BMP-2-Driven Osteogenesis: A Comparative Analysis of Porcine BMSCs and ASCs and the Role of TGF-β and FGF Signaling
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Porcine ASC and BMSC Harvest
2.2. Methods of MSC Preparation
2.2.1. Isolation of pACS
2.2.2. Isolation of pBMCs
2.3. Phenotypical Analysis of pMSCs Using Flow Cytometry
2.4. Seeding and Osteogenic Differentiation of pASCs and pBMSCs
2.5. Analysis of Osteogenic Differentiation Detection Using Alizarin Red S Staining and Quantification Using Cetylpyridinium Chloride
2.6. Western Blot Analysis of Protein Expression in pACS
2.7. Statistical Analysis
3. Results
3.1. Characterization of pASCs and pBMSCs
3.2. Comparison of Osteogenic Differentiation of pASCs and pBMSCs with Addition of BMP-2
3.2.1. Osteogenic Differentiation of pASCs with or Without BMP-2
3.2.2. Osteogenic Differentiation of pBMSCs with or Without BMP-2
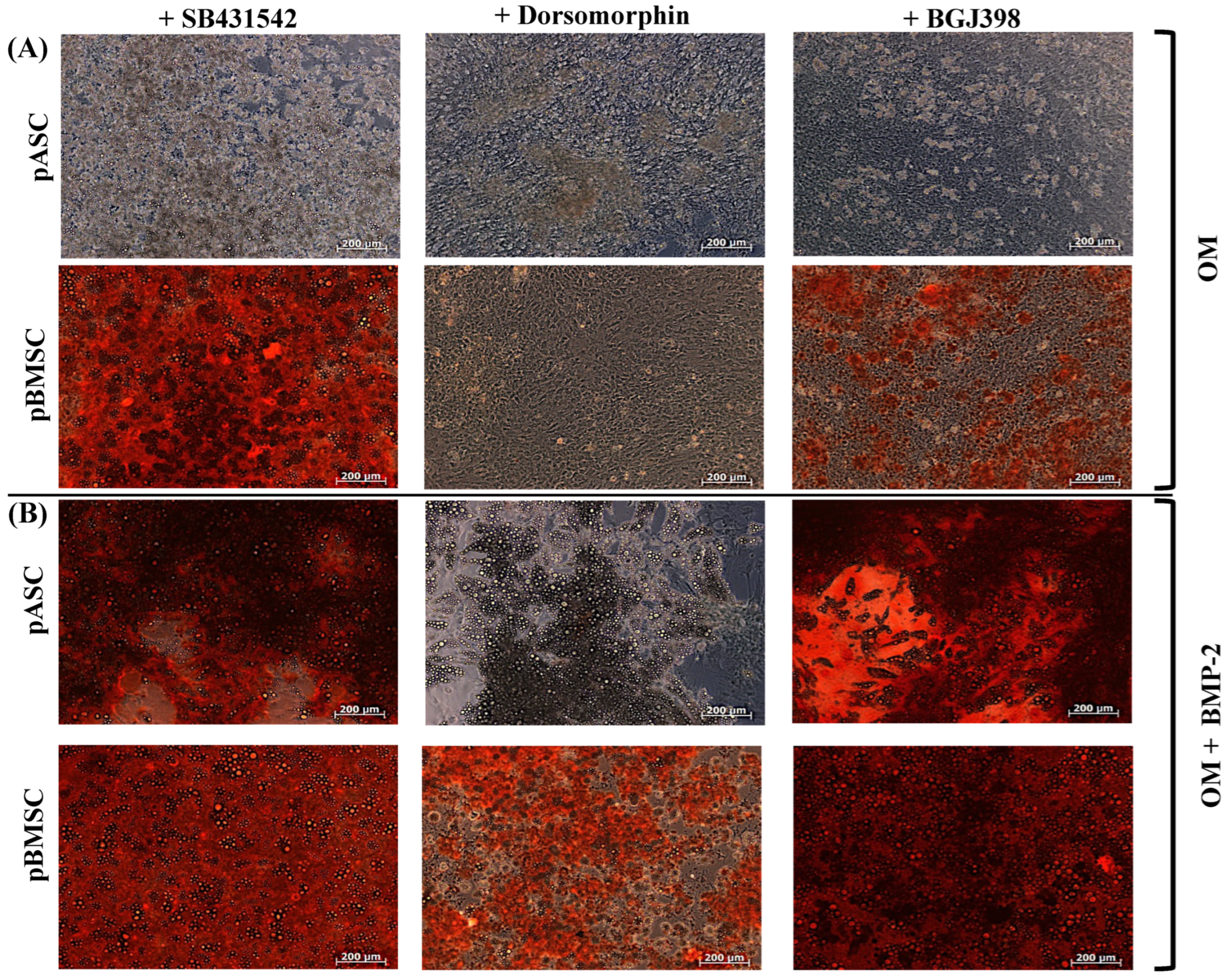
3.3. Comparison of Osteogenic Differentiation of pASCs and pBMSCs Under Specific Inhibition from TGF-β, BMP, and FGF Signaling
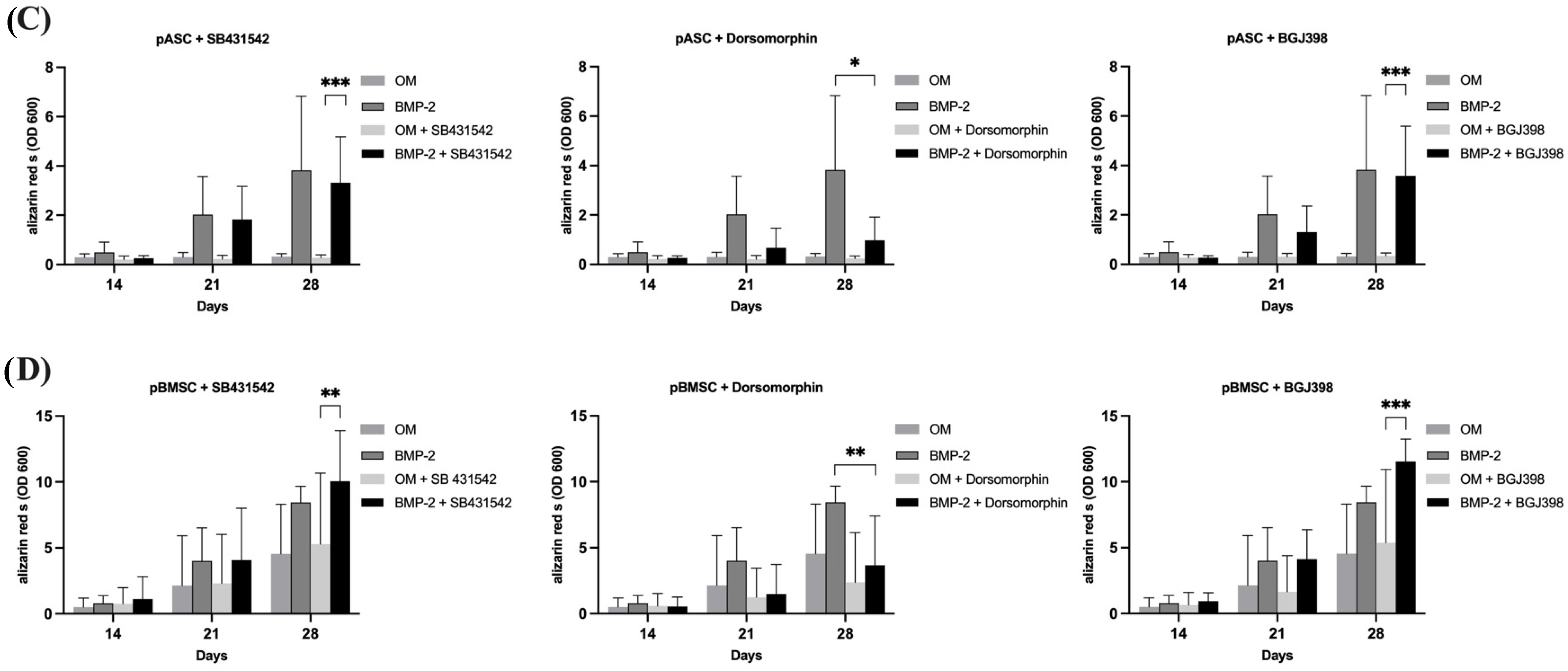
3.3.1. Impact of TGF-β Signaling on Osteogenic Differentiation of pASCs and pBMSCs
3.3.2. Impact of BMP Signaling on Osteogenic Differentiation of pASCs and pBMSCs
3.3.3. Impact of FGF Signaling on Osteogenic Differentiation of pASCs and pBMSCs
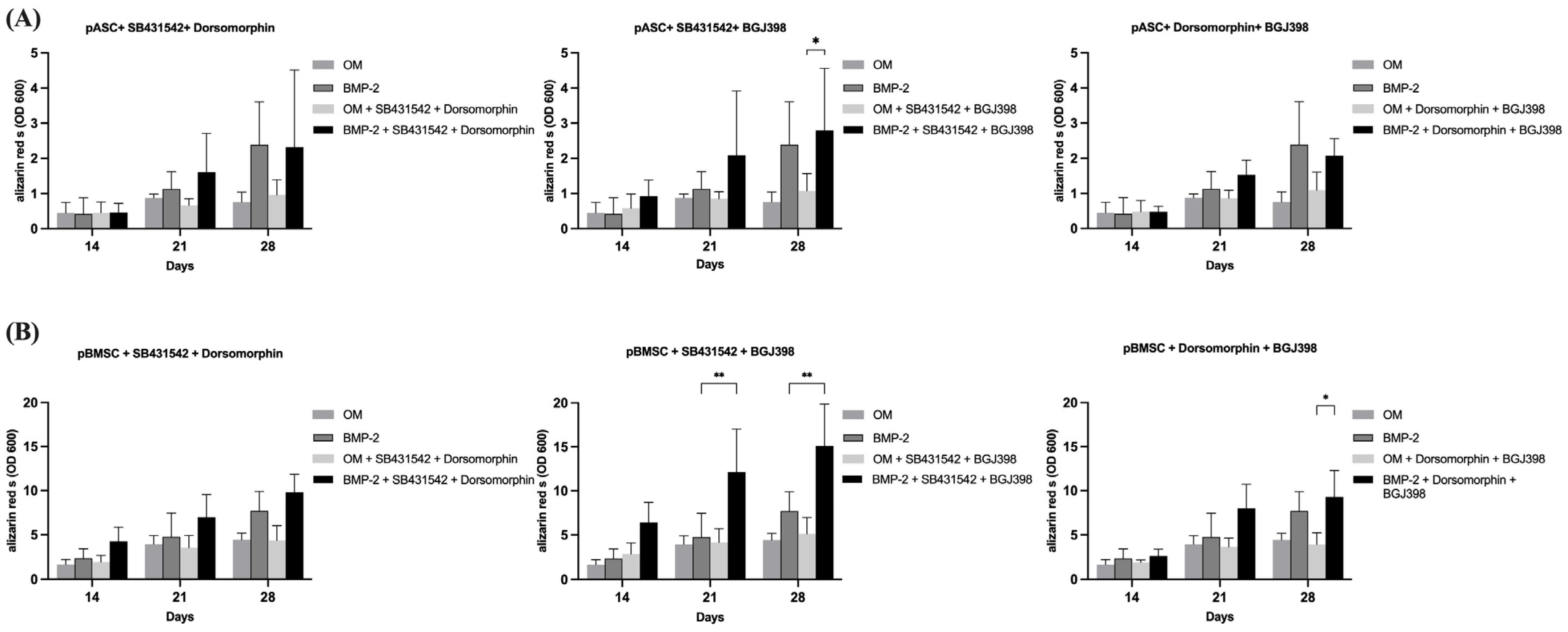
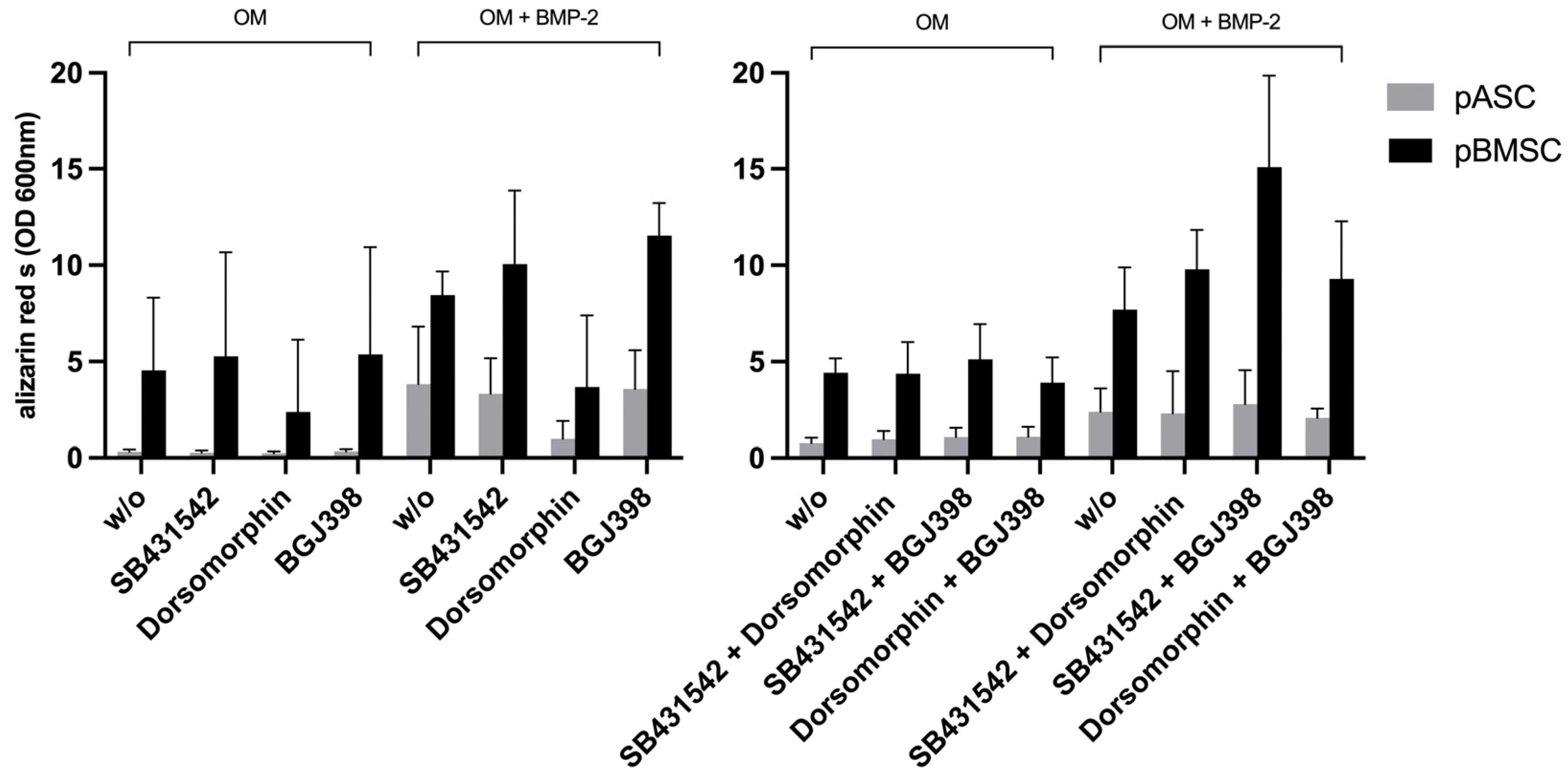
3.3.4. Impact of the Simultaneous Inhibition of TGF-β and BMP Signaling on Osteogenic Differentiation of pASCs and pBMSCs
3.3.5. Impact of Simultaneous Inhibition of TGF-β and FGF Signaling on Osteogenic Differentiation of pASCs and pBMSCs
3.3.6. Impact of Simultaneous Inhibition of FGF and BMP Signaling on Osteogenic Differentiation of pASCs and pBMSCs
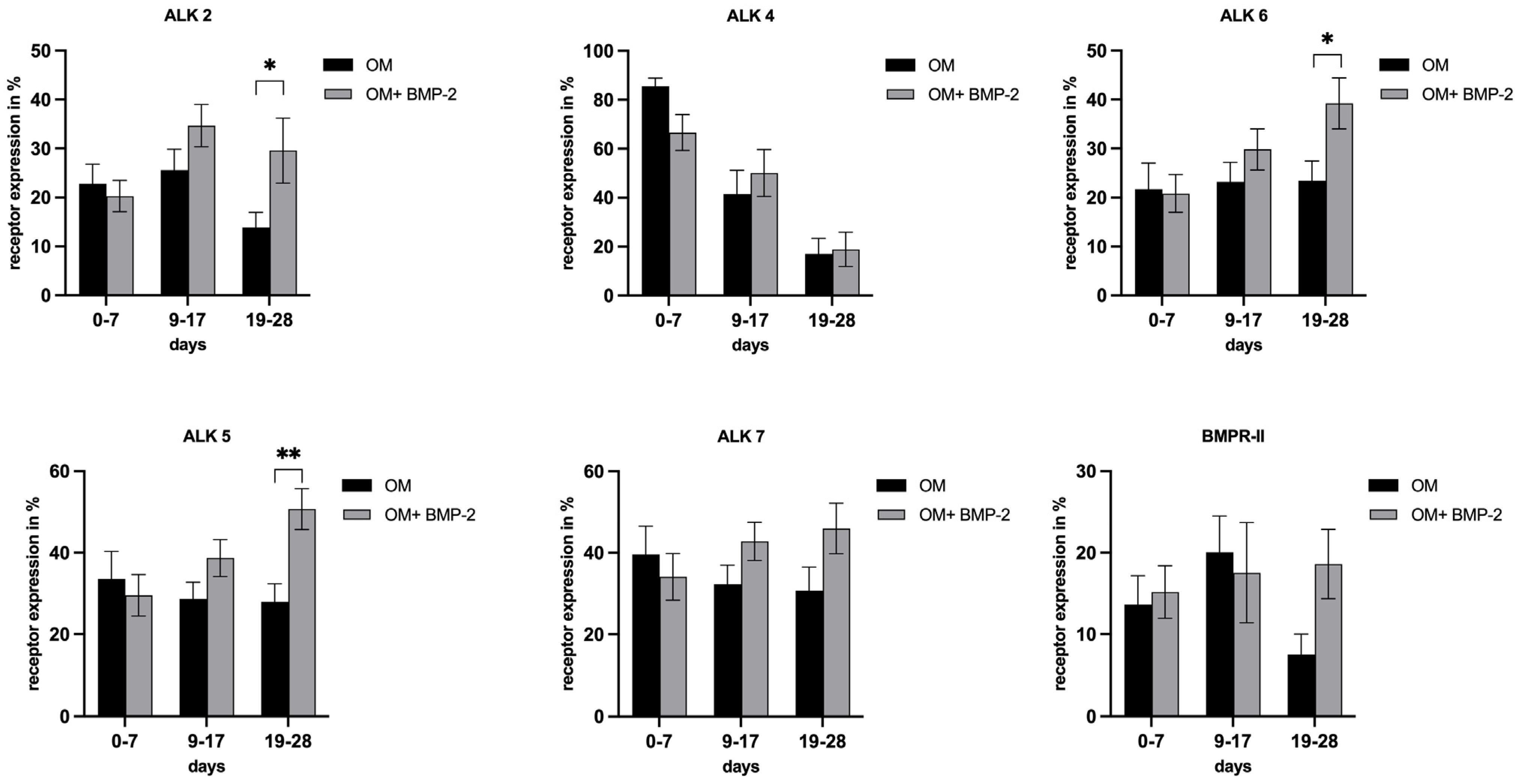
3.4. Evaluation of Receptor Expression in pASCs in the Course of Osteogenic Differentiation
3.5. Analysis of Key Protein Expressions of TGF-β, BMP, Wnt, MAPK, and FGFR Signaling in pASCs
4. Discussion
pp38, a Regulatory Signaling for BMP-2 Induced Osteogenic Differentiation in pASC?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| pBMSC | porcine bone marrow-derived stromal cell |
| pASC | porcine adipose-derived stromal cell |
| TGF | transforming growth factor |
| FGF | fibroblast growth factor |
| BMP | bone morphogenic protein |
| MAPK | mitogen-activated protein kinase |
| pp38 | phosphorylated p38 |
| ALK | anaplastic lymphoma kinase |
| OM | osteogenic medium |
| DMEM | Dulbecco’s Modified Eagle Medium |
| LANUV | Landesamt für Natur, Umwelt und Klima Norrhein-Westfalen |
References
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold Design for Bone Regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed]
