MaxEnt Modeling of the Impacts of Human Activities and Climate Change on the Potential Distribution of Plantago in China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition and Processing of Plantago
2.2. Environmental Data Sources
2.3. Preprocessing of Environmental Factors
2.4. Model Accuracy Test
2.5. Changes in Spatial Patterns and Center-of-Distribution Analyses in Species Habitat Areas
3. Results
3.1. Accuracy and Evaluation of the Model
3.2. Dominant Environmental Factors Affecting Distribution
3.3. Potential Habitat Areas of the Genus Plantago Under Current and Future Climate Scenarios
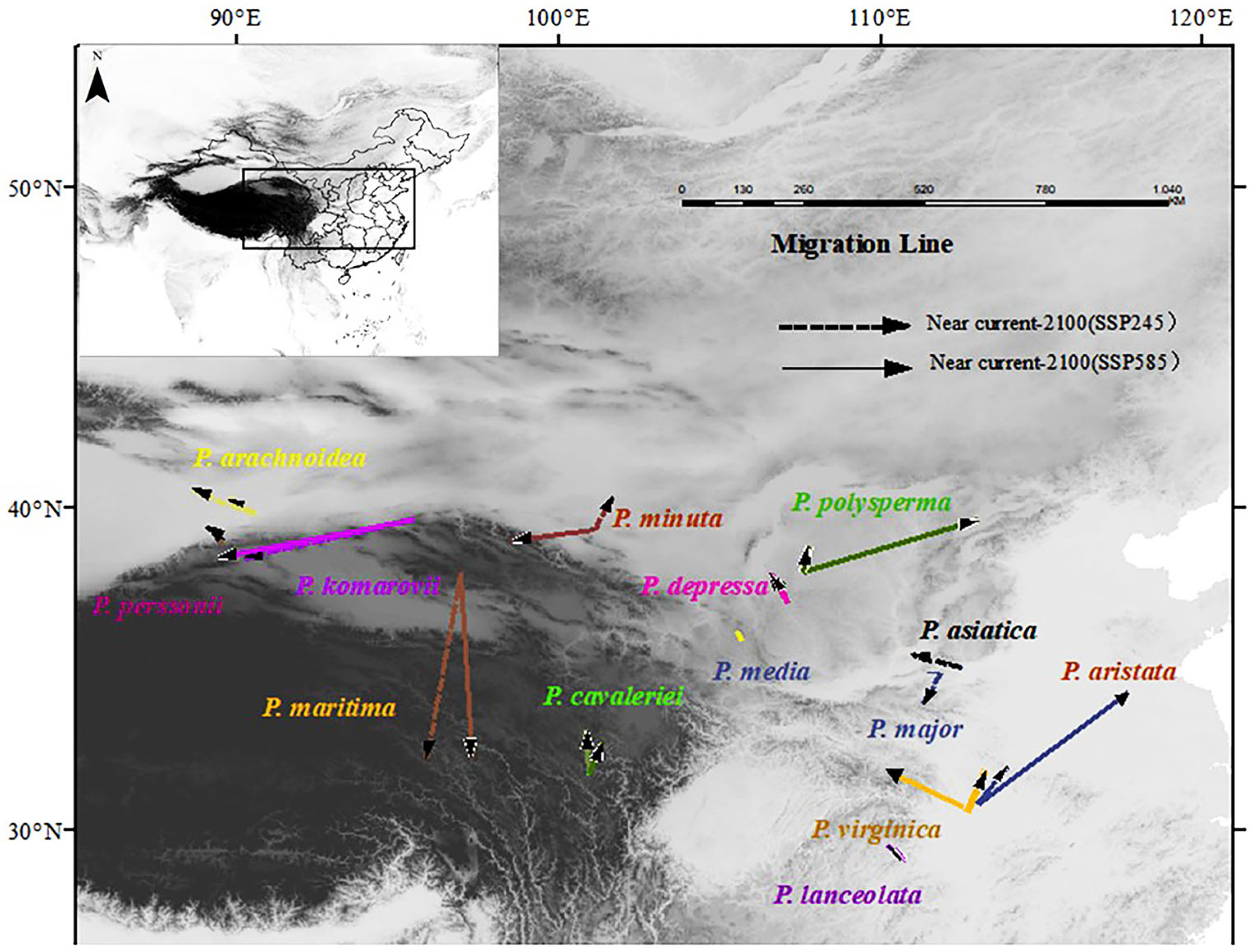
3.4. Changes in the Spatial Distribution of the Genus Under Different Climate Scenarios
3.5. Changes in the Distribution Centroid in the Potential Distribution Area of the Genus Plantago Under Climate Scenarios
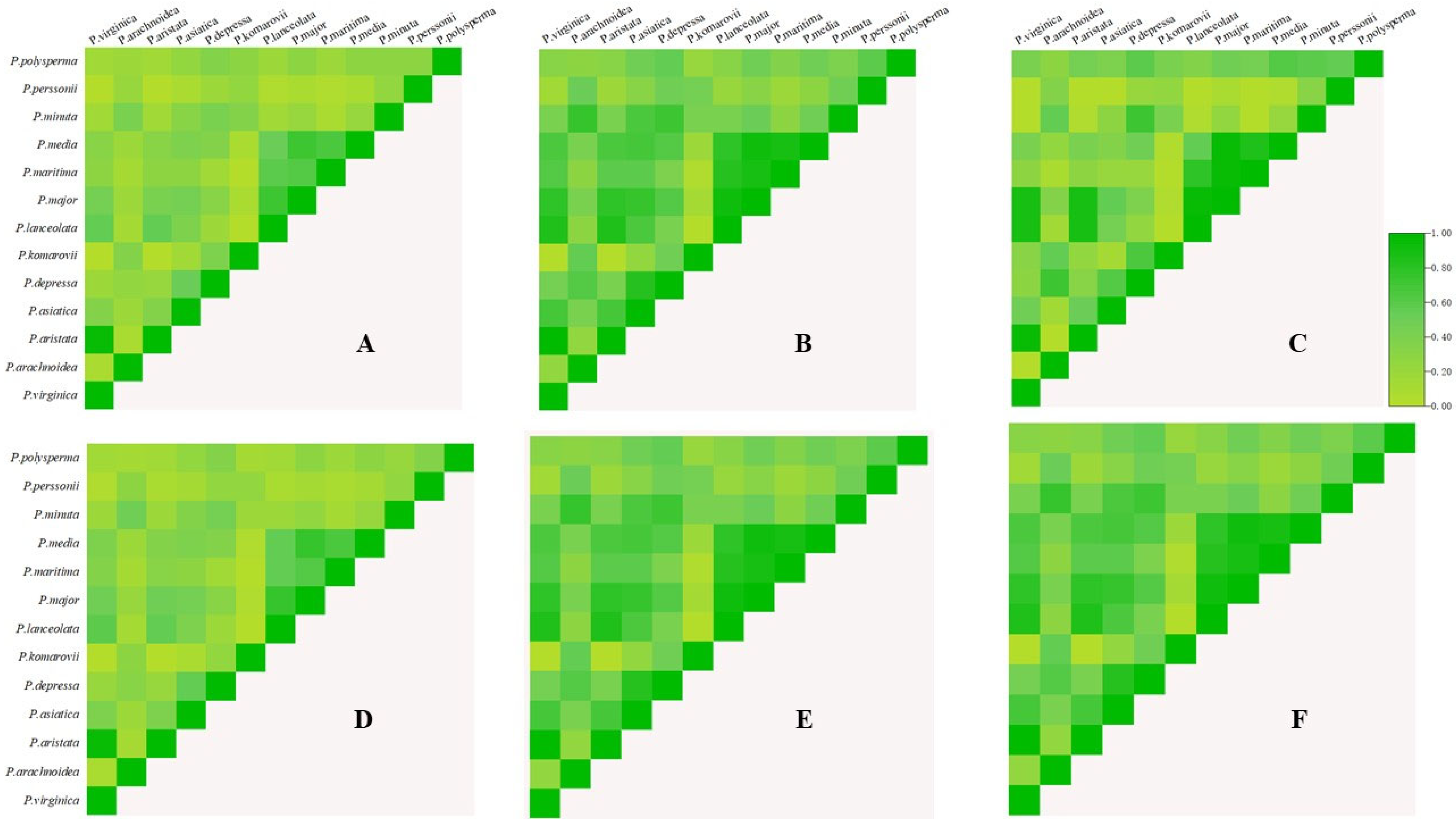
3.6. Niche Comparisons
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mi, X.C.; Feng, G.; Hu, Y.B.; Zhang, J.; Chen, L.; Corlett, R.T.; Hughes, A.C.; Pimm, S.; Schmid, B.; Shi, S.H.; et al. The global significance of biodiversity science in China: An overview. Natl. Sci. Rev. 2021, 8, nwab032. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ruiz, E.P.; Lacher, T.E., Jr. Climate change, range shifts, and the disruption of a pollinator-plant complex. Sci. Rep. 2019, 9, 14048. [Google Scholar] [CrossRef]
- Cepic, M.; Bechtold, U.; Wilfing, H. Modelling human influences on biodiversity at a global scale: A human ecology perspective. Ecol. Model. 2022, 465, 109854. [Google Scholar] [CrossRef]
- Lear, L.; Hesse, E.; Buckling, A. Disturbances can facilitate prior invasions more than subsequent invasions in microbial communities. Ecol. Lett. 2024, 27, e14493. [Google Scholar] [CrossRef]
- Campbell, C.; Russo, L.; Albert, R.; Buckling, A.; Shea, K. Whole community invasions and the integration of novel ecosystems. PLoS Comput. Biol. 2022, 18, e1010151. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, R.; Sheng, S.; Fu, H.H.; Wang, X.Y. Integrated metabolomic and transcriptomic profiling revealed coping mechanisms of the edible and medicinal homologous plant Plantago asiatica L. cadmium resistance. Open Life Sci. 2022, 17, 1347–1359. [Google Scholar] [CrossRef]
- Thuiller, W. Ecological niche modelling. Curr. Biol. 2024, 34, R225–R229. [Google Scholar] [CrossRef]
