Functional Characterization of MIP_07528 of Mycobacterium indicus pranii for Tyrosine Phosphatase Activity Displays Sensitivity to Oxidative Inactivation and Plays a Role in Immunomodulation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. In Silico Characterization of MIP_07528 as a Putative PtpB
2.2. Bacterial Strains and Culture Conditions
2.3. Assessing Phosphatase Activity in Mycobacterium Indicus Pranii
2.4. Cloning and Expression of MIP_07528 and ptpB-Mtb in Heterologous Host
2.5. Validation of rMIP_07528 Using Anti-His Antibody
2.6. In Vitro Enzymatic Characterization of Purified rMIP_07528 and rPtpB-Mtb
2.7. Oxidative Inactivation Susceptibility Profile of Recombinant MIP_07528 Protein and rPtpB-Mtb
2.8. Evaluation of Immunomodulatory Effects of rMIP_07528 on THP-1 Cells
3. Results
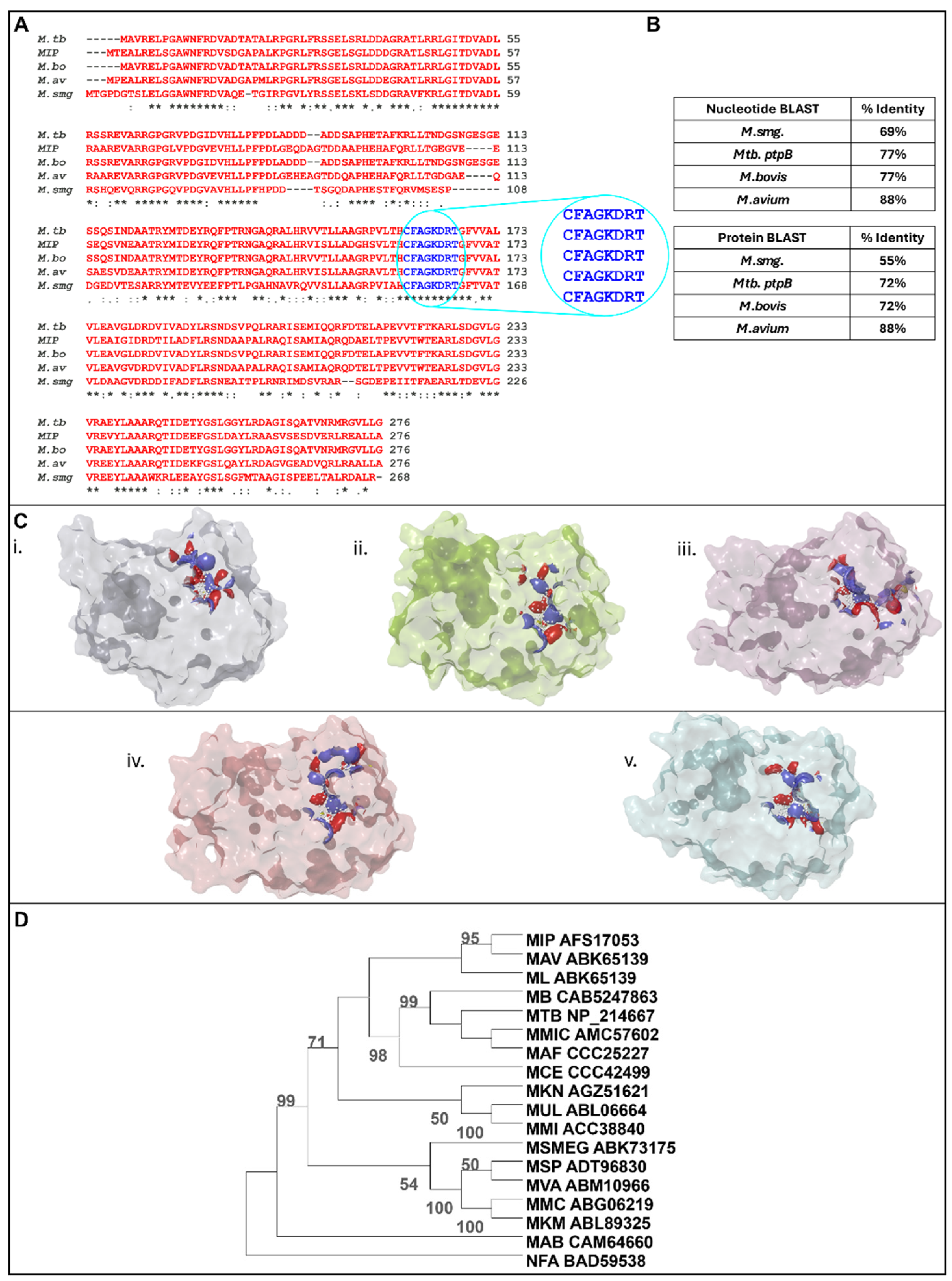
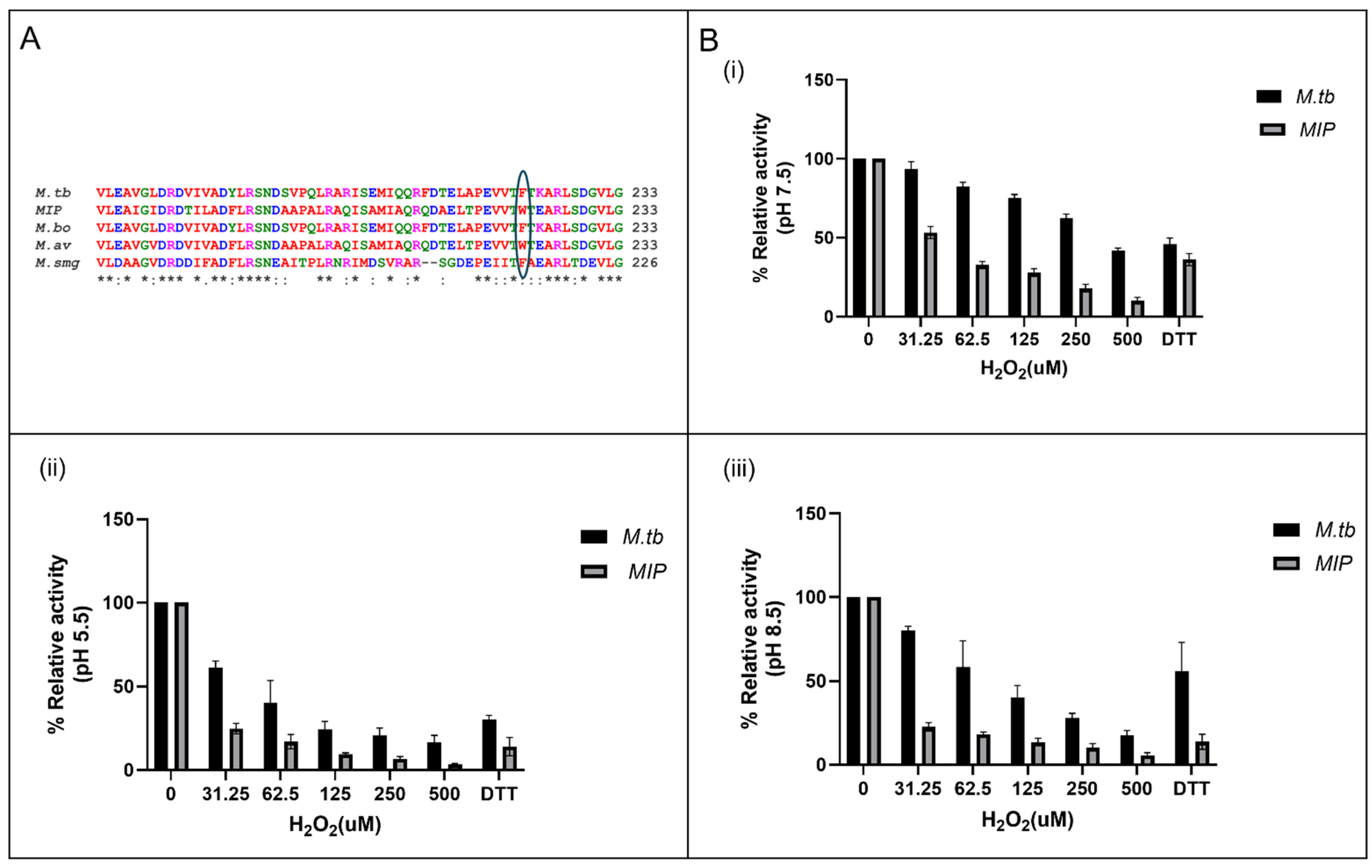
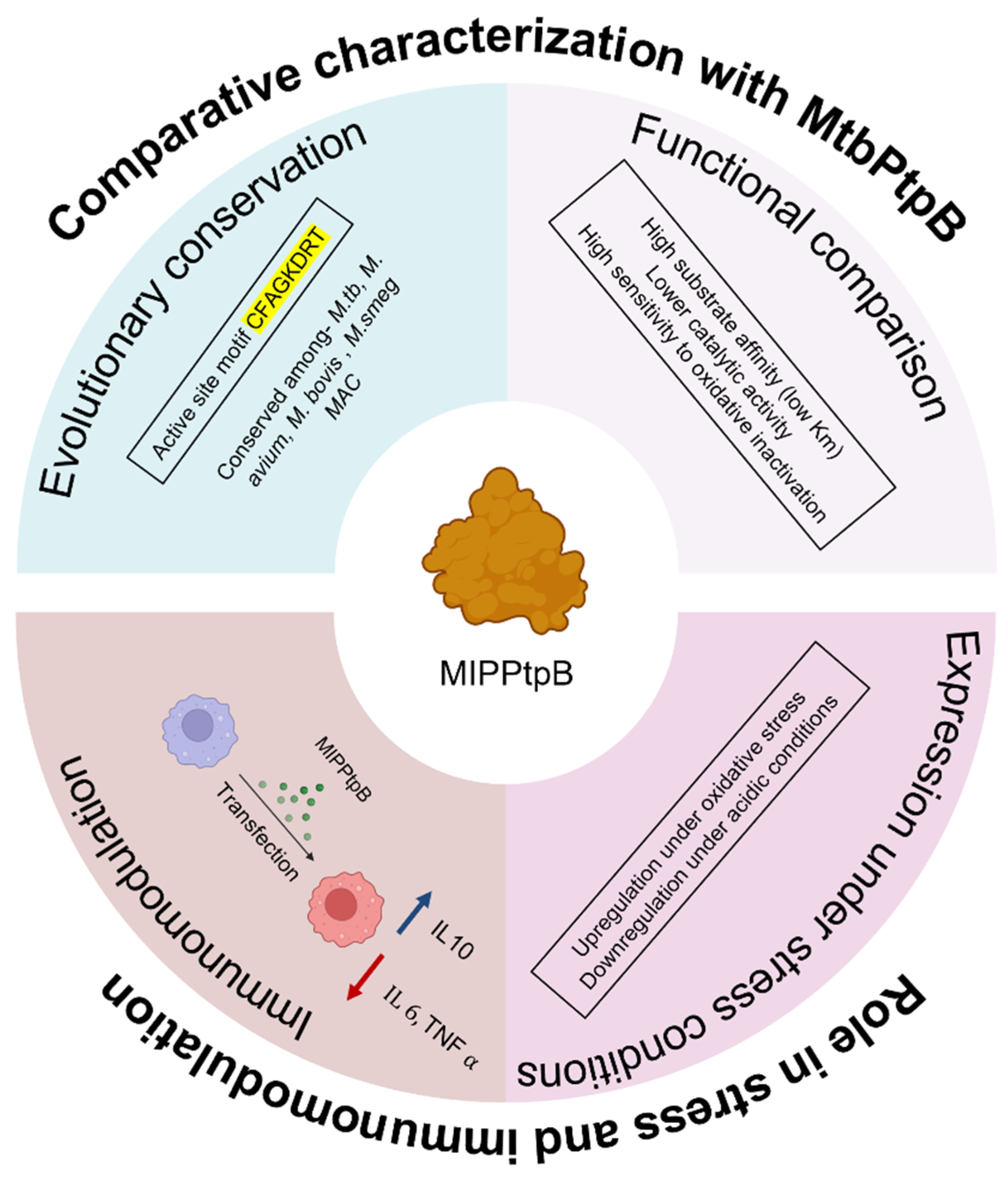
3.1. In Silico Analysis Identifies MIP_07528 as a Conserved Mycobacterial PtpB Ortholog
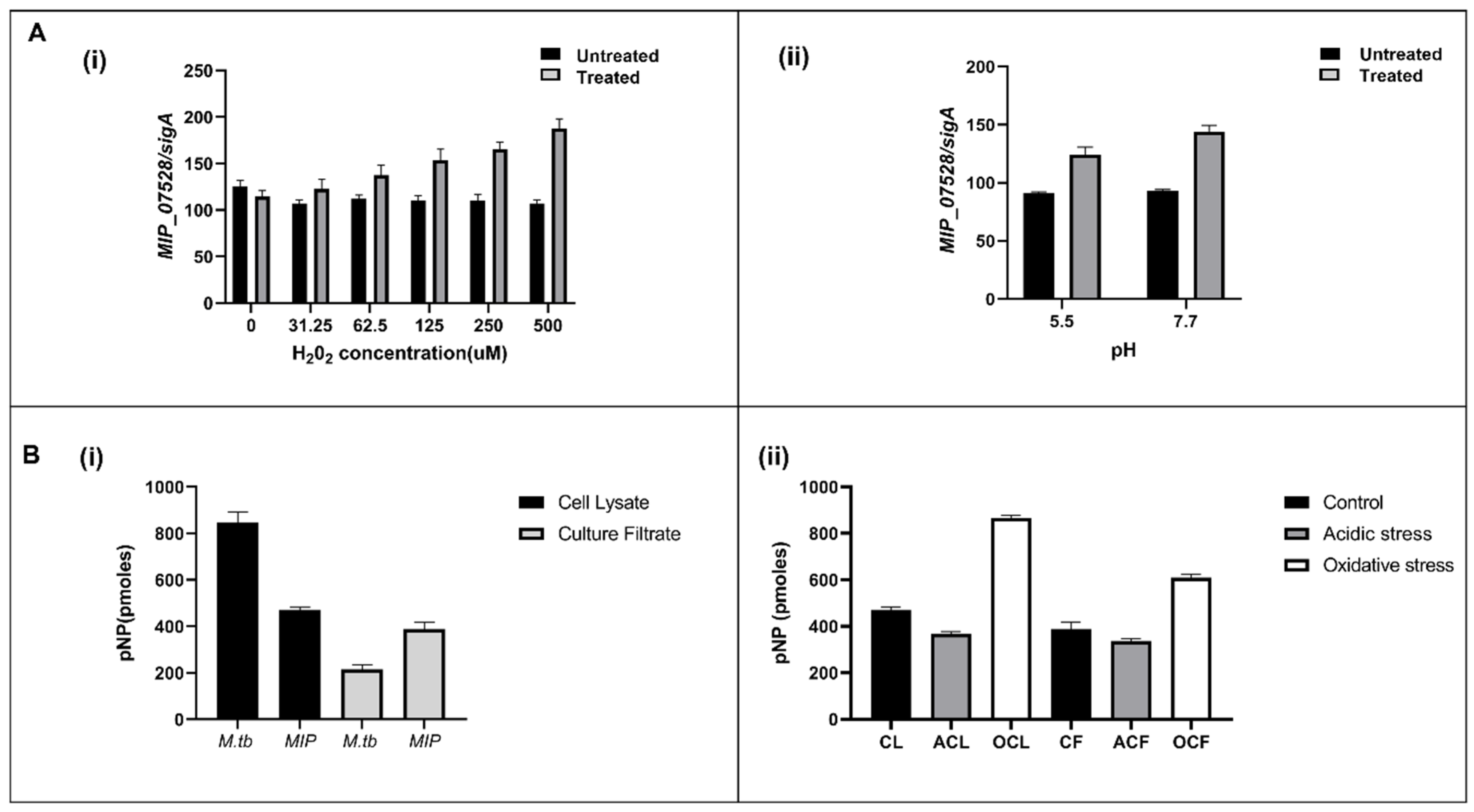
3.2. Cell Lysate and Culture Filtrate Has Phosphatase Activity in MIP
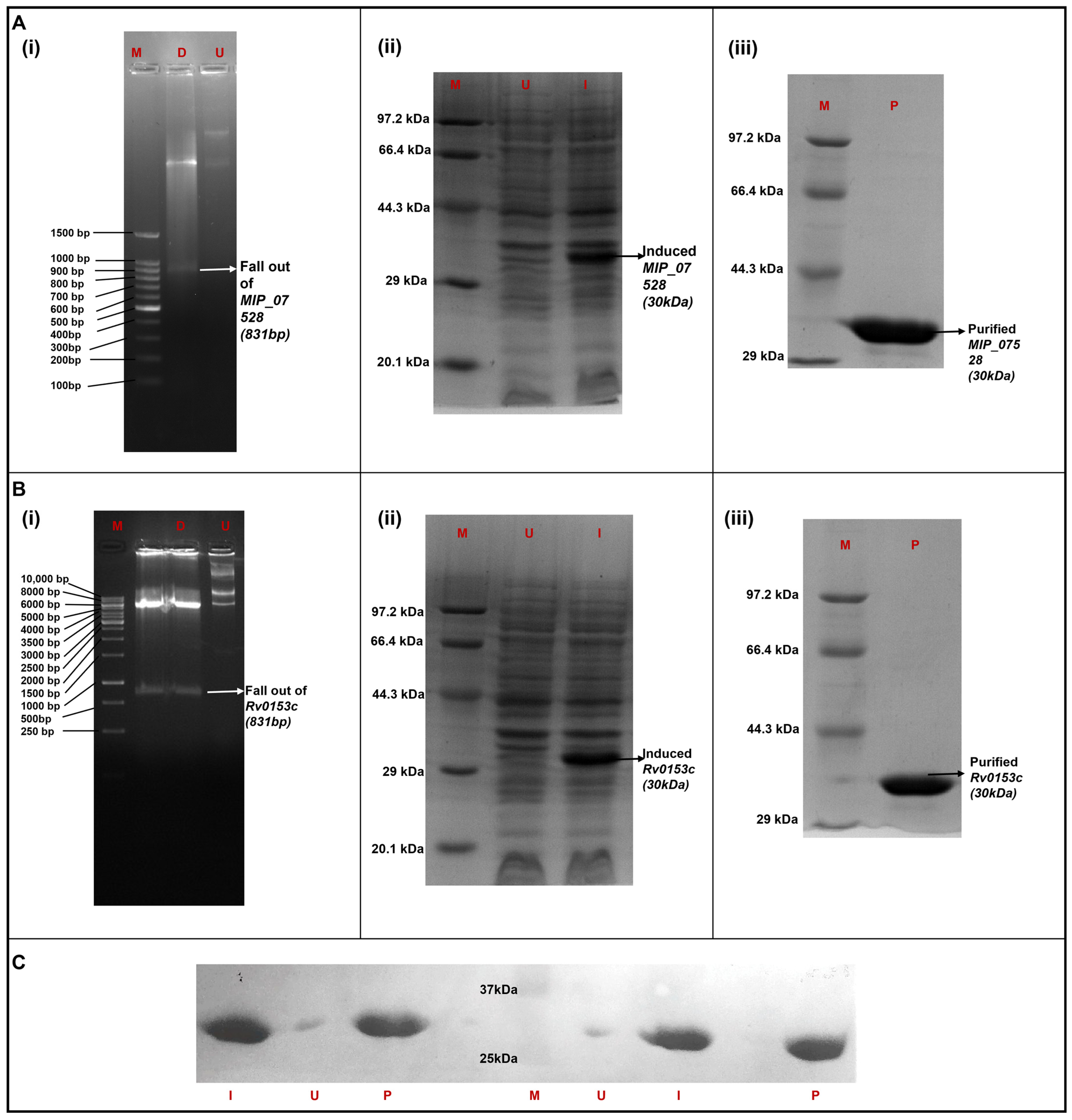
3.3. Heterologous Expression and Purification of MIP_07528 and Rv0153c (ptpB-Mtb) in E. coli
3.4. MIP_07528 Protein Displays Phosphatase Activity