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma 2018, 32, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar] [PubMed]
- Busch, A.; Wegner, A.; Haversath, M.; Jäger, M. Bone Substitutes in Orthopaedic Surgery: Current Status and Future Perspectives. Z. Für. Orthop. Unfallchirurgie 2021, 159, 304–313. [Google Scholar] [CrossRef]
- Mohiuddin, O.A.; Campbell, B.; Poche, J.N.; Ma, M.; Rogers, E.; Gaupp, D.; Harrison, M.A.A.; Bunnell, B.A.; Hayes, D.J.; Gimble, J.M. Decellularized Adipose Tissue Hydrogel Promotes Bone Regeneration in Critical-Sized Mouse Femoral Defect Model. Front. Bioeng. Biotechnol. 2019, 7, 211. [Google Scholar] [CrossRef]
- Jungbluth, P.; Wild, M.; Grassmann, J.; Ar, E.; Sager, M.; Herten, M.; Jäger, M.; Becker, J.; Windolf, J.; Hakimi, M. Platelet-rich plasma on calcium phosphate granules promotes metaphyseal bone healing in mini-pigs: Platelet-Rich Plasma on Calcium Phosphate Granules. J. Orthop. Res. 2010, 28, 1448–1455. [Google Scholar] [CrossRef]
- Clough, B.H.; McCarley, M.R.; Krause, U.; Zeitouni, S.; Froese, J.J.; McNeill, E.P.; Chaput, C.D.; Sampson, H.W.; A Gregory, C. Bone Regeneration With Osteogenically Enhanced Mesenchymal Stem Cells and Their Extracellular Matrix Proteins. J. Bone Miner. Res. 2015, 30, 83–94. [Google Scholar] [CrossRef]
- Gelinsky, M.; Dittrich, R.; Bernhardt, A.; Despang, F. Biomimetische Calciumphosphat-Biokeramiken mit parallel orientierten Kanalporen: Ein Knochenersatzmaterial mit Osteonen-artiger Mikrostruktur. In Proceedings of the Dtsch Kongr Für Orthop Unfallchirurgie 75 Jahrestag Dtsch Ges Für Unfallchirurgie, 97. Tagung der Deutschen Gesellschaft für Orthopädie und Orthopädische Chirurgie: 52. Tagung des Berufsverbandes der Fachärzte für Orthopädie, Berlin, Germany, 25–28 October 2011. [Google Scholar] [CrossRef]
- Rahman, G.; Frazier, T.P.; Gimble, J.M.; Mohiuddin, O.A. The Emerging Use of ASC/Scaffold Composites for the Regeneration of Osteochondral Defects. Front. Bioeng. Biotechnol. 2022, 10, 893992. [Google Scholar] [CrossRef]
- Robey, P.G. “Mesenchymal stem cells”: Fact or fiction, and implications in their therapeutic use. F1000Research 2017, 6, 524. [Google Scholar] [CrossRef]
- Jungbluth, P.; Spitzhorn, L.-S.; Grassmann, J.; Tanner, S.; Latz, D.; Rahman, S.; Bohndorf, M.; Wruck, W.; Sager, M.; Grotheer, V.; et al. Human iPSC-derived iMSCs improve bone regeneration in mini-pigs. Bone Res. 2019, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Unger, M.; Van Griensven, M.; Balmayor, E.R. Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine. Eur. J. Med. Res. 2017, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Kim, B.S.; Kim, J.D.; Choi, Y.C.; Lee, H.Y.; Cho, Y.W. In Vitro Cartilage Tissue Engineering Using Adipose-Derived Extracellular Matrix Scaffolds Seeded with Adipose-Derived Stem Cells. Tissue Eng. Part A 2012, 18, 80–92. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Chen, Y.; Yuan, L.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020, 2020, 1–26. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. [Google Scholar] [CrossRef] [PubMed]
