Integration of Transcriptomics and Metabolomics Reveals Mechanisms of High-Temperature Stress Tolerance in the Hepatopancreas of Penaeus monodon
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Shrimp and Rearing Conditions
2.2. High-Temperature Stress and Sample Collection
2.3. -Transcriptomic Analysis
2.3.1. RNA Extraction and Library Construction
2.3.2. Bioinformatics Analysis of the Transcriptome
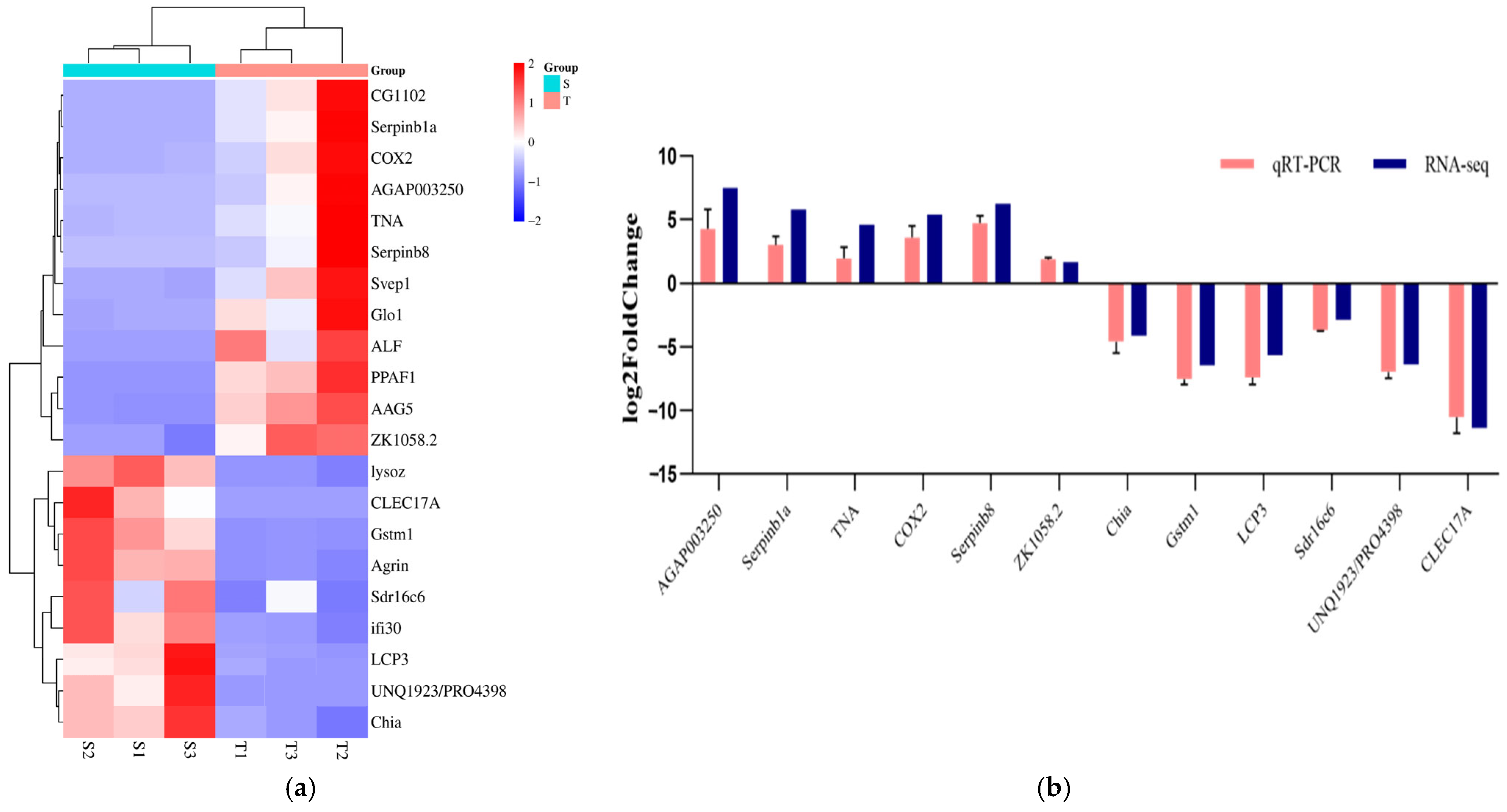
2.3.3. qRT-PCR Verification
2.4. Metabolomics Analysis
2.4.1. Metabolite Extraction and LC-MS Analysis
2.4.2. Bioinformatics Analysis of the Metabolome
2.5. Combined Analysis of Hepatopancreatic Transcriptome and Metabolome
3. Results
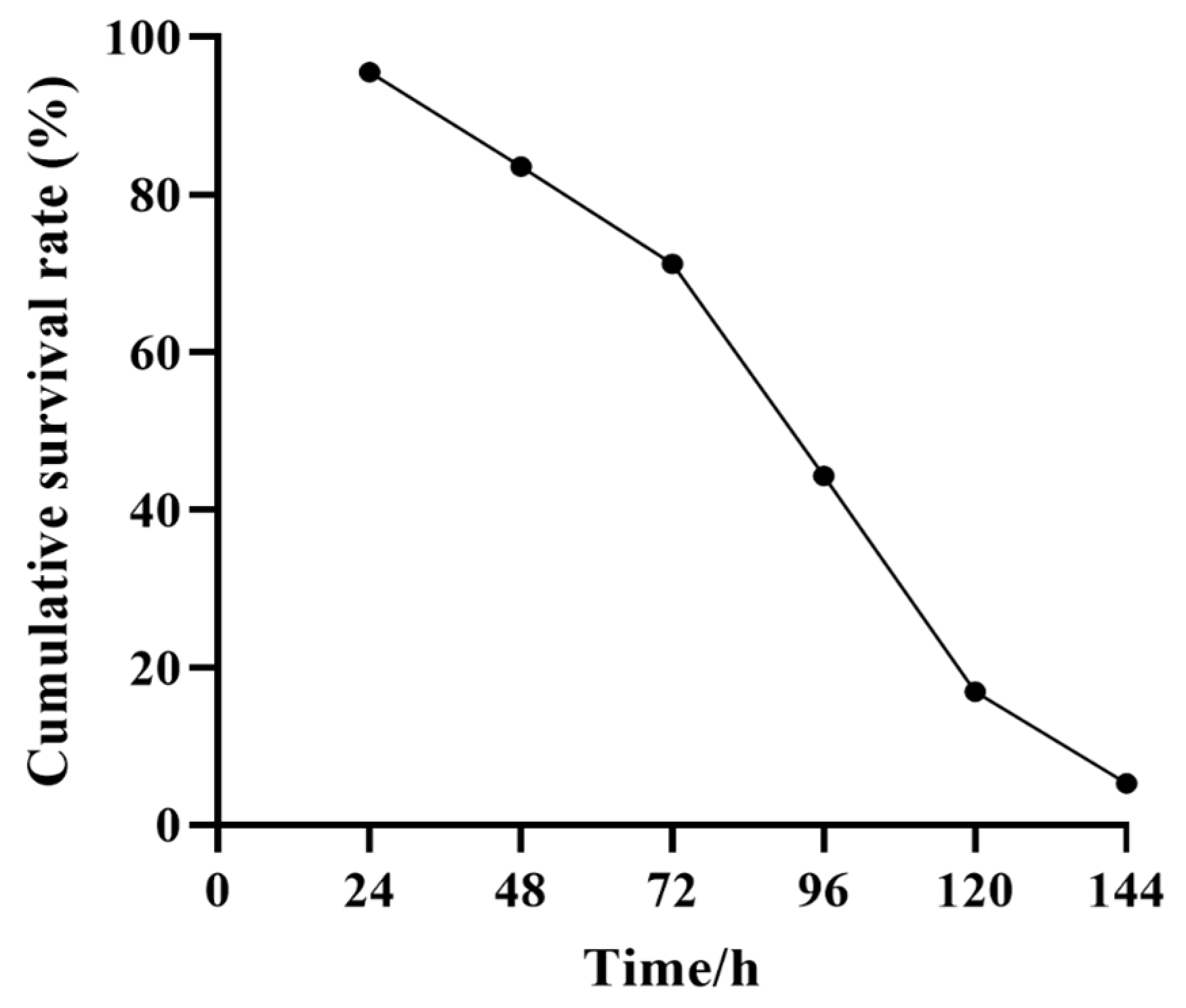
3.1. The Cumulative Survival Rate of P. Monodon
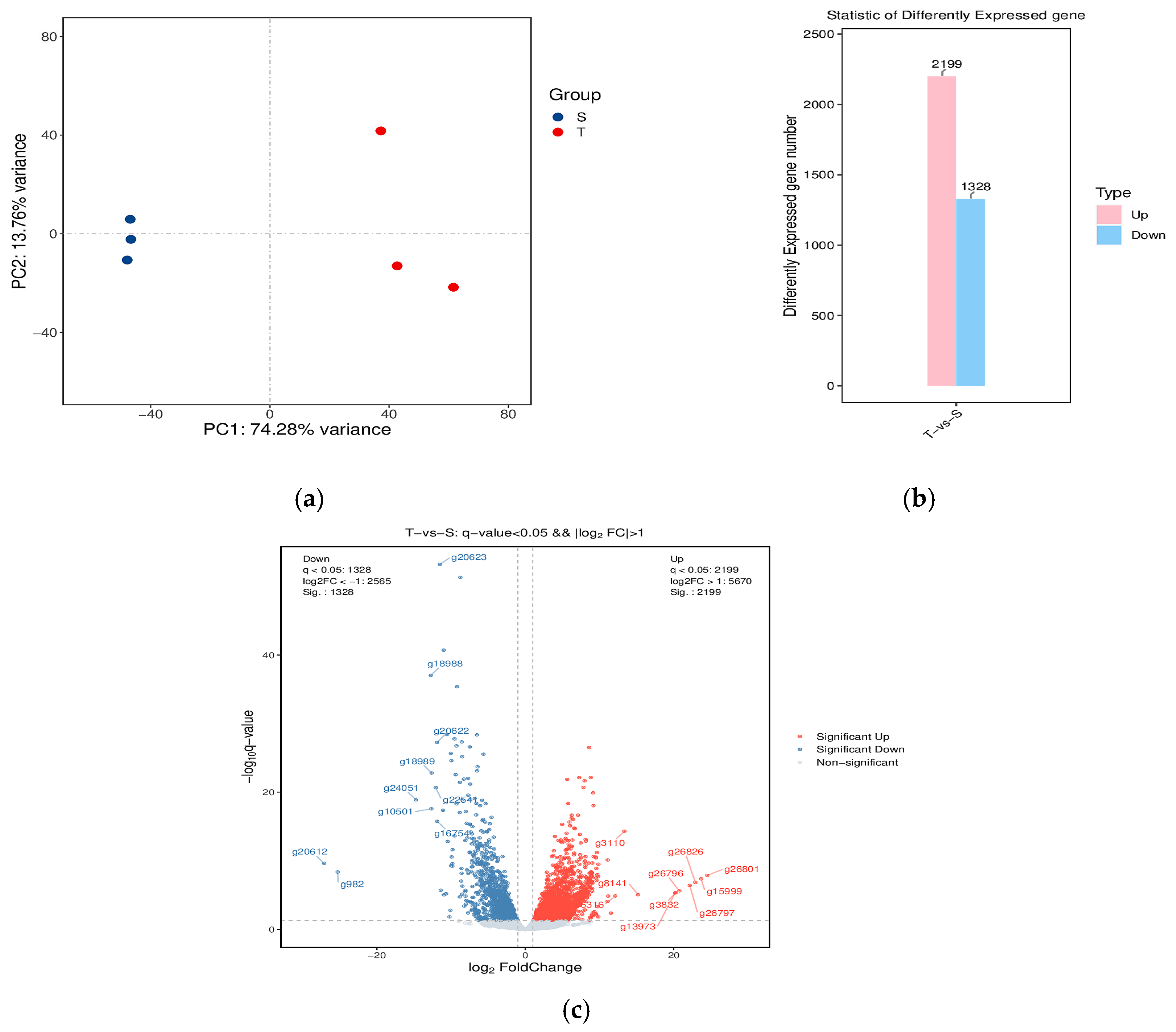
3.2. Hepatopancreas Transcriptomic Analysis
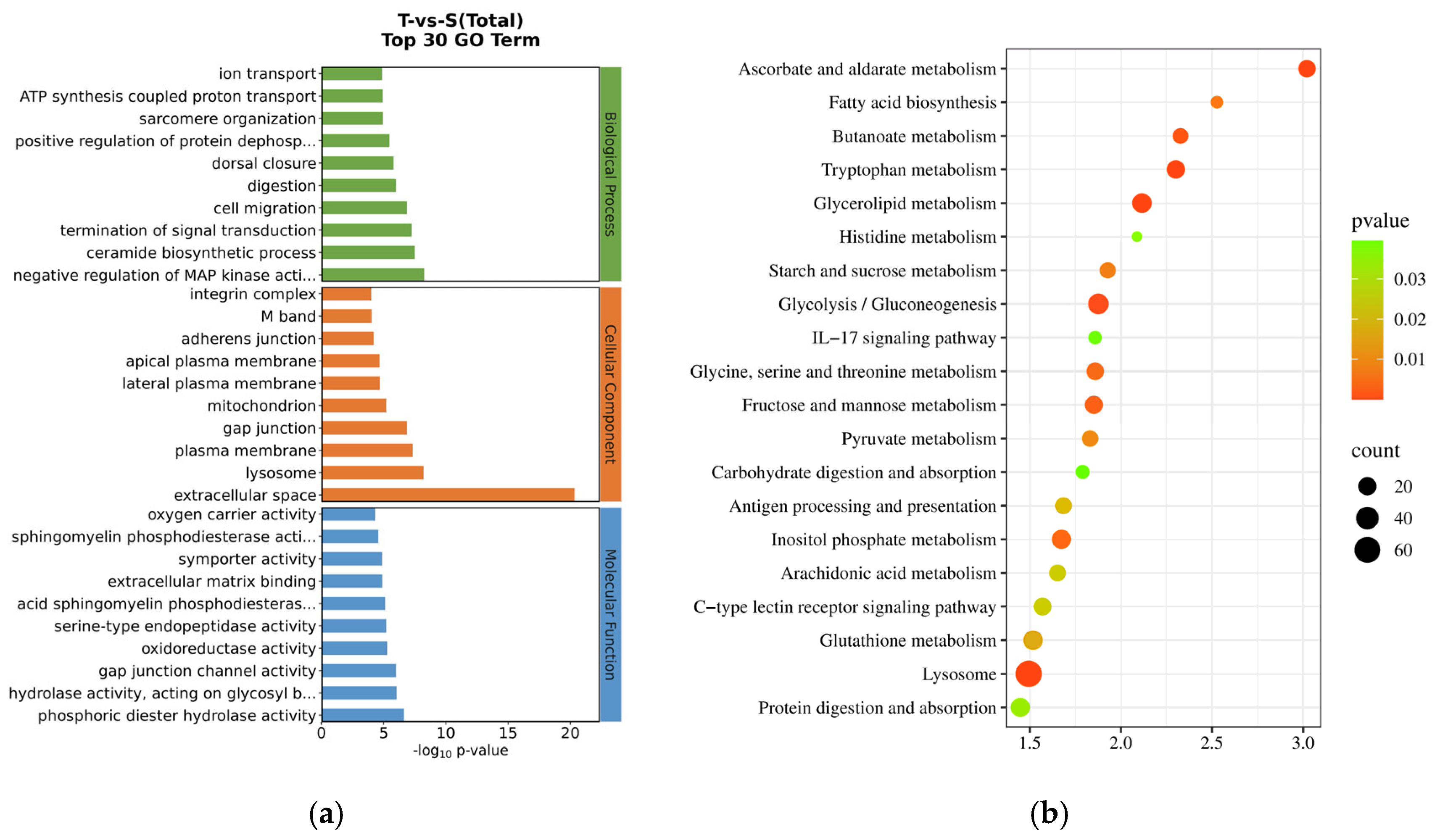
3.2.1. Identification and Functional Annotation of the DEGs
3.2.2. Analysis of Specific Genes for Immunity and Oxidative Stress
3.3. Hepatopancreas Metabolomics Analysis
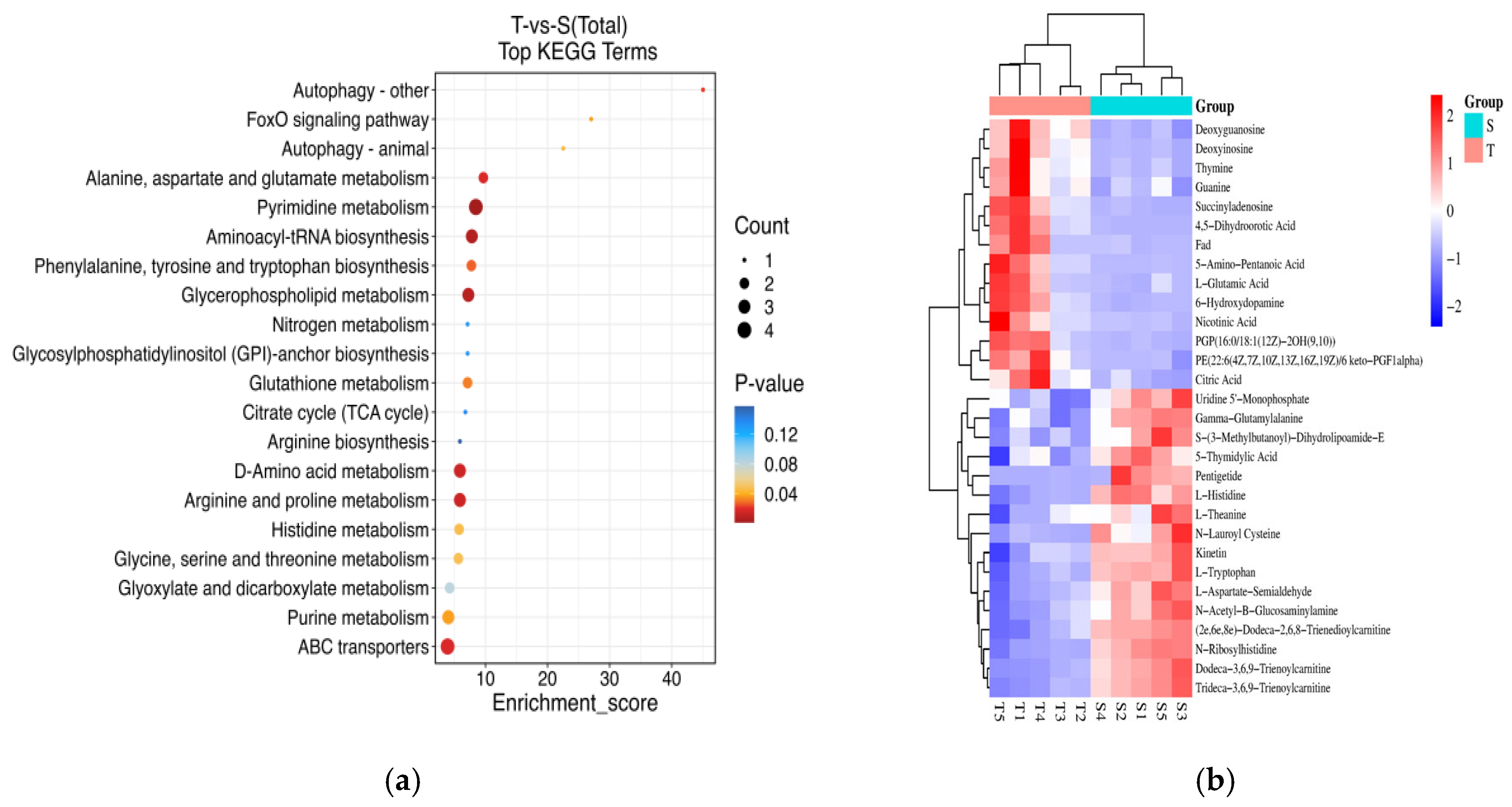
3.3.1. Identification and Functional Analysis of Differential Metabolites
3.3.2. Recognition of Metabolite Markers
3.4. Combined Transcriptome and Metabolome Analysis
4. Discussion
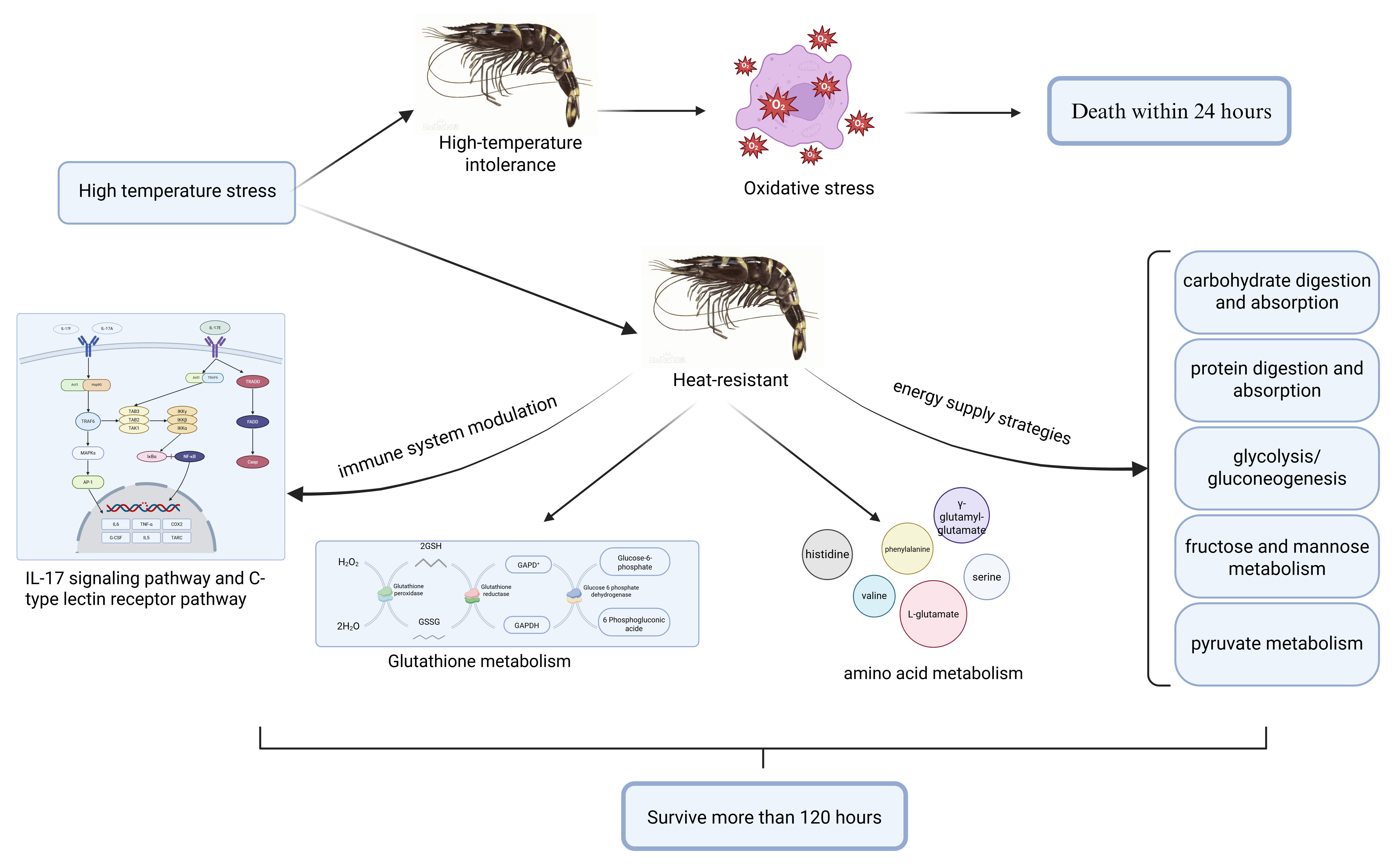
4.1. Energy Metabolism Regulation Is Necessary for the Black Tiger Shrimp to Adapt to High-Temperature Stress
4.2. The Regulation of Innate Immune System Is Necessary for the Black Tiger Shrimp to Adapt to High-Temperature Stress
4.3. Amino Acid Metabolism Is Necessary for the Black Tiger Shrimp to Adapt to High-Temperature Stress
4.4. Antioxidant Response and Detoxification Is Necessary for the Black Tiger Shrimp to Adapt to High-Temperature Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pörtner, H.O.; Knust, R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 2007, 315, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, M.; Lu, Z.; Liu, Y.; Li, B.; Gao, Z.; Shen, Z. High-temperature stress will put the thermo-sensitive teleost yellow catfish (Tachysurus fulvidraco) in danger through reducing reproductivity. Ecotoxicol. Environ. Saf. 2022, 239, 113638. [Google Scholar] [CrossRef]
