs especially for anurans in China. Asian Herpetol. Res. 2018, 9, 165–174. [Google Scholar]
- Byrne, P.; Roberts, J.; Simmons, L. Sperm competition selects for increased testes mass in Australian frogs. J. Evol. Biol. 2002, 15, 347–355. [Google Scholar] [CrossRef]
- Jin, L.; Mi, Z.P.; Liao, W.B. Altitudinal variation in male reproductive investment in a polyandrous frog species (Hyla gongshanensis jingdongensis). Anim. Biol. 2016, 66, 289–303. [Google Scholar] [CrossRef]
- Qiu, D.J.; Yu, X.; Mao, J.Z.; Long, J. A Comparison of Somatic Condition and Testis Mass in Black-Spectacled Toad (Duttaphrynus melanostictus) between Two Populations at Different Altitudes. Pakistan J. Zool. 2020, 52, 2347. [Google Scholar] [CrossRef]
- Hettyey, A.; Laurila, A.; Herczeg, G.; Jönsson, K.I.; Kovács, T.; Merilä, J. Does testis weight decline towards the Subarctic? A case study on the common frog, Rana temporaria. Naturwissenschaften 2005, 92, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Hettyey, A.; Roberts, J.D. Sperm traits of the quacking frog, Crinia georgiana: Intra-and interpopulation variation in a species with a high risk of sperm competition. Behav. Ecol. Sociobiol. 2006, 59, 389–396. [Google Scholar] [CrossRef]
- Tang, T.; Luo, Y.; Huang, C.H.; Liao, W.B.; Huang, W.C. Variation in somatic condition and testis mass in Feirana quadranus along an altitudinal gradient. Anim. Biol. 2018, 68, 277–288. [Google Scholar] [CrossRef]
- Furness, A.I.; Venditti, C.; Capellini, I. Terrestrial reproduction and parental care drive rapid evolution in the trade-off between offspring size and number across amphibians. PLoS Biol. 2022, 20, e3001495. [Google Scholar] [CrossRef]
- Liao, W.; Lu, X.; Jehle, R. Altitudinal variation in maternal investment and trade-offs between egg size and clutch size in the Andrew’s toad. J. Zool. 2014, 293, 84–91. [Google Scholar] [CrossRef]
- Gould, J.; Beranek, C.; Valdez, J.; Mahony, M. Quantity versus quality: A balance between egg and clutch size among Australian amphibians in relation to other life-history variables. Austral Ecol. 2022, 47, 685–697. [Google Scholar] [CrossRef]
- Ma, L.; Hou, C.; Jiang, Z.W.; Du, W.G. Divergent effects of climate change on the egg-laying opportunity of species in cold and warm regions. Conserv Biol. 2023, 37, e14056. [Google Scholar] [CrossRef]
- Pettersen, A.K.; White, C.R.; Bryson-Richardson, R.J.; Marshall, D.J.; Auer, S. Linking life-history theory and metabolic theory explains the offspring size-temperature relationship. Ecol. Lett. 2019, 56, 518–526. [Google Scholar] [CrossRef]
- Dziminski, M.A.; Vercoe, P.E.; Roberts, J.D. Variable offspring provisioning and fitness: A direct test in the field. Funct. Ecol. 2009, 23, 164–171. [Google Scholar] [CrossRef]
- Crump, M.L. Intraclutch egg size variability in Hyla crucifer (Anura: Hylidae). Copeia 1984, 1984, 302–308. [Google Scholar] [CrossRef]
- Robinson, C.; Elvidge, C.; Frank, R.A.; Headley, J.V.; Hewitt, L.M.; Little, A.; Robinson, S.A.; Trudeau, V.; Vander Meulen, I.; Orihel, D. Naphthenic acid fraction compounds reduce the reproductive success of wood frogs (Rana sylvatica) by affecting offspring viability. Environ. Pollut. 2023, 316, 120455. [Google Scholar]
- Liao, W.B.; Lu, X. Proximate mechanisms leading to large male-mating advantage in the Andrew’s toad, Bufo andrewsi. Behaviour 2011, 148, 1087–1102. [Google Scholar] [CrossRef]