- Vater, C.; Kasten, P.; Stiehler, M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011, 7, 463–477. [Google Scholar] [CrossRef]
- Shui, C.; Spelsberg, T.C.; Riggs, B.L.; Khosla, S. Changes in RUNX2/CBFA1 Expression and Activity During Osteoblastic Differentiation of Human Bone Marrow Stromal Cells. J. Bone Miner. Res. 2003, 18, 213–221. [Google Scholar] [CrossRef]
- Tada, H.; Nemoto, E.; Foster, B.L.; Somerman, M.J.; Shimauchi, H. Phosphate increases bone morphogenetic protein-2 expression through cAMP-dependent protein kinase and ERK1/2 pathways in human dental pulp cells. Bone 2011, 48, 1409–1416. [Google Scholar] [CrossRef]
- Noort, W.A.; Oerlemans, M.I.F.J.; Rozemuller, H.; Feyen, D.; Jaksani, S.; Stecher, D.; Naaijkens, B.; Martens, A.C.; Bühring, H.J.; Doevendans, P.A.; et al. Human versus porcine mesenchymal stromal cells: Phenotype, differentiation potential, immunomodulation and cardiac improvement after transplantation. J. Cell. Mol. Med. 2012, 16, 1827–1839. [Google Scholar] [CrossRef]
- Bayraktar, S.; Jungbluth, P.; Deenen, R.; Grassmann, J.; Schneppendahl, J.; Eschbach, D.; Scholz, A.; Windolf, J.; Suschek, C.V.; Grotheer, V. Molecular- and microarray-based analysis of diversity among resting and osteogenically induced porcine mesenchymal stromal cells of several tissue origin: Molecular- and microarray-based analysis of diversity among resting and osteogenically induced porcine MSCs of several tissue. J. Tissue Eng. Regen. Med. 2018, 12, 114–128. [Google Scholar] [CrossRef]
- Chang, Y.; Ping, A.; Chang, C.; Betz, V.M.; Cai, L.; Ren, B. Lactoferrin Mediates Enhanced Osteogenesis of Adipose-Derived Stem Cells: Innovative Molecular and Cellular Therapy for Bone Repair. Int. J. Mol. Sci. 2023, 24, 1749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, J.; Zhou, Y.; Wu, G. The Roles of Bone Morphogenetic Proteins and Their Signaling in the Osteogenesis of Adipose-Derived Stem Cells. Tissue Eng. Part B Rev. 2014, 20, 84–92. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP Signaling in Osteoblast Differentiation and Bone Formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Prudovsky, I. Cellular Mechanisms of FGF-Stimulated Tissue Repair. Cells 2021, 10, 1830. [Google Scholar] [CrossRef]
- Fakhry, A.; Ratisoontorn, C.; Vedhachalam, C.; Salhab, I.; Koyama, E.; Leboy, P.; Pacifici, M.; Kirschner, R.E.; Nah, H.-D. Effects of FGF-2/-9 in calvarial bone cell cultures: Differentiation stage-dependent mitogenic effect, inverse regulation of BMP-2 and noggin, and enhancement of osteogenic potential. Bone 2005, 36, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Charles, L.F.; Woodman, J.L.; Ueno, D.; Gronowicz, G.; Hurley, M.M.; Kuhn, L.T. Effects of low dose FGF-2 and BMP-2 on healing of calvarial defects in old mice. Exp. Gerontol. 2015, 64, 62–69. [Google Scholar] [CrossRef]