- Huang, T.; Gu, W.; Liu, E.; Wang, B.; Wang, G.; Dong, F.; Guo, F.; Jiao, W.; Sun, Y.; Wang, X.; et al. miR-301b-5p and its target gene nfatc2ip regulate inflammatory responses in the liver of rainbow trout (Oncorhynchus mykiss) under high temperature stress. Ecotoxicol. Environ. Saf. 2022, 242, 113915. [Google Scholar] [CrossRef]
- Li, H.; Yu, H.; Zhang, X.; Huang, W.; Zhang, C.; Wang, C.; Gao, Q.; Dong, S. Temperature acclimation improves high temperature tolerance of rainbow trout (Oncorhynchus mykiss) by improving mitochondrial quality and inhibiting apoptosis in liver. Sci. Total Environ. 2024, 912, 169452. [Google Scholar] [CrossRef]
- Lim, M.Y.; Bernier, N.J. Intergenerational plasticity to cycling high temperature and hypoxia affects offspring stress responsiveness and tolerance in zebrafish. J. Exp. Biol. 2023, 226, jeb245583. [Google Scholar] [CrossRef]
- Lee, D.; Kim, K.; Park, J.; Lee, J.; Kim, J. High water temperature-mediated immune gene expression of olive flounder, Paralichthys olivaceus according to pre-stimulation at high temperatures. Environ. Toxicol. Pharmacol. 2023, 101, 104159. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, X.; Wang, Q.; Ma, A.; Zhao, T.; Qiao, X.; Li, M. Digital RNA-seq analysis of the cardiac transcriptome response to thermal stress in turbot Scophthalmus maximus. J. Therm. Biol. 2022, 104, 103141. [Google Scholar] [CrossRef]
- Xiong, D.; Duan, Y.; Xu, J.; Zhan, A.; Chen, C.; Zhang, J. Physiological responses in gills of Litopenaeus vannamei exposed to the combined stress of temperature and ammonia. J. South. Agric. 2020, 51, 2296–2303. [Google Scholar] [CrossRef]
- Zhu, M.; Yao, C. The impact of temperature on the oxygen metabolism and energy metabolism in the hepatopancreas of shrimp Litopenaeus vannamei. J. Fish. China 2015, 39, 669–678. [Google Scholar] [CrossRef]
- Zhu, X.; Zheng, X.; Xing, Y.; Huang, J.; Dong, H.; Zhang, J. Study on tributyrin enhancing anti periodic high temperature stress ability of gill tissue in Litopenaeus vannamei. South China Fish. Sci. 2024, 20, 66–75. [Google Scholar] [CrossRef]
- Earhart, M.L.; Blanchard, T.S.; Harman, A.A.; Schulte, P.M. Hypoxia and High Temperature as Interacting Stressors: Will Plasticity Promote Resilience of Fishes in a Changing World? Biol. Bull. 2022, 243, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Heise, K.; Puntarulo, S.; Nikinmaa, M.; Abele, D.; Pörtner, H. Oxidative stress during stressful heat exposure and recovery in the North Sea eelpout Zoarces viviparus L. J. Exp. Biol. 2006, 209, 353–363. [Google Scholar] [CrossRef]