- Gollmann, B.; Gollmann, G. Geographic variation of larval traits in the Australian frog Geocrinia victoriana. Herpetologica 1996, 52, 181–187. [Google Scholar]
- Cejp, B.; Griebeler, E.M. Body Mass Shapes Most Life History Traits and a Fast-Slow Continuum in Amphibians. Ecol. Evol. 2024, 14, e70377. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, C.; Liao, W.B. No evidence for trade-off between clutch size and egg size in the spot-legged treefrog (Polypedates megacephalus). North-West J. Zool. 2017, 13, 58–62. [Google Scholar]
- Yu, T.L.; Xu, Y.; Busam, M.; Deng, Y.H. Maternal investment decreases under stressful environments in 11 plateau brown frog (Rana kukunoris) populations. Ethol. Ecol. Evol. 2018, 30, 168–177. [Google Scholar] [CrossRef]
- Chen, W.; Tang, Z.; Fan, X.; Wang, Y.; Pike, D. Maternal investment increases with altitude in a frog on the Tibetan Plateau. J. Evol. Biol. 2013, 26, 2710–2715. [Google Scholar] [CrossRef]
- Hastings, T.P.; Hossack, B.R.; Fishback, L.; Davenport, J.M. Using physiological conditions to assess current and future habitat use of a Subarctic frog. Integr. Zool. 2023, 18, 2–14. [Google Scholar]
- Liedtke, H.C.; Wiens, J.J.; Gomez-Mestre, I. The evolution of reproductive modes and life cycles in amphibians. Nat. Commun. 2022, 13, 7039. [Google Scholar] [CrossRef]
- Lei, Z.X.; Yan, C.C.; Jin, L.; Li, J.T.; Yan, C.Z.; Liao, W.B. Genomic evidence for climatic adaptation in Fejervarya multistriata. Divers. Distrib. 2024, 30, e13796. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, C.; Liao, W.B. Anuran interorbital distance variation: The role of ecological and behavioral factors. Integr. Zool. 2022, 17, 777–786. [Google Scholar]
- Zhang, X.; Niu, Y.; Men, S.; Tang, X.; Storey, K.B.; Chen, Q. High-altitude Adaptation of Xizang Plateau Frogs, Nanorana parkeri from a Perspective of the Gut Microbiome. Asian Herpetol. Res. 2024, 15, 214–224. [Google Scholar] [CrossRef]
- Mcwhinnie, R.B.; Sckrabulis, J.P.; Raffel, T.R. Temperature and mass scaling affect cutaneous and pulmonary respiratory performance in a diving frog. Integr. Zool. 2021, 16, 712–728. [Google Scholar]
- Walls, S.; Barichivich, W.; Brown, M. Drought, Deluge and Declines: The Impact of Precipitation Extremes on Amphibians in a Changing Climate. Biology. 2013, 2, 399–418. [Google Scholar]
- Rudin-Bitterli, T.S.; Mitchell, N.J.; Evans, J.P. Extensive geographical variation in testes size and ejaculate traits in a terrestrial-breeding frog. Biol. Lett. 2020, 16, 20200411. [Google Scholar] [CrossRef] [PubMed]
- Ficetola, G.F.; Maiorano, L. Contrasting effects of temperature and precipitation change on amphibian phenology, abundance and performance. Oecologia 2016, 181, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Shen, T.; Mu, L.; Liu, J.; Li, S.; Diao, Y.; Su, H. Geographical Variation in the Advertisement Calls of Leptobrachella ventripunctata (Anura: Megophryidae) in Southwestern China. Asian Herpetol. Res. 2024, 15, 140–151. [Google Scholar] [CrossRef]
- Wang, X.Y.; Huang, Y.; Zhong, M.J.; Yang, S.N.; Yang, X.; Jiang, J.P.; Hu, J.H. Environmental stress shapes life-history variation in the swelled-vented frog (Feirana quadranus). Evol. Ecol. 2019, 33, 435–448. [Google Scholar]
- IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/search?query=Rana%20quadranus&searchType=species (accessed on 12 July 2025).