- Quarto, N.; Longaker, M.T. FGF-2 Inhibits Osteogenesis in Mouse Adipose Tissue-Derived Stromal Cells and Sustains their Proliferative and Osteogenic Potential State. Tissue Eng. 2006, 12, 1405–1418. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Ge, C. Control of the Osteoblast Lineage by Mitogen-Activated Protein Kinase Signaling. Curr. Mol. Biol. Rep. 2017, 3, 122–132. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, G.; Wu, X.; Xie, J.; Yang, X.; Li, T. Disruption of Wnt/β-catenin Signaling in Odontoblasts and Cementoblasts Arrests Tooth Root Development in Postnatal Mouse Teeth. Int. J. Biol. Sci. 2013, 9, 228–236. [Google Scholar] [CrossRef]
- Duan, P.; Bonewald, L.F. The role of the wnt/β-catenin signaling pathway in formation and maintenance of bone and teeth. Int. J. Biochem. Cell Biol. 2016, 77, 23–29. [Google Scholar] [CrossRef]
- Jeong, S.; An, B.; Kim, J.-H.; Han, H.-W.; Heo, H.-R.; Ha, K.-S.; Han, E.-T.; Park, W.S.; Hong, S.-H. BMP4 and perivascular cells promote hematopoietic differentiation of human pluripotent stem cells in a differentiation stage-specific manner. Exp. Mol. Med. 2020, 52, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Luong, L.N.; Ramaswamy, J.; Kohn, D.H. Effects of osteogenic growth factors on bone marrow stromal cell differentiation in a mineral-based delivery system. Biomaterials 2012, 33, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Grotheer, V.; Skrynecki, N.; Oezel, L.; Windolf, J.; Grassmann, J. Osteogenic differentiation of human mesenchymal stromal cells and fibroblasts differs depending on tissue origin and replicative senescence. Sci. Rep. 2021, 11, 11968. [Google Scholar] [CrossRef]
- Ho, D.M.; Whitman, M. TGF-β signaling is required for multiple processes during Xenopus tail regeneration. Dev. Biol. 2008, 315, 203–216. [Google Scholar] [CrossRef]
- Dahl, L.K. A Simple and Sensitive Histochemical Method for Calcium. Exp. Biol. Med. 1952, 80, 474–479. [Google Scholar] [CrossRef]
- Gregory, C.A.; Grady Gunn, W.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Laping, N.J.; Grygielko, E.; Mathur, A.; Butter, S.; Bomberger, J.; Tweed, C.; Martin, W.; Fornwald, J.; Lehr, R.; Harling, J.; et al. Inhibition of Transforming Growth Factor (TGF)-β1–Induced Extracellular Matrix with a Novel Inhibitor of the TGF-β Type I Receptor Kinase Activity: SB-431542. Mol. Pharmacol. 2002, 62, 58–64. [Google Scholar] [CrossRef]