- González-Ruiz, R.; Leyva-Carrillo, L.; Peregrino-Uriarte, A.B.; Yepiz-Plascencia, G. The combination of hypoxia and high temperature affects heat shock, anaerobic metabolism, and pentose phosphate pathway key components responses in the white shrimp (Litopenaeus vannamei). Cell Stress Chaperon 2023, 28, 493–509. [Google Scholar] [CrossRef]
- Ndong, D.; Chen, Y.; Lin, Y.; Vaseeharan, B.; Chen, J. The immune response of tilapia Oreochromis mossambicus and its susceptibility to Streptococcus iniae under stress in low and high temperatures. Fish Shellfish Immun. 2007, 22, 686–694. [Google Scholar] [CrossRef]
- Huang, D.; Ren, M.; Liang, H.; Ge, X.; Xu, H.; Wu, L. Transcriptome analysis of the effect of high-temperature on nutrient metabolism in juvenile grass carp (Ctenopharyngodon idellus). Gene 2022, 809, 146035. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, J.; Zhang, X.; Yu, Y.; Li, F. Comparative transcriptomic analysis unveils a network of energy reallocation in Litopenaeus vannamei responsive to heat-stress. Ecotoxicol. Environ. Saf. 2022, 238, 113600. [Google Scholar] [CrossRef]
- Luo, L.; Huang, J.; Liu, D.; Jiang, S.; Zhou, F.; Jiang, S.; Yang, Q.; Li, Y.; Li, T.; Tan, L.; et al. Comparative transcriptome analysis of differentially expressed genes and pathways in Procambarus clarkii (Louisiana crawfish) at different acute temperature stress. Genomics 2022, 114, 110415. [Google Scholar] [CrossRef]
- Viant, M.R. Recent developments in environmental metabolomics. Mol. Biosyst. 2008, 4, 980–986. [Google Scholar] [CrossRef]
- Fang, M.; Lei, Z.; Ruilin, M.; Jing, W.; Leqiang, D. High temperature stress induced oxidative stress, gut inflammation and disordered metabolome and microbiome in tsinling lenok trout. Ecotoxicol. Environ. Saf. 2023, 266, 115607. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Quan, J.; Lu, J.; Zhao, G.; Sun, J. Metabonomics analysis reveals the protective effect of nano-selenium against heat stress of rainbow trout (Oncorhynchus mykiss). J. Proteom. 2022, 259, 104545. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Lai, J.; Liu, Y.; Song, M.; Li, F.; Gong, Q. Integrated biochemical, transcriptomic and metabolomic analyses provide insight into heat stress response in Yangtze sturgeon (Acipenser dabryanus). Ecotoxicol. Environ. Saf. 2023, 249, 114366. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Ma, A.; Yang, S.; Huang, Z. Integrated metabolome and transcriptome analyses revealing the effects of thermal stress on lipid metabolism in juvenile turbot Scophthalmus maximus. J. Therm. Biol. 2021, 99, 102937. [Google Scholar] [CrossRef]