- Lissovsky, A.A.; Dudov, S.V. Species-distribution modeling: Advantages and limitations of application of the species distribution modeling methods. 2. MaxEnt. Biol. Bull. Rev. 2020, 81, 135–146. [Google Scholar]
- Ngarega, B.K.; Chaibva, P.; Masocha, V.F.; Saina, J.K.; Khine, P.K.; Schneider, H. Application of MaxEnt modeling to evaluate the climate change effects on the geographic distribution of Lippia javanica (Burm. f.) Spreng in Africa. Environ. Monit. Assess. 2024, 196, 62. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, X.Z.; Sun, Y.J.; Liu, Y.H. Species distribution modeling based on MaxEnt to inform biodiversity conservation in the Central Urban Area of Chongqing Municipality. Ecol. Indic. 2024, 158, 111491. [Google Scholar] [CrossRef]
- Luo, J.; Ma, Y.Y.; Liu, Y.; Zhu, D.P.; Guo, X.Z. Predicting Polygonum capitatum distribution in China across climate scenarios using MaxEnt modeling. Sci. Rep. 2024, 14, 20020. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-G.; Huang, J.-H.; He, X.L.-S.; Luo, R.; Ge, H.Z.; Wolowicz, A.N.; Wawrzkiewicz, M.; Gladysz-Plaska, A.; Li, B.; Yu, Q.X.; et al. Impacts of heavy metals and medicinal crops on ecological systems, environmental pollution, cultivation, and production processes in China. Ecotox Envrion. safe. 2021, 219, 112336. [Google Scholar] [CrossRef]
- Wang, H.T.; Zhou, Y.M.; Chen, Y.; Wang, Q.X.; Jiang, L.F.; Luo, Y.Q. Allelopathic potential of invasive Plantago virginica on four lawn species. PLoS ONE 2015, 10, e0125433. [Google Scholar] [CrossRef] [PubMed]
- Primack, R.B.; Antonovics, J. Experimental ecological genetics in Plantago. V. Components of seed yield in the ribwort plantain Plantago lanceolata L. Evolution 1981, 35, 1069–1079. [Google Scholar]
- Luo, X.; Zheng, Y.; Xu, X.H.; Xiao, R.; Guo, H. The impacts of warming and nitrogen addition on competitive ability of native and invasive populations of Plantago virginica. J. Plant Ecol. 2020, 13, 676–682. [Google Scholar] [CrossRef]
- Gioria, M.; Hulme, P.E.; Richardson, D.M.; Pyšek, P. Why are invasive plants successful? Annu. Rev. Plant Biol. 2023, 74, 635–670. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Rabus, B.; Eineder, M.; Roth, A.; Bamler, R. The Shuttle Radar Topography Mission—A New Class of Digital Elevation Models Acquired by Spaceborne Radar. ISPRS J. Photogramm. 2003, 57, 241–262. [Google Scholar] [CrossRef]
- Gomes, V.H.F.; Ijff, S.D.; Raes, N.; Amaral, I.L.; Salomao, R.P.; Coelho, L.D.; Matos, F.D.D.; Castilho, C.V.; Lima, D.D.; Lopez, D.C. Species distribution modelling: Contrasting presence-only models with plot abundance data. Sci. Rep. 2018, 8, 1003. [Google Scholar] [CrossRef]