- Pal, S.K.; Rosenberg, J.E.; Hoffman-Censits, J.H.; Berger, R.; Quinn, D.I.; Galsky, M.D.; Wolf, J.; Dittrich, C.; Keam, B.; Delord, J.-P.; et al. Efficacy of BGJ398, a Fibroblast Growth Factor Receptor 1–3 Inhibitor, in Patients with Previously Treated Advanced Urothelial Carcinoma with FGFR3 Alterations. Cancer Discov. 2018, 8, 812–821. [Google Scholar] [CrossRef]
- Carluccio, M.; Ziberi, S.; Zuccarini, M.; Giuliani, P.; Caciagli, F.; Di Iorio, P.; Ciccarelli, R. Adult mesenchymal stem cells: Is there a role for purine receptors in their osteogenic differentiation? Purinergic Signal. 2020, 16, 263–287. [Google Scholar] [CrossRef]
- Huang, Z.; Nelson, E.R.; Smith, R.L.; Goodman, S.B. The Sequential Expression Profiles of Growth Factors from Osteroprogenitors to Osteoblasts In Vitro. Tissue Eng. 2007, 13, 2311–2320. [Google Scholar] [CrossRef]
- Huang, Z.; Nelson, E.R.; Smith, R.L.; Goodman, S.B. Repair of Large Bone Defects with the Use of Autologous Bone Marrow Stromal Cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef]
- Stockmann, P.; Park, J.; von Wilmowsky, C.; Nkenke, E.; Felszeghy, E.; Dehner, J.-F.; Schmitt, C.; Tudor, C.; Schlegel, K.A. Guided bone regeneration in pig calvarial bone defects using autologous mesenchymal stem/progenitor cells—A comparison of different tissue sources. J. Cranio-Maxillofac. Surg. 2012, 40, 310–320. [Google Scholar] [CrossRef]
- Lendeckel, S.; Jödicke, A.; Christophis, P.; Heidinger, K.; Wolff, J.; Fraser, J.K.; Hedrick, M.H.; Berthold, L.; Howaldt, H.-P. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: Case report. J. Cranio-Maxillofac. Surg. 2004, 32, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.I.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, S.K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef]
- Martini, F.; Pellati, A.; Mazzoni, E.; Salati, S.; Caruso, G.; Contartese, D.; De Mattei, M. Bone Morphogenetic Protein-2 Signaling in the Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells Induced by Pulsed Electromagnetic Fields. Int. J. Mol. Sci. 2020, 21, 2104. [Google Scholar] [CrossRef]
- Kloen, P.; Lauzier, D.; Hamdy, R.C. Co-expression of BMPs and BMP-inhibitors in human fractures and non-unions. Bone 2012, 51, 59–68. [Google Scholar] [CrossRef]
- Rodríguez-Carballo, E.; Gámez, B.; Ventura, F. p38 MAPK Signaling in Osteoblast Differentiation. Front. Cell Dev. Biol. 2016, 4, 40. [Google Scholar] [CrossRef]
- Wan, M.; Li, C.; Zhen, G.; Jiao, K.; He, W.; Jia, X.; Wang, W.; Shi, C.; Xing, Q.; Chen, Y.; et al. Injury-Activated Transforming Growth Factor β Controls Mobilization of Mesenchymal Stem Cells for Tissue Remodeling. Stem Cells 2012, 30, 2498–2511. [Google Scholar] [CrossRef]