- Huang, J.H. Penaeus Monodon Breeding and Culture Technology; South China University of Technology Press: Guangzhou, China, 2018. [Google Scholar]
- Qin, Y.; Jiang, S.; Huang, J.; Zhou, F.; Yang, Q.; Jiang, S.; Yang, L. C-type lectin response to bacterial infection and ammonia nitrogen stress in tiger shrimp (Penaeus monodon). Fish Shellfish Immun. 2019, 90, 188–198. [Google Scholar] [CrossRef]
- Jiravanichpaisal, P.; Söderhäll, K.; Söderhäll, I. Effect of water temperature on the immune response and infectivity pattern of white spot syndrome virus (WSSV) in freshwater crayfish. Fish Shellfish Immun. 2004, 17, 265–275. [Google Scholar] [CrossRef]
- Magouz, F.I.; Moustafa, E.M.; Abo-Remela, E.M.; Halawa, M.R.; Barakaat, P.M.; Omar, A.A. Summer mortality syndrome bacterial pathogens in farmed Nile tilapia (Oreochromis niloticus). Open Vet. J. 2024, 14, 53–69. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Chen, F.; Qi, H.; Chen, L.; Sung, Y.Y.; Huang, Y.; Lv, A.; Hu, X. Phenotypic and genomic characterization of a Vibrio parahaemolyticus strain causing disease in Penaeus vannamei provides insights into its niche adaptation and pathogenic mechanism. Microb. Genom. 2021, 7, 000549. [Google Scholar] [CrossRef]
- Nandy, T.; Baag, S.; Mandal, S. Impact of elevated temperature on physiological energetics of Penaeus monodon post larvae: A mesocosm study. J. Therm. Biol. 2021, 97, 102829. [Google Scholar] [CrossRef]
- Fan, R.; Li, Y.; Jiang, S.; Jiang, S.; Yang, Q.; Yang, L.; Huang, J.; Zhou, F. cDNA cloning and expression analysis of glutaredoxin 3 in black tiger shrimp Penaeus monodon. Aquac. Int. 2021, 29, 2661–2679. [Google Scholar] [CrossRef]
- Liu, B.; Xie, J.; Ge, X.; Xu, P.; Wang, A.; He, Y.; Zhou, Q.; Pan, L.; Chen, R. Effects of anthraquinone extract from Rheum officinale Bail on the growth performance and physiological responses of Macrobrachium rosenbergii under high temperature stress. Fish Shellfish Immunol. 2010, 29, 49–57. [Google Scholar] [CrossRef]