- Lu, K.; He, Y.M.; Mao, W.; Du, Z.Y.; Wang, L.J.; Liu, G.M.; Feng, W.J.; Duan, Y.Z. Potential geographical distribution and shifts of Artemisia ordosica in China under future climate change. Chin. J. Appl. Ecol. 2020, 31, 3758–3766. [Google Scholar]
- Zhang, H.; Zhao, H.; Wang, H. Potential geographical distribution of Populus euphratica in China under future climate change scenarios based on Maxent model. Acta Ecol. Sin. 2020, 40, 6552–6563. [Google Scholar]
- Wang, R.; Li, Q.; Feng, C.; Shi, Z.P. Predicting potential ecological distribution of Locusta migratoria tibetensis in China using MaxEnt ecological niche modeling. Acta Ecol. Sin. 2017, 37, 8556–8566. [Google Scholar]
- Li, Y.C.; Li, M.Y.; Li, C.; Liu, Z.Z. Optimized MaxEnt model predictions of climate change impacts on the suitable distribution of Cunninghamia lanceolata in China. Forests 2020, 11, 302. [Google Scholar] [CrossRef]
- Su, Q.T.; Du, Z.X.; Zhou, B.; Liao, Y.H.; Wang, C.C.; Xiao, Y.A. Analysis of potential distribution areas of Impatiens davidii and its pollinators in China. Chin. J. Plant Ecol. 2022, 46, 785–796. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Ning, Y.L.; Li, J.X.; Shi, Z.L.; Zhang, Q.Z.; Li, L.Q.; Kang, B.Y.; Du, Z.B.; Luo, J.C.; He, M.X. Invasion stage and competition intensity co-drive reproductive strategies of native and invasive saltmarsh plants: Evidence from field data. Sci. Total Environ. 2024, 954, 176383. [Google Scholar] [CrossRef]
- Puşcaş, M.; Taberlet, P.; Choler, P. No positive correlation between species and genetic diversity in European alpine grasslands dominated by Carex curvula. Divers. Distrib. 2008, 14, 852–861. [Google Scholar] [CrossRef]
- Yahara, T.; Donoghue, M.; Hendry, A. Direct and indirect impacts of climatic change on species diversity and genetic diversity. IOP Conf. Ser. Earth Environ. Sci. 2009, 8, 7. [Google Scholar] [CrossRef]
- Zu, K.L.; Wang, Z.H.; Zhu, X.Y.; Lenoir, J.; Shrestha, N.; Lyu, T.; Luo, A.; Li, Y.Q.; Ji, C.J.; Peng, S.J. Upward shift and elevational range contractions of subtropical mountain plants in response to climate change. Sci. Total Environ. 2021, 783, 146896. [Google Scholar] [CrossRef]
- Huebner, C.D. Effects of Global Climate Change on Regeneration of Invasive Plant Species from Seeds. In Plant Regeneration from Seeds; Academic Pres: Cambridge, MA, USA, 2022; pp. 243–257. [Google Scholar]