- Zhang, L.; Yang, J.; Lu, Y.; Lu, X.; Chen, X. Aquatic eggs are fertilised by multiple males not engaged in amplexus in a stream-breeding frog. Behav. Processes. 2012, 91, 304–307. [Google Scholar]
- Chang, L.M.; Chen, Q.H.; Wang, B.; Liu, J.Y.; Zhang, M.H.; Zhu, W.; Jiang, J.P. Single cell RNA analysis uncovers the cell differentiation and functionalization for air breathing of frog lung. Commun. Biol. 2024, 7, 665. [Google Scholar] [CrossRef]
- Fu, Y.P.; Song, Y.L.; Yang, C.; Liu, X.Y.; Liu, Y.N.; Huang, Y. Relationship between brain size and digestive tract length support the expensive-tissue hypothesis in Feirana quadranus. Front Ecol. Evol. 2022, 10, 982590. [Google Scholar]
- Wang, B.; Jiang, J.P.; Xie, F.; Li, C. Postglacial colonization of the Qinling Mountains: Phylogeography of the swelled vent frog (Feirana quadranus). PloS ONE 2012, 7, e41579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Zhang, L.P.; Yu, D.N.; Storey, K.B.; Zheng, R.Q. Complete mitochondrial genomes of Nanorana taihangnica and N. yunnanensis (Anura: Dicroglossidae) with novel gene arrangements and phylogenetic relationship of Dicroglossidae. BMC Evol. Biol. 2018, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Pottier, P.; Kearney, M.R.; Wu, N.C.; Gunderson, A.R.; Rej, J.E.; Rivera-Villanueva, A.N.; Pollo, P.; Burke, S.; Drobniak, S.M.; Nakagawa, S. Vulnerability of amphibians to global warming. Nature 2025, 639, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, Q.; Cheng, Y.; Tang, K.; Sun, L.; Huang, Y.; Zhao, L.; Liang, D.; Wang, X.; Hu, J. Resurvey After 20 Years: Updating the Distribution, Population, and Potential Threats of the Chinting Alpine Toad. Asian Herpetol. Res. 2024, 15, 73–81. [Google Scholar] [CrossRef]
- Yang, L.; Huang, L.; Zhang, H.; Lee, P.; Zhang, N.; Cai, R.; Li, E.; Pan, T.; Wu, X. Habitat Suitability and Distribution Pattern Response to Global Climate Change in a Widespread Species, the Asiatic Toad (Bufo gargarizans). Asian Herpetol. Res. 2023, 14, 138–146. [Google Scholar] [CrossRef]
- Sun, J.H.; Lin, H.X.; Li, H.; Hu, J.H. Morphological Variation in Sichuan Spiny Frogs Along Geographic and Environmental Gradients. Asian Herpetol. Res. 2025, 16, 66–74. [Google Scholar]
- Ping, Y.J.; Cheng, C.; Long, J.; Li, Z.; Bo, L.W. No Evidence for Significant Effect of Body Size and Age on Male Mating Success in the Spot-legged Treefrog. Asian Herpetol. Res. 2016, 7, 41–45. [Google Scholar]
- Sinsch, U. Skeletochronological assessment of demographic life-history traits in amphibians. J. Herpetol. 2015, 25, 5–13. [Google Scholar]
- Yue, Y.F.; Jin, L.; Mai, C.L.; Huang, X.F.; Liao, W.B. No evidence for the compensation hypothesis in the swelled vent frog (Feirana quadranus). Asian Herpetol. Res. 2020, 11, 225–229. [Google Scholar]
- Ju, Y.L.; Jun, Z.M.; Bo, L.W. Age Structure of Two Species of Odorous Frogs (Odorrana margaretae and Odorrana grahami). Asian Herpetol. Res. 2021, 12, 308–314. [Google Scholar]
- Li, S.T.; Wu, X.; Li, D.Y.; Lou, S.L.; Mi, Z.P.; Liao, W.B. Body size variation of odorous frogs (Odorrana grahami) across altitudinal gradients. J. Herpetol. 2013, 23, 187–192. [Google Scholar]