- Madeira, C.; Leal, M.C.; Diniz, M.S.; Cabral, H.N.; Vinagre, C. Thermal stress and energy metabolism in two circumtropical decapod crustaceans: Responses to acute temperature events. Mar. Environ. Res. 2018, 141, 148–158. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Zhang, H.; Ji, Q.; Song, L.; Qiu, L.; Zhou, Z.; Wang, M.; Wang, L. Immune response and energy metabolism of Chlamys farreri under Vibrio anguillarum challenge and high temperature exposure. Fish Shellfish Immun. 2012, 33, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, L.; Meng, J.; Qi, H.; Qu, T.; Xu, F.; Zhang, L. Molecular Basis for Adaptation of Oysters to Stressful Marine Intertidal Environments. Annu. Rev. Anim. Biosci. 2016, 4, 357–381. [Google Scholar] [CrossRef]
- Liu, H.; Pan, L.; Shen, J.; Tan, B.; Dong, X.; Yang, Q.; Chi, S.; Zhang, S. Effects of Carbohydrase Supplementation on Growth Performance, Intestinal Digestive Enzymes and Flora, Glucose Metabolism Enzymes, and glut2 Gene Expression of Hybrid Grouper (Epinephelus fuscoguttatus♀ × E. lanceolatus♂) Fed Different CHO/L Ratio Diets. Metabolites 2023, 13, 98. [Google Scholar] [CrossRef]
- Blanco, A.M.; Bertucci, J.I.; Sánchez-Bretaño, A.; Delgado, M.J.; Valenciano, A.I.; Unniappan, S. Ghrelin modulates gene and protein expression of digestive enzymes in the intestine and hepatopancreas of goldfish (Carassius auratus) via the GHS-R1a: Possible roles of PLC/PKC and AC/PKA intracellular signaling pathways. Mol. Cell. Endocrinol. 2017, 442, 165–181. [Google Scholar] [CrossRef]
- Andersen, S.M.; Waagbø, R.; Espe, M. Functional amino acids in fish nutrition, health and welfare. Front. Biosci. 2016, 8, 143–169. [Google Scholar]
- Jiang, J.; Xu, J.; Ye, L.; Sun, M.; Jiang, Z.; Mao, M. Identification of differentially expressed genes in gills of tiger puffer (Takifugu rubripes) in response to low-salinity stress. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 243–244, 110437. [Google Scholar] [CrossRef]
- Liu, J.; Jin, P.; Li, M.; Yi, X.; Tian, Y.; Zhang, Z.; Liu, J.; Shi, L. The energy metabolism of the freshwater leech Whitmania pigra in response to fasting. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2024, 274, 110999. [Google Scholar] [CrossRef]
- Li, Y.; Pan, L.; Yu, J. The injection of one recombinant C-type lectin (LvLec) induced the immune response of hemocytes in Litopenaeus vannamei. Fish Shellfish Immun. 2022, 124, 324–331. [Google Scholar] [CrossRef]