- Yang, W.J.; Sun, S.X.; Wang, N.X.; Sun, S.X.; Fan, P.X.; You, C.; Wang, R.Q.; Zheng, P.M.; Wang, H. Dynamics of the distribution of invasive alien plants (Asteraceae) in China under climate change. Sci. Total Environ. 2023, 903, 166260. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, A.; Kaur, S.; Kohli, R.K.; Ravinder, K.K. Plant invasion and climate change: A global overview. In Plant Invasions and Global Climate Change; Springer: Berlin/Heidelberg, Germany, 2023; pp. 3–30. [Google Scholar]
- Chen, Y.Y.; Yang, X.R.; Zhang, T.W.; Zhao, Y.P.; Sun, Y.G.; Ma, M.J. Warming Promotes Divergent Shift in Sequential Phenophases of Alpine Meadow Plants. Agric. For. Meteorol. 2025, 368, 110521. [Google Scholar] [CrossRef]
- Kelley, A.L. The role thermal physiology plays in species invasion. Conserv. Physiol. 2014, 2, cou045. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.-Q.; Zou, C.B.; Wan, L.-Y.; Johnson, J.H.; Li, J.; Lan, Z.; Qi, S.S.; Dai, Z.C.; Zhang, H.Y.; Du, D.L. Interactive Effect of Climate Warming and Nitrogen Deposition May Shift the Dynamics of Native and Invasive Species. J. Plant Ecol. 2021, 14, 84–95. [Google Scholar] [CrossRef]
- Lin, Z.-H.; Wu, C.-H.; Ho, C.-K. Warming neutralizes host-specific competitive advantages between a native and invasive herbivore. Sci. Rep. 2018, 8, 11130. [Google Scholar] [CrossRef]
- Zheng, W.; Cao, K. Impacts of future climate change on the geographical distribution of four Litsea species. Guihaia 2020, 40, 1584–1594. [Google Scholar]
- Zhang, L.; Jiang, B.; Meng, Y.; Jia, Y.; Xu, Q.; Pan, Y.Z. The influence of climate change on the distribution of Hibiscus mutabilis in China: MaxEnt model-based prediction. Plants 2024, 13, 1744. [Google Scholar] [CrossRef]
- Xu, W.-B.; Svenning, J.-C.; Chen, G.-K.; Zhang, M.G.; Huang, J.H.; Chen, B.; Ordonez, A.; Ma, K.P. Human activities have opposing effects on distributions of narrow-ranged and widespread plant species in China. Proc. Natl. Acad. Sci. USA 2019, 116, 26674–26681. [Google Scholar] [CrossRef]
- Mamantov, M.A.; Gibson-Reinemer, D.K.; Linck, E.B.; Sheldon, K.S. Climate-driven range shifts of montane species vary with elevation. Glob. Ecol. Biogeogr. 2021, 30, 784–794. [Google Scholar] [CrossRef]
- Baltazar-Soares, M.; Brans, K.I.; Eizaguirre, C. Human-induced evolution: Signatures, processes and mechanisms underneath anthropogenic footprints on natural systems. Glob. Change Biol. 2021, 14, 2335–2341. [Google Scholar] [CrossRef]