- Liao, W.B.; Lu, X. Adult body size= f (initial size+ growth rate× age): Explaining the proximate cause of Bergman’s cline in a toad along altitudinal gradients. Evol. Ecol. 2012, 26, 579–590. [Google Scholar] [CrossRef]
- Castanet, J. Introduction to the skeletochronological method in amphibians and reptiles. Ann. Sci. Nat. Zool. 1990, 11, 191–196. [Google Scholar]
- Liao, W.B.; Luo, Y.; Lou, S.L.; Lu, D.; Jehle, R. Geographic variation in life-history traits: Growth season affects age structure, egg size and clutch size in Andrew’s toad (Bufo andrewsi). Front. Zool. 2016, 13, 6. [Google Scholar]
- Min, M.; Yan, H.; Ping, M.Z.; Hong, L.Y.; Quan, Z.C. Skeletochronological Study of Age, Longevity and Growth in a Population of Rana nigromaculata (Amphibia: Anura) in Sichuan, China. Asian Herpetol. Res. 2012, 3, 258–264. [Google Scholar] [CrossRef]
- Bordin, R.O.; Fernandes, C.E.d.S.; Franco-Belussi, L.; Leão, T.R.F.; Sanabria, M. Sperm morphology and testicular histology of the polyandric species Leptodactylus podicipinus (Anura: Leptodactylidae) from an urban environment. Anat. Rec. 2022, 305, 3532–3542. [Google Scholar] [CrossRef]
- Zeng, Y.; Lou, S.L.; Liao, W.B.; Jehle, R. Evolution of sperm morphology in anurans: Insights into the roles of mating system and spawning location. BMC Evol. Biol. 2014, 14, 104. [Google Scholar] [CrossRef]
- Salles, N.M.; Zara, F.J.; Prado, C.P. Differences in sperm morphology in foam-nesting leptodactyline frogs (Anura, Leptodactylidae). Acta. Zool. 2017, 98, 1–12. [Google Scholar]
- Dumont, J.N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J. Morphol. 1972, 136, 153–179. [Google Scholar] [CrossRef]
- Kuramoto, M. Correlations of Quantitative Parameters of Fecundity in Amphibians. Evol. Int. J. Org. Evol. 1978, 32, 287–296. [Google Scholar] [CrossRef]
- Sun, Z.J.; Zhao, T.; Hu, S.W.; Chen, G.F.; Zhu, W.; Peng, X.W.; Su, S.Q. Integrating climate change and fine-scale habitat suitability to assess amphibian range shift in Mount Emei, China. Front. Zool. 2025, 22, 16. [Google Scholar] [CrossRef]
- Mi, C.; Huettmann, F.; Li, X.; Jiang, Z.; Du, W.; Sun, B. Effects of climate and human activity on the current distribution of amphibians in China. Conserv. Biol. 2022, 36, e13964. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.F.; Jin, Y.T. Altitudinal variation in reproductive strategy of the toad-headed lizard, Phrynocephalus vlangalii in North Tibet Plateau (Qinghai). Amphib. -Reptil. 2007, 28, 509–515. [Google Scholar]
- Durán, F.; Méndez, M.A.; Correa, C. The Atacama toad (Rhinella atacamensis) exhibits an unusual clinal pattern of decreasing body size towards more arid environments. BMC Zool. 2021, 6, 25. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Scali, S.; Denoël, M.; Montinaro, G.; Vukov, T.D.; Zuffi, M.A.; Padoa-Schioppa, E. Ecogeographical variation of body size in the newt Triturus carnifex: Comparing the hypotheses using an information-theoretic approach. Global Ecol. Biogeogr. 2010, 19, 485–495. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Clim. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Zhong, M.-J.; Zhang, J.; Si, X.-F.; Yang, S.-N.; Jiang, J.-P.; Hu, J.-H. Multidimensional amphibian diversity and community structure along a 2 600 m elevational gradient on the eastern margin of the Qinghai-Tibetan Plateau. Zool. Res. 2022, 43, 40–51. [Google Scholar] [CrossRef]