- Sun, J.; Lan, J.; Zhao, X.; Vasta, G.R.; Wang, J. Binding of a C-type lectin’s coiled-coil domain to the Domeless receptor directly activates the JAK/STAT pathway in the shrimp immune response to bacterial infection. PLoS Pathog. 2017, 13, e1006626. [Google Scholar] [CrossRef]
- Wang, X.; Vasta, G.R.; Wang, J. The functional relevance of shrimp C-type lectins in host-pathogen interactions. Dev. Comp. Immunol. 2020, 109, 103708. [Google Scholar] [CrossRef]
- Mcgeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Amatya, N.; Garg, A.V.; Gaffen, S.L. IL-17 Signaling: The Yin and the Yang. Trends Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Zheng, J.; Zuo, H.; Li, C.; Niu, S.; Yang, L.; Yan, M.; Weng, S.; He, J.; Xu, X. Identification and characterization of an interleukin-16-like gene from pacific white shrimp Litopenaeus vannamei. Dev. Comp. Immunol. 2017, 74, 49–59. [Google Scholar] [CrossRef]
- Chang, S.H.; Dong, C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell. Signal. 2011, 23, 1069–1075. [Google Scholar] [CrossRef]
- Kumar, V.; Sahu, N.P.; Pal, A.K.; Kumar, S.; Sinha, A.K.; Ranjan, J.; Baruah, K. Modulation of key enzymes of glycolysis, gluconeogenesis, amino acid catabolism, and TCA cycle of the tropical freshwater fish Labeo rohita fed gelatinized and non-gelatinized starch diet. Fish Physiol. Biochem. 2010, 36, 491–499. [Google Scholar] [CrossRef]
- Xu, Z.; Cao, J.; Qin, X.; Qiu, W.; Mei, J.; Xie, J. Toxic Effects on Bioaccumulation, Hematological Parameters, Oxidative Stress, Immune Responses and Tissue Structure in Fish Exposed to Ammonia Nitrogen: A Review. Animals 2021, 11, 3304. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Ye, S.; Bureau, D.P.; Liu, H.; Yin, J.; Mou, Z.; Lin, H.; Hao, F. Global metabolic responses of the lenok (Brachymystax lenok) to thermal stress. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 29, 308–319. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Wang, L.; Ahn, Y.J.; Asmis, R. Sexual dimorphism in glutathione metabolism and glutathione-dependent responses. Redox Biol. 2020, 31, 101410. [Google Scholar] [CrossRef]
- Calabrese, G.; Morgan, B.; Riemer, J. Mitochondrial Glutathione: Regulation and Functions. Antioxid. Redox Sign. 2017, 27, 1162–1177. [Google Scholar] [CrossRef]