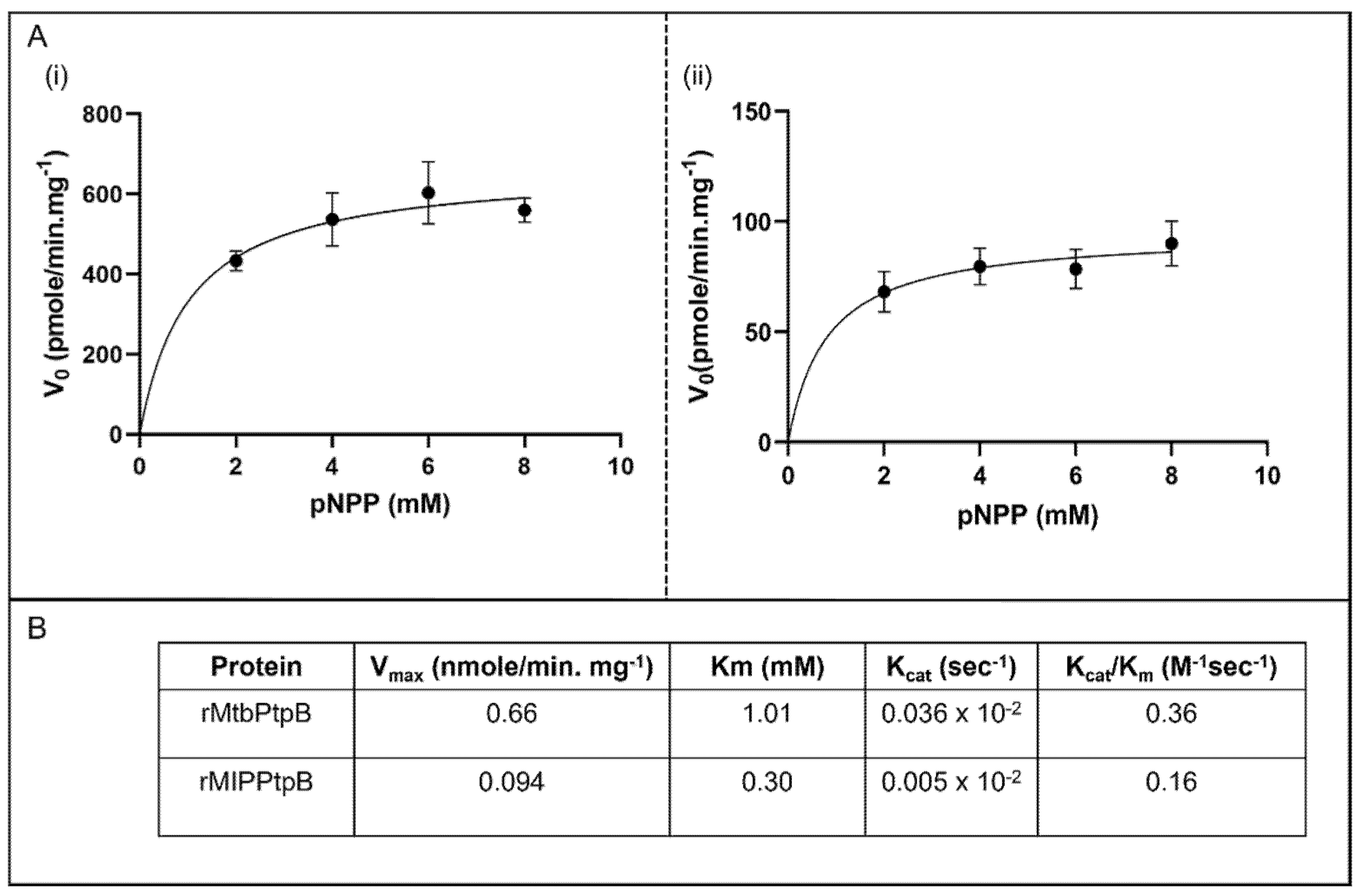
3.5. Kinetic Analysis Reveals Superior Catalytic Efficiency of rMIP_07528 Protein over rPtpB-Mtb
3.6. Enhanced Susceptibility to Oxidative Inactivation of rMIP_07528 Compared to rPtpBMtb
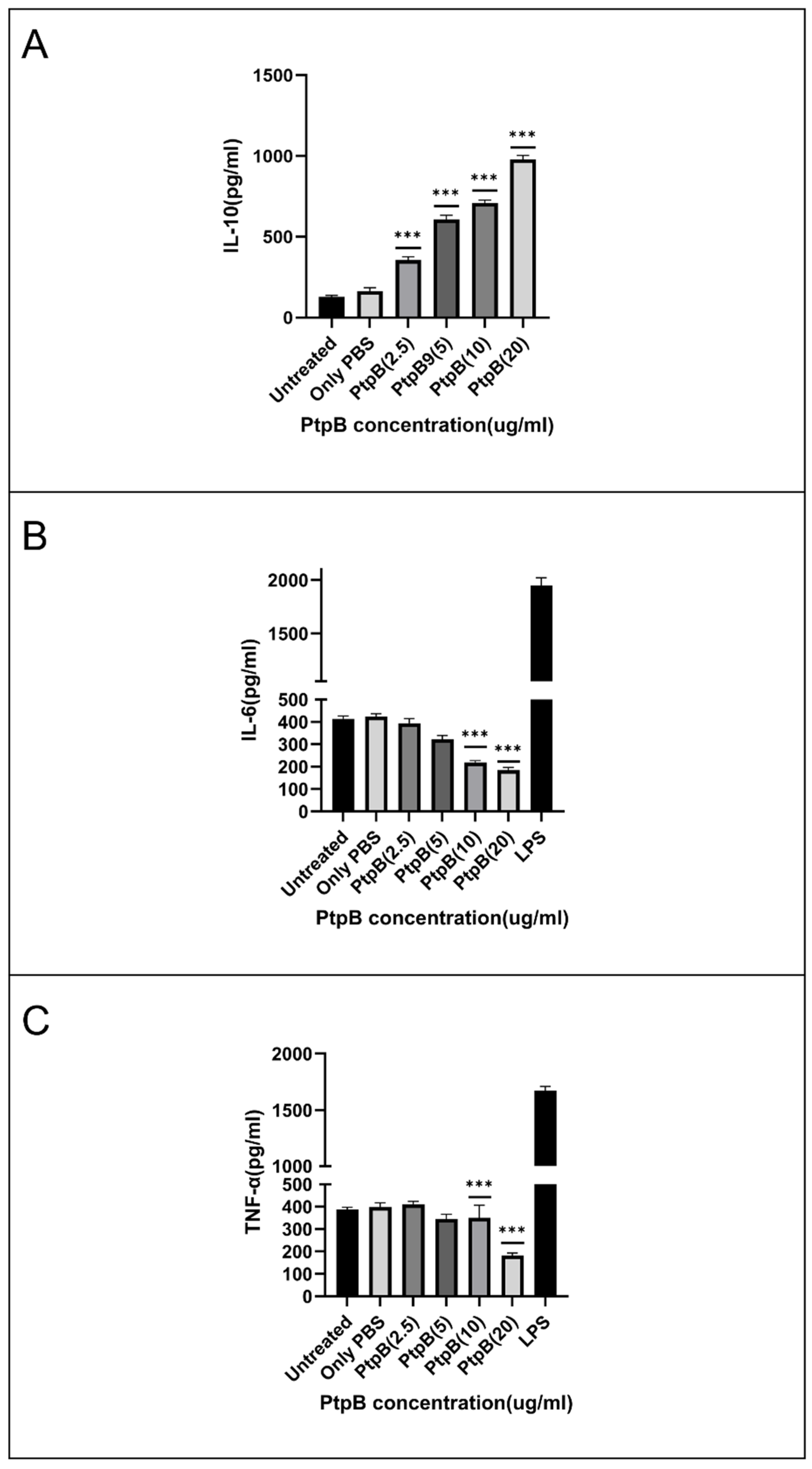
3.7. rMIP_07528 Suppresses Pro-Inflammatory Cytokines in Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stefan, K.; Gordon, R.; Rolig, A.; Honkala, A.; Tailor, D.; Davis, L.E.; Modi, R.I.; Joshipura, M.; Khamar, B.; Malhotra, S.V. Mycobacterium w—A promising immunotherapeutic intervention for diseases. Front. Immunol. 2024, 15, 1450118. [Google Scholar] [CrossRef]