- Chen, C.J.; Wang, G.A.; Li, J.Z.; Jia, Y.F.; Chen, Z.X. Examination of the negative correlation between leaf 815N and the N: P ratio across a northeast–southwest transect in China. Sci. Total Environ. 2023, 85, 163843. [Google Scholar] [CrossRef]
- Sorgog, K.; Kamo, M. Quantifying the precision of ecological risk: Conventional assessment factor method vs. species sensitivity distribution method. Ecotoxicol. Environ. Saf. 2019, 183, 109494. [Google Scholar] [CrossRef]
- Qi, Y.; Lian, X.H.; Wang, H.W.; Zhang, J.L.; Yang, R. Dynamic mechanism between human activities and ecosystem services: A case study of Qinghai lake watershed, China. Ecol. Indic. 2020, 117, 106528. [Google Scholar] [CrossRef]
- Humphreys, A.M.; Govaerts, R.; Ficinski, S.Z.; Lughadha, E.N.; Vorontsova, M.S. Global dataset shows geography and life form predict modern plant extinction and rediscovery. Nat. Ecol. Evol. 2019, 3, 1043–1047. [Google Scholar] [CrossRef]
- Zhang, J.; Miao, L.; Wu, P.; Yang, X.; Guo, W.; Li, S.; Feng, G. Effects of anthropogenic activities and climate factors on the distribution of invasive alien species in China. Sci. Sin. Vitae 2023, 53, 543–550. [Google Scholar] [CrossRef]
- Su, Q.T.; Du, Z.X.; Luo, Y.; Luo, Y.; Zhou, B.; Xiao, Y.A.; Zou, Z.R. MaxEnt modeling for predicting the potential geographical distribution of Hydrocera triflora since the last interglacial and under future climate scenarios. Biology 2024, 13, 745. [Google Scholar] [CrossRef]
- Su, Q.T.; Du, Z.X.; Xue, Y.X.; Li, H.; Zhang, Y.X.; Zhang, S.J.; Huang, X.Y.; Zhou, B.; Qian, H.; Xiao, Y.A.; et al. Habitat suitability modeling of endemic genus Chimonanthus in China under climate change. Forests 2024, 15, 1625. [Google Scholar] [CrossRef]
- Jabloński, P.G.; Borowiec, M.; Nowakowski, J.J.; Stawarczyk, T. Ecological niche partitioning in a fragmented landscape between two highly specialized avian flush-pursuit foragers in the Andean zone of sympatry. Sci. Rep. 2020, 10, 22024. [Google Scholar] [CrossRef]
- Bhadra, P.; Maitra, S.; Shankar, T.; Hossain, A.; Praharaj, S.; Aftab, T. Climate Change Impact on Plants: Plant Responses and Adaptations; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–24. [Google Scholar]
- Torres, N.; Herrera, I.; Fajardo, L.; Bustamante, R.O. Meta-analysis of the impact of plant invasions on soil microbial communities. BMC Ecol. Evol. 2021, 21, 172. [Google Scholar] [CrossRef]
- Xu, X.; Wolfe, L.; Diez, J.; Zheng, Y.; Guo, H.; Hu, S. Differential germination strategies of native and introduced populations of the invasive species Plantago virginica. NeoBiota 2019, 43, 101–118. [Google Scholar] [CrossRef]