- Null, R.C.T.R.; Team, R.; Null, R.C.T.; Core Writing, T.; Null, R.; Team, R.; Null, R.D.C.T.; Core, R.; Team, R.; Team, R.D.C. R: A language and environment for statistical computing. Computing 2011, 1, 12–21. [Google Scholar]
- Møller, A.P.; Swaddle, J.P. Asymmetry, Developmental Stability and Evolution; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Anderson, D.R.; Burnham, K.P. Avoiding pitfalls when using information-theoretic methods. J. Wildl. Manag. 2002, 66, 912–918. [Google Scholar] [CrossRef]
- Bai, Z.; Wu, Q.; Zhou, M.; Tian, Y.; Sun, J.; Jiang, F.; Xu, Y.-P. An Integrated Framework for NDVI and LAI Forecasting with Climate Factors: A Case Study in Oujiang River Basin, Southeast China. Forests 2025, 16, 1075. [Google Scholar] [CrossRef]
- Aiello, L.C.; Wheeler, P. The expensive-tissue hypothesis: The brain and the digestive system in human and primate evolution. Curr. Anthrop. 1995, 36, 199–221. [Google Scholar]
- Garland, T., Jr.; Downs, C.J.; Ives, A.R. Trade-offs (and constraints) in organismal biology. Physiol. Biochem. Zool. 2022, 95, 82–112. [Google Scholar] [PubMed]
- Li, Y.; Cohen, J.M.; Rohr, J.R. Review and synthesis of the effects of climate change on amphibians. Integr. Zool. 2013, 8, 145–161. [Google Scholar] [PubMed]
- Jin, L.; Yang, S.N.; Liao, W.B.; Lüpold, S. Altitude underlies variation in the mating system, somatic condition, and investment in reproductive traits in male Asian grass frogs (Fejervarya limnocharis). Behav. Ecol. Sociobiol. 2016, 70, 1197–1208. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Y.Y.; Liao, W.B. A comparison of testes size and sperm length between Polypedates megacephalus populations at different altitudes. J. Herpetol. 2016, 26, 249–252. [Google Scholar]
- Valencia-Aguilar, A.; Ringler, E.; Lüpold, S.; Guayasamin, J.M.; Prado, C.P. Evolutionary trade-offs between testes size and parenting in Neotropical glassfrogs. Proc. R. Soc. B 2024, 291, 20240054. [Google Scholar] [CrossRef]
- Byrne, P.G.; Simmons, L.W.; Roberts, J.D. Sperm competition and the evolution of gamete morphology in frogs. Proc. Biol. Sci. 2003, 270, 2079–2086. [Google Scholar] [CrossRef]
- Chen, C.; Pfennig, K.S. Female toads engaging in adaptive hybridization prefer high-quality heterospecifics as mates. Science 2020, 367, 1377–1379. [Google Scholar] [CrossRef]
- Liu, Y.H.; Liao, W.B.; Zhou, C.Q.; Mi, Z.P.; Mao, M. Asymmetry of Testes in Guenther’s Frog, Hylarana guentheri (Anuar: Ranidae). Asian Herpetol. Res. 2011, 02, 234–239. [Google Scholar] [CrossRef]
- Liao, W.B.; Huang, Y.; Zeng, Y.; Zhong, M.J.; Luo, Y.; Lüpold, S. Ejaculate evolution in external fertilizers: Influenced by sperm competition or sperm limitation? Evolution 2018, 72, 4–17. [Google Scholar]
- Agrawal, A.A. Phenotypic Plasticity in the Interactions and Evolution of Species. Science 2001, 294, 321–326. [Google Scholar] [CrossRef]
- Dongen, S.V. Fluctuating asymmetry and developmental instability in evolutionary biology: Past, present and future. J. Evol. Biol. 2006, 19, 1727–1743. [Google Scholar] [CrossRef]