- Eroglu, A.; Dogan, Z.; Kanak, E.G.; Atli, G.; Canli, M. Effects of heavy metals (Cd, Cu, Cr, Pb, Zn) on fish glutathione metabolism. Environ. Sci. Pollut. Res. Int. 2015, 22, 3229–3237. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, A.; Ma, S.; Lee, W.; Lee, S.; Yoon, D.; Kim, D.; Kim, S. Glutathione Injection Alleviates the Fluctuation of Metabolic Response under Thermal Stress in Olive Flounder, Paralichthys olivaceus. Metabolites 2019, 10, 3. [Google Scholar] [CrossRef]
- Mahanty, A.; Purohit, G.K.; Banerjee, S.; Karunakaran, D.; Mohanty, S.; Mohanty, B.P. Proteomic changes in the liver of Channa striatus in response to high temperature stress. Electrophoresis 2016, 37, 1704–1717. [Google Scholar] [CrossRef]
- Schleger, I.C.; Pereira, D.M.C.; Resende, A.C.; Romão, S.; Herrerias, T.; Neundorf, A.K.A.; de Souza, M.R.D.P.; Donatti, L. Metabolic responses in the gills of Yellowtail Lambari Astyanax lacustris under low- and high-temperature thermal stress. J. Aquat. Anim. Health 2024, 36, 16–31. [Google Scholar] [CrossRef]


| Pathway/Gene Symbol | Gene All Name | log2FoldChange | p-Value | Up/Down |
|---|---|---|---|---|
| Glutathione metabolism | ||||
| GLCL | glutamate-cysteine ligase catalytic subunit-like | 1.264 | 0.00276 | UP |
| Ggct | gamma-glutamylcyclotransferase-like | 2.413 | 0.00943 | UP |
| GST1 | glutathione S-transferase 1-like | −5.980 | 1.63 × 10−8 | DOWN |
| ICDH | Isocitrate dehydrogenase (NADP) | −1.652 | 0.00183 | DOWN |
| Rrm1 | ribonucleoside-diphosphate reductase large subunit-like | −7.808 | 3.03 × 10−16 | DOWN |
| Lysosome | ||||
| MLA1 | CD63 antigen-like | 4.658 | 1.98 × 10−11 | UP |
| CTSC | cathepsin C | 4.561 | 3.72 × 10−5 | UP |
| NPC1 | NPC intracellular cholesterol transporter 1-like | −4.530 | 0.00011 | DOWN |
| CTSD | lysosomal aspartic protease-like | −4.764 | 6.26 × 10−10 | DOWN |
| GLB1 | beta-galactosidase-like | −4.198 | 1.22 × 10−13 | DOWN |
| Glycolysis/Gluconeogenesis | ||||
| CG10160 | L-lactate dehydrogenase-like isoform X1 | 3.236 | 9.52 × 10−6 | UP |
| TEgg056i02.1 | aldoketoreductase-like protein | 5.122 | 0.00208 | UP |
| G6pt | glucose-6-phosphatase-like | −6.920 | 1.24 × 10−15 | DOWN |
| ALDH3 | aldehyde dehydrogenase, dimeric NADP-preferring-like | −1.712 | 0.00442 | DOWN |
| Aldhx | aldehyde dehydrogenase X, mitochondrial-like isoform X2 | −4.764 | 3.61 × 10−10 | DOWN |
| Glycine, serine, and threonine metabolism | ||||
| Tdh | L-threonine 3-dehydrogenase, mitochondrial-like | 5.340 | 0.00113 | UP |
| LPIPOX | peroxisomal sarcosine oxidase-like | 2.781 | 0.00026 | UP |