- Wong, M.K. Misrepresentation of invasive species in the mass media with images of unrelated organisms. Conserv. Biol. 2024, 38, e14382. [Google Scholar] [CrossRef]
- Carey, C.J.; Blankinship, J.C.; Eviner, V.T.; Malmstrom, C.M.; Hart, S.C. Invasive plants decrease microbial capacity to nitrify and denitrify compared to native California grassland communities. Biol. Invasions. 2017, 19, 2941–2957. [Google Scholar] [CrossRef]
- Penczykowski, R.M.; Sieg, R.D. Plantago spp. as models for studying the ecology and evolution of species interactions across environmental gradients. Am. Nat. 2021, 198, 158–176. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, M.A.; Weiskopf, S.R.; Bertrand, R.; Carter, S.L.; Comte, L.; Eaton, M.J.; Johnson, C.G.; Lenoir, J.; Lynch, A.J.; Miller, B.W.; et al. Climate change and the global redistribution of biodiversity: Substantial variation in empirical support for expected range shifts. Environ. Evid. 2023, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Chukwuma, E.C.; Oyebanji, O.O.; Chukwuma, D.M.; Ayodele, A.E.; Tiamiyu, B.B.; Bolarinwa, K.A.A.; Adeyemi, S.B.; Abdulquadri, S.B. Predicting the potential impact of environmental factors on the distribution of Triplochiton scleroxylon (Malvaceae): An economically important tree species in Nigeria. Acta Ecol. Sin. 2023, 43, 1101–1111. [Google Scholar] [CrossRef]


| Variable | Description |
|---|---|
| bio1 | Mean annual air temperature |
| bio2 | Monthly mean |
| bio3 | Isothermality |
| bio4 | Variation in temperature seasonlity |
| bio5 | Maximum temperature of warmest month |
| bio6 | Minimum temperature of coldest month |
| bio7 | Temperature annual range |
| bio8 | Mean temperature of wettest quarter |
| bio9 | Mean temperature of driest quarter |
| bio10 | Mean temperature of warmest quarter |
| bio11 | Mean temperature of coldest quarter |
| bio12 | Mean annual precipitation |
| bio13 | Precipitation of wettest month |
| bio14 | Precipitation of the driest month |
| bio15 | Variation in precipitation seasonlity |
| bio16 | Precipitation of wettest quarter |
| bio17 | Precipitation of driest quarter |
| bio18 | Precipitation of warmest quarter |
| bio19 | Precipitation of coldest quarter |
| elev | Elevation |
| aspect | Influences microclimatic conditions critical to plant growth |
| ha | Human activity factor |
| slope | Slope |
| Species | Training AUC | Test AUC |
|---|---|---|