- Parker, G.; Ball, M. Sperm competition, mating rate and the evolution of testis and ejaculate sizes: A population model. Biol. Lett. 2005, 1, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.A.; Pizzari, T. Sperm competition and ejaculate economics. Biol. Rev. 2010, 85, 897–934. [Google Scholar] [CrossRef] [PubMed]
- Stockley, P.; Gage, M.; Parker, G.; Møller, A. Sperm competition in fishes: The evolution of testis size and ejaculate characteristics. Am. Nat. 1997, 149, 933–954. [Google Scholar] [CrossRef] [PubMed]
- Gomendio, M.; Roldan, E.R. Sperm competition influences sperm size in mammals. Proc. Roy. Soc. London Ser. B Biol. Sci. 1991, 243, 181–185. [Google Scholar]
- Miaud, C.; Guyétant, R.; Elmberg, J. Variations in life-history traits in the common frog Rana temporaria (Amphibia: Anura): A literature review and new data from the French Alps. J. Zool. 1999, 249, 61–73. [Google Scholar] [CrossRef]
- Lüddecke, H. Variation and trade-off in reproductive output of the Andean frog Hyla labialis. Oecologia 2002, 130, 403–410. [Google Scholar] [CrossRef]
- Yu, T.L.; Deng, Y.H. Geographic variation in maternal investment and trade-offs between egg size and clutch size in an endemic toad of the Qinghai-Tibet plateau. Sci. Rep. 2020, 10, 6838. [Google Scholar] [CrossRef]
- Liao, W.B.; Lu, X. Male mate choice in the Andrew’s toad Bufo andrewsi: A preference for larger females. J. Ethol. 2008, 27, 413–417. [Google Scholar] [CrossRef]
- Rastogi, R.K.; Pinelli, C.; Polese, G.; D’Aniello, B.; Chieffi-Baccari, G. Hormones and Reproductive Cycles in Anuran Amphibians. Horm. Reprod. Vertebr. 2011, 2, 219–233. [Google Scholar]
- Kahrl, A.F.; Snook, R.R.; Fitzpatrick, J.L. Fertilization mode drives sperm length evolution across the animal tree of life. Nat. Ecol. Evol. 2021, 5, 1153–1164. [Google Scholar] [CrossRef]
- Deng, W.Y.; Jin, L.; Qiu, D.J.; Yan, C.Z.; Liao, W.B. Geographic variation in organ size in a toad (Duttaphrynus melanostictus). Animals 2023, 13, 2645. [Google Scholar] [CrossRef]
- Pierson, T.W.; Blake-Sinclair, J.; Rittenburg, L.T.; Kalki, Y. Geographic variation in the reproductive phenology of a widespread amphibian. J. Herpetol. 2023, 57, 125–132. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, L.; Huang, S.; He, M.; Huang, Y. Reproductive Strategies of the Swelled Vent Frog (Nanorana quadranus): Testicular Size, Sperm Traits, and Fecundity Responses to Geographical Gradients. Biology 2025, 14, 1224. https://doi.org/10.3390/biology14091224
Lyu L, Huang S, He M, Huang Y. Reproductive Strategies of the Swelled Vent Frog (Nanorana quadranus): Testicular Size, Sperm Traits, and Fecundity Responses to Geographical Gradients. Biology. 2025; 14(9):1224. https://doi.org/10.3390/biology14091224
Chicago/Turabian StyleLyu, Lulu, Shuang Huang, Miao He, and Yan Huang. 2025. "Reproductive Strategies of the Swelled Vent Frog (Nanorana quadranus): Testicular Size, Sperm Traits, and Fecundity Responses to Geographical Gradients" Biology 14, no. 9: 1224. https://doi.org/10.3390/biology14091224
APA StyleLyu, L., Huang, S., He, M., & Huang, Y. (2025). Reproductive Strategies of the Swelled Vent Frog (Nanorana quadranus): Testicular Size, Sperm Traits, and Fecundity Responses to Geographical Gradients. Biology, 14(9), 1224. https://doi.org/10.3390/biology14091224