- Oshimori, N.; Fuchs, E. Paracrine TGF-β Signaling Counterbalances BMP-Mediated Repression in Hair Follicle Stem Cell Activation. Cell Stem Cell 2012, 10, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.; Chen, H.; Yao, S.; Lai, X.; Qiu, Y.; Cai, J.; Huang, Y.; Wei, X.; Guan, Y.; et al. Inhibition of TGFβ improves hematopoietic stem cell niche and ameliorates cancer-related anemia. Stem Cell Res. Ther. 2021, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Weng, T.; Zhang, J.; Wang, J.; Li, W.; Wan, H.; Lan, Y.; Cheng, X.; Hou, N.; Liu, H.; et al. Smad4 is required for maintaining normal murine postnatal bone homeostasis. J. Cell Sci. 2007, 120, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.C.; Yu, P.B. Applications of small molecule BMP inhibitors in physiology and disease. Cytokine Growth Factor Rev. 2009, 20, 409–418. [Google Scholar] [CrossRef]
- Konecny, G.E.; Kolarova, T.; O’Brien, N.A.; Winterhoff, B.; Yang, G.; Qi, J.; Qi, Z.; Venkatesan, N.; Ayala, R.; Luo, T.; et al. Activity of the Fibroblast Growth Factor Receptor Inhibitors Dovitinib (TKI258) and NVP-BGJ398 in Human Endometrial Cancer Cells. Mol. Cancer Ther. 2013, 12, 632–642. [Google Scholar] [CrossRef]
- Kuhn, L.T.; Ou, G.; Charles, L.; Hurley, M.M.; Rodner, C.M.; Gronowicz, G. Fibroblast Growth Factor-2 and Bone Morphogenetic Protein-2 Have a Synergistic Stimulatory Effect on Bone Formation in Cell Cultures from Elderly Mouse and Human Bone. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1170–1180. [Google Scholar] [CrossRef]
- Fujimura, K.; Bessho, K.; Okubo, Y.; Kusumoto, K.; Segami, N.; Iizuka, T. The effect of fibroblast growth factor-2 on the osteoinductive activity of recombinant human bone morphogenetic protein-2 in rat muscle. Arch. Oral Biol. 2002, 47, 577–584. [Google Scholar] [CrossRef]
- Holtzhausen, A.; Golzio, C.; How, T.; Lee, Y.; Schiemann, W.P.; Katsanis, N.; Blobe, G.C. Novel bone morphogenetic protein signaling through Smad2 and Smad3 to regulate cancer progression and development. FASEB J. 2014, 28, 1248–1267. [Google Scholar] [CrossRef]
- Wang, W.; Rigueur, D.; Lyons, K.M. TGFβ as a gatekeeper of BMP action in the developing growth plate. Bone 2020, 137, 115439. [Google Scholar] [CrossRef]
- Cho, T.; Gerstenfeld, L.C.; Einhorn, T.A. Differential Temporal Expression of Members of the Transforming Growth Factor β Superfamily During Murine Fracture Healing. J. Bone Miner. Res. 2002, 17, 513–520. [Google Scholar] [CrossRef]
- Ramoshebi, L.N.; Matsaba, T.N.; Teare, J.; Renton, L.; Patton, J.; Ripamonti, U. Tissue engineering: TGF-[beta] superfamily members and delivery systems in bone regeneration. Expert Rev. Mol. Med. 2002, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Wang, W.; Xu, J.; Zhao, D.; Dong, Q.; Li, L.; Yang, X.; Duan, X.; Liang, Y.; Xiao, Y.; et al. Fibroblast growth factor 2 inhibits bone morphogenetic protein 9-induced osteogenic differentiation of mesenchymal stem cells by repressing Smads signaling and subsequently reducing Smads dependent up-regulation of ALK1 and ALK2. Int. J. Biochem. Cell Biol. 2013, 45, 1639–1646. [Google Scholar] [CrossRef]