| P. arachnoidea | 0.998 | 0.991 |
| P. aristata | 0.920 | 0.919 |
| P. asiatica | 0.879 | 0.875 |
| P. cavaleriei | 0.988 | 0.990 |
| P. depressa | 0.912 | 0.917 |
| P. komarovii | 0.992 | 0.997 |
| P. lanceolata | 0.846 | 0.847 |
| P. major | 0.829 | 0.828 |
| P. maritima | 0.909 | 0.911 |
| P.media | 0.843 | 0.842 |
| P. minuta | 0.983 | 0.976 |
| P. perssonii | 0.999 | 0.978 |
| P. polysperma | 0.972 | 0.942 |
| P. virginica | 0.920 | 0.923 |
| Species | elev | aspect | ha | slope | bio01 | bio03 | bio04 | bio09 | bio10 | bio11 | bio14 | bio15 | bio18 | bio19 | Suitability More Than 50% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. arachnoidea | 38.8 | — | — | 20.8 | — | — | 10.8 | — | — | — | — | — | — | — | 690–3520 m |
| P. aristata. | — | — | — | — | — | — | 15 | — | — | — | 42 | — | 58.8 | — | 52.98–97.20 mm |
| P. asiatica | — | — | 17.4 | — | — | — | 17.5 | — | — | — | — | — | — | — | 266.10–279.29 mm |
| P. cavaleriei | 10.7 | — | — | 20.8 | — | — | — | — | — | — | — | — | 33.5 | — | 5.067–5.26 °C |
| P. depressa | — | — | 27.4 | — | — | — | — | 27.9 | — | — | — | 20.7 | — | — | 1.00–1.96 °C |
| P. komarovii | 44 | — | — | — | 20.4 | — | — | 15.5 | — | — | — | — | — | — | 1603.12–1617.25 m |
| P. lanceolata | — | — | 45.7 | — | — | — | — | — | — | 11.6 | — | — | — | 26.8 | 26.94–27.37 mm |
| P. major | — | — | 50.4 | — | 9.3 | — | — | — | — | 19.8 | 24.7 | 14.4 | — | — | 68.4–72.3 |
| P. maritima | — | — | 32 | — | 10.3 | — | — | — | — | — | 30.2 | — | — | — | 45.49–46.104 |
| P. media | — | — | 42 | — | 19.5 | — | — | — | — | — | 24.2 | — | — | — | 39.372–207.67 |
| P. minuta | — | — | 21.6 | — | — | — | — | — | — | 17.7 | — | — | — | 22.4 | 10.24–17.92 mm |
| P. perssonii | — | 41.4 | — | — | — | — | — | — | — | 16.6 | — | — | — | 22.4 | 53.4–54.672 |
| P. polysperma | — | — | — | — | — | 59.3 | — | — | 24 | — | — | 11.2 | — | — | 22.67–22.24 |
| P. virginica | — | — | 14.9 | — | — | — | 15.1 | — | — | — | 41 | — | — | — | 51.79–97.20 mm |
| Species | Current | 2030S | 2050S | 2070S | 2090S | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SSP126 | SSP245 | SSP585 | SSP126 | SSP245 | SSP585 | SSP126 | SSP245 | SSP585 | SSP126 | SSP245 | SSP585 | ||
| P.arachnoida | 66.1 | 66.1 | 86.2 | 72.5 | 91.2 | 77.2 | 95.1 | 95.9 | 98.7 | 86.1 | 95.8 | 98.3 | 173.2 |
| P. aristata | 135.1 | 119.1 | 135.7 | 137.6 | 116.5 | 132.1 | 147.0 | 126.3 | 137.9 | 179.2 | 131.4 | 164.4 | 145.2 |