| bhmt | betaine--homocysteine S-methyltransferase 1-like | −6.325 | 2.47 × 10−10 | DOWN |
| DAO | D-amino-acid oxidase-like | −2.561 | 3.36 × 10−8 | DOWN |
| AGT1 | serine--pyruvate aminotransferase-like isoform X1 | −2.175 | 7.15 × 10−6 | DOWN |
| C-type lectin receptor signaling pathway | ||||
| COX2 | cyclooxygenase | 5.416 | 3.55 × 10−8 | UP |
| CG12559 | ERK | 4.871 | 0.01191 | UP |
| FYN | tyrosine-protein kinase SRK3-like | 5.898 | 0.00017 | UP |
| DDB_G0277917 | putative calmodulin-like | −1.112 | 0.00971 | DOWN |
| MLT | putative mucosa-associated lymphoid tissue lymphoma translocation protein 1-like | −1.346 | 0.01094 | DOWN |
| IL-17 signaling pathway | ||||
| CG11992 | NF-κB transcription factor Relish | 1.748 | 0.00083 | UP |
| mapk15 | putative mitogen-activated protein kinase 15-like | 6.033 | 0.00114 | UP |
| Traf6 | tumor necrosis factor receptor-associated factor 6 | 2.107 | 0.00236 | UP |
| CG5680 | c-jun N-terminal kinase (JNK) | 2.065 | 0.00745 | UP |
| T05E11.3 | Endoplasmin (Hsp90b1) | −1.408 | 0.00224 | DOWN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhao, P.; Yang, L.; Li, Y.; Huang, Z.; Yang, Q.; Yang, Y.; Shi, J.; Chen, Y.; Huang, J. Integration of Transcriptomics and Metabolomics Reveals Mechanisms of High-Temperature Stress Tolerance in the Hepatopancreas of Penaeus monodon. Biology 2025, 14, 591. https://doi.org/10.3390/biology14060591
Liu L, Zhao P, Yang L, Li Y, Huang Z, Yang Q, Yang Y, Shi J, Chen Y, Huang J. Integration of Transcriptomics and Metabolomics Reveals Mechanisms of High-Temperature Stress Tolerance in the Hepatopancreas of Penaeus monodon. Biology. 2025; 14(6):591. https://doi.org/10.3390/biology14060591
Chicago/Turabian StyleLiu, Li, Peng Zhao, Lishi Yang, Yundong Li, Zhong Huang, Qibin Yang, Yukai Yang, Jianzhi Shi, Yibiao Chen, and Jianhua Huang. 2025. "Integration of Transcriptomics and Metabolomics Reveals Mechanisms of High-Temperature Stress Tolerance in the Hepatopancreas of Penaeus monodon" Biology 14, no. 6: 591. https://doi.org/10.3390/biology14060591
APA StyleLiu, L., Zhao, P., Yang, L., Li, Y., Huang, Z., Yang, Q., Yang, Y., Shi, J., Chen, Y., & Huang, J. (2025). Integration of Transcriptomics and Metabolomics Reveals Mechanisms of High-Temperature Stress Tolerance in the Hepatopancreas of Penaeus monodon. Biology, 14(6), 591. https://doi.org/10.3390/biology14060591