- Biver, E.; Soubrier, A.-S.; Thouverey, C.; Cortet, B.; Broux, O.; Caverzasio, J.; Hardouin, P. Fibroblast growth factor 2 inhibits up-regulation of bone morphogenic proteins and their receptors during osteoblastic differentiation of human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2012, 427, 737–742. [Google Scholar] [CrossRef]
- Quarto, N.; Wan, D.C.; Longaker, M.T. Molecular mechanisms of FGF-2 inhibitory activity in the osteogenic context of mouse adipose-derived stem cells (mASCs). Bone 2008, 42, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, M.; Boccafoschi, F.; Leigheb, M.; Cannas, M.F. Effect of different growth factors on human osteoblasts activities: A possible application in bone regeneration for tissue engineering. Biomol. Eng. 2007, 24, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Sudheer, S.; Bhushan, R.; Fauler, B.; Lehrach, H.; Adjaye, J. FGF Inhibition Directs BMP4-Mediated Differentiation of Human Embryonic Stem Cells to Syncytiotrophoblast. Stem Cells Dev. 2012, 21, 2987–3000. [Google Scholar] [CrossRef]
- Moustakas, A.; Heldin, C.H. From mono- to oligo-Smads: The heart of the matter in TGF-β signal transduction: Figure 1. Genes Dev. 2002, 16, 1867–1871. [Google Scholar] [CrossRef]
- Salazar, V.S.; Zarkadis, N.; Huang, L.; Watkins, M.; Kading, J.; Bonar, S.; Norris, J.; Mbalaviele, G.; Civitelli, R. Postnatal Ablation of Osteoblast Smad4 Enhances Proliferative Responses to Canonical Wnt Signaling via Interactions with β-catenin. J. Cell Sci. 2013, 126, 5598–5609. [Google Scholar] [CrossRef]
- Maeda, K.; Kobayashi, Y.; Koide, M.; Uehara, S.; Okamoto, M.; Ishihara, A.; Kayama, T.; Saito, M.; Marumo, K. The Regulation of Bone Metabolism and Disorders by Wnt Signaling. Int. J. Mol. Sci. 2019, 20, 5525. [Google Scholar] [CrossRef]
- Mbalaviele, G.; Sheikh, S.; Stains, J.P.; Salazar, V.S.; Cheng, S.-L.; Chen, D.; Civitelli, R. β-Catenin and BMP-2 synergize to promote osteoblast differentiation and new bone formation. J. Cell. Biochem. 2005, 94, 403–418. [Google Scholar] [CrossRef]
- Vlashi, R.; Zhang, X.; Wu, M.; Chen, G. Wnt signaling: Essential roles in osteoblast differentiation, bone metabolism and therapeutic implications for bone and skeletal disorders. Genes Dis. 2023, 10, 1291–1317. [Google Scholar] [CrossRef] [PubMed]
- Ikpegbu, E.; Basta, L.; Clements, D.N.; Fleming, R.; Vincent, T.L.; Buttle, D.J.; Pitsillides, A.A.; Staines, K.A.; Farquharson, C. FGF-2 promotes osteocyte differentiation through increased E11/podoplanin expression. J. Cell. Physiol. 2018, 233, 5334–5347. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Marie, P.J. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev. 2015, 29, 1463–1486. [Google Scholar] [CrossRef]