| P. asiatica | 257.7 | 166.8 | 247.8 | 219.9 | 219.1 | 267.2 | 218.2 | 249.9 | 246.2 | 278.0 | 242.5 | 221.3 | 193.8 |
| P. cavaleriei | 275.9 | 266.8 | 223.0 | 209.5 | 216.8 | 231.3 | 224.2 | 159.0 | 160.8 | 153.0 | 162.5 | 140.2 | 133.7 |
| P. depressa | 291.1 | 240.8 | 275.0 | 276.1 | 248.5 | 270.5 | 223.6 | 220.8 | 242.7 | 276.7 | 266.7 | 250.1 | 242.9 |
| P. komarovii | 55.6 | 12.8 | 20.7 | 15.7 | 25.5 | 59.5 | 41.3 | 44.4 | 25.5 | 164.6 | 149.5 | 18.5 | 24.2 |
| P. lanceolata | 117.5 | 232.8 | 142.6 | 131.9 | 136.5 | 141.1 | 157.8 | 150.7 | 158.8 | 168.8 | 149.3 | 164.3 | 138.3 |
| P. major | 275.2 | 296.2 | 284.9 | 273.8 | 280.0 | 293.9 | 305.6 | 277.7 | 318.4 | 323.0 | 323.0 | 320.2 | 321.4 |
| P. maritima | 232.3 | 145.8 | 266.1 | 214.7 | 187.1 | 175.1 | 147.0 | 214.7 | 218.1 | 165.6 | 236.8 | 116.7 | 105.7 |
| P. media | 117.8 | 139.9 | 226.3 | 209.0 | 220.9 | 199.0 | 199.0 | 207.4 | 199.7 | 199.9 | 212.2 | 198.5 | 118.2 |
| P. minuta | 159.2 | 11.5 | 165.5 | 205.0 | 200.5 | 188.6 | 188.6 | 180.7 | 198.6 | 191.8 | 199.2 | 174.3 | 159.2 |
| P. perssonii | 59.0 | 61.7 | 22.5 | 22.2 | 24.9 | 58.0 | 28.0 | 136.4 | 130.1 | 101.4 | 124.0 | 119.9 | 111.5 |
| P. polysperma | 290.7 | 292.7 | 247.8 | 272.4 | 227.5 | 260.6 | 213.0 | 109.1 | 236.7 | 264.9 | 209.7 | 274.4 | 95.1 |
| P. virginica | 136.5 | 117.8 | 247.8 | 131.7 | 107.7 | 124.4 | 175.5 | 127.4 | 151.1 | 147.2 | 150.3 | 153.9 | 105.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, D.; Zhou, B.; Xiao, H.; Zhang, Y.; Zhang, S.; Su, Q.; Yan, X. MaxEnt Modeling of the Impacts of Human Activities and Climate Change on the Potential Distribution of Plantago in China. Biology 2025, 14, 564. https://doi.org/10.3390/biology14050564
Liao D, Zhou B, Xiao H, Zhang Y, Zhang S, Su Q, Yan X. MaxEnt Modeling of the Impacts of Human Activities and Climate Change on the Potential Distribution of Plantago in China. Biology. 2025; 14(5):564. https://doi.org/10.3390/biology14050564
Chicago/Turabian StyleLiao, Da, Bing Zhou, Haiyan Xiao, Yuxin Zhang, Shujian Zhang, Qitao Su, and Xiaohong Yan. 2025. "MaxEnt Modeling of the Impacts of Human Activities and Climate Change on the Potential Distribution of Plantago in China" Biology 14, no. 5: 564. https://doi.org/10.3390/biology14050564
APA StyleLiao, D., Zhou, B., Xiao, H., Zhang, Y., Zhang, S., Su, Q., & Yan, X. (2025). MaxEnt Modeling of the Impacts of Human Activities and Climate Change on the Potential Distribution of Plantago in China. Biology, 14(5), 564. https://doi.org/10.3390/biology14050564