- Higuchi, C.; Myoui, A.; Hashimoto, N.; Kuriyama, K.; Yoshioka, K.; Yoshikawa, H.; Itoh, K. Continuous Inhibition of MAPK Signaling Promotes the Early Osteoblastic Differentiation and Mineralization of the Extracellular Matrix. J. Bone Miner. Res. 2002, 17, 1785–1794. [Google Scholar] [CrossRef]
- Elkhenany, H.; Amelse, L.; Caldwell, M.; Abdelwahed, R.; Dhar, M. Impact of the source and serial passaging of goat mesenchymal stem cells on osteogenic differentiation potential: Implications for bone tissue engineering. J. Anim. Sci. Biotechnol. 2016, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Gallea, S.; Lallemand, F.; Atfi, A.; Rawadi, G.; Ramez, V.; Spinella-Jaegle, S.; Kawai, S.; Faucheu, C.; Huet, L.; Baron, R.; et al. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone 2001, 28, 491–498. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Sun, Y.; Wang, B.; Xiong, Y.; Lin, W.; Wei, Q.; Wang, H.; He, W.; Li, G. Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 2017, 8, 275. [Google Scholar] [CrossRef]
- Rosina, M.; Langone, F.; Giuliani, G.; Perpetuini, A.C.; Reggio, A.; Calderone, A.; Fuoco, C.; Castagnoli, L.; Gargioli, C.; Cesareni, G. Osteogenic differentiation of skeletal muscle progenitor cells is activated by the DNA damage response. Sci. Rep. 2019, 9, 5447. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Shim, J.-H.; Zou, W.; Sitara, D.; Schweitzer, M.; Hu, D.; Lotinun, S.; Sano, Y.; Baron, R.; Park, J.M.; et al. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J. Clin. Investig. 2010, 120, 2457–2473. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taday, R.; Jungbluth, P.; Zensen, S.; Krakau, T.; Windolf, J.; Hoffmann, M.J.; Grotheer, V. BMP-2-Driven Osteogenesis: A Comparative Analysis of Porcine BMSCs and ASCs and the Role of TGF-β and FGF Signaling. Biology 2025, 14, 610. https://doi.org/10.3390/biology14060610
Taday R, Jungbluth P, Zensen S, Krakau T, Windolf J, Hoffmann MJ, Grotheer V. BMP-2-Driven Osteogenesis: A Comparative Analysis of Porcine BMSCs and ASCs and the Role of TGF-β and FGF Signaling. Biology. 2025; 14(6):610. https://doi.org/10.3390/biology14060610
Chicago/Turabian StyleTaday, Roman, Pascal Jungbluth, Sebastian Zensen, Thomas Krakau, Joachim Windolf, Michèle J. Hoffmann, and Vera Grotheer. 2025. "BMP-2-Driven Osteogenesis: A Comparative Analysis of Porcine BMSCs and ASCs and the Role of TGF-β and FGF Signaling" Biology 14, no. 6: 610. https://doi.org/10.3390/biology14060610
APA StyleTaday, R., Jungbluth, P., Zensen, S., Krakau, T., Windolf, J., Hoffmann, M. J., & Grotheer, V. (2025). BMP-2-Driven Osteogenesis: A Comparative Analysis of Porcine BMSCs and ASCs and the Role of TGF-β and FGF Signaling. Biology, 14(6), 610. https://doi.org/10.3390/biology14060610