- Saini, V.; Raghuvanshi, S.; Khurana, J.P.; Ahmed, N.; Hasnain, S.E.; Tyagi, A.K.; Tyagi, A.K. Massive gene acquisitions in Mycobacterium indicus pranii provide a perspective on mycobacterial evolution. Nucleic Acids Res. 2012, 40, 10832–10850. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Saqib, M.; Singh, B.; Pal, L.; Nishikanta, A.; Bhaskar, S. Mycobacterium indicus pranii Induced Memory T-Cells in Lung Airways Are Sentinels for Improved Protection Against M.tb Infection. Front. Immunol. 2019, 10, 2359. [Google Scholar] [CrossRef] [PubMed]
- Talwar, G.P.; Gupta, J.C.; Mustafa, A.S.; Kar, H.K.; Katoch, K.; Parida, S.K.; Reddi, P.P.; Ahmed, N.; Saini, V.; Gupta, S. Development of a potent invigorator of immune responses endowed with both preventive and therapeutic properties. Biologics 2017, 11, 55–63. [Google Scholar] [CrossRef]
- Sur, P.K.; Dastidar, A.G. Role of mycobacterium w as adjuvant treatment of lung cancer (non-small cell lung cancer). J. Indian Med. Assoc. 2003, 118, 120. [Google Scholar]
- Belani, C.P.; Desai, D.; Khamar, B.M. Open-label, randomized multicenter phase II clinical trial of a toll-like receptor-2 (TLR2) agonist mycobacterium w (Cadi-05) in combination with paclitaxel plus cisplatin versus paclitaxel plus cisplatin in advanced non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2011, 29, 7501. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Mukhopadhyay, S. Bladder preserving approach for muscle invasive bladder cancer--role of mycobacterium w. J. Indian Med. Assoc. 2003, 101, 559–560. [Google Scholar]
- Subramaniam, M.; Arshad, N.M.; Mun, K.S.; Malagobadan, S.; Awang, K.; Nagoor, N.H. Anti-Cancer Effects of Synergistic Drug-Bacterium Combinations on Induced Breast Cancer in BALB/c Mice. Biomolecules 2019, 9, 626. [Google Scholar] [CrossRef]
- Sehgal, I.S.; Agarwal, R.; Aggarwal, A.N.; Jindal, S.K. A randomized trial of Mycobacterium w in severe sepsis. J. Crit. Care 2015, 30, 85–89. [Google Scholar] [CrossRef]
- Sehgal, I.S.; Basumatary, N.M.; Dhooria, S.; Prasad, K.T.; Muthu, V.; Aggarwal, A.N.; Pal, A.; Desai, M.; Chaudhry, D.; Supe, P.D.; et al. A Randomized Trial of Mycobacterium w in Severe Presumed Gram-Negative Sepsis. Chest 2021, 160, 1282–1291. [Google Scholar] [CrossRef]
- Kaur, A.; Brar, B.K.; Kumar, S.; Brar, S.K.; Boparai, A.S.; Puri, N. A Randomized Comparative Study of MIP and MMR Vaccine for the Treatment of Cutaneous Warts. Indian J. Dermatol. 2021, 66, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Sandhu, K.; Kaur, I. Role of Mycobacterium w vaccine in the management of psoriasis. Br. J. Dermatol. 2005, 152, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Katoch, K.; Sarin, R.; Balambal, R.; Kumar Jain, N.; Patel, N.; Murthy, K.J.R.; Singla, N.; Saha, P.K.; Khanna, A.; et al. Efficacy and Safety of Mycobacterium indicus pranii as an adjunct therapy in Category II pulmonary tuberculosis in a randomized trial. Sci. Rep. 2017, 7, 3354. [Google Scholar] [CrossRef]
- Patel, N.; Trapathi, S.B. Improved cure rates in pulmonary tuberculosis category II (retreatment) with mycobacterium w. J. Indian Med. Assoc. 2003, 680, 682. [Google Scholar]
- Kharkar, R. Immune recovery in HIV with Mycobacterium W. J. Indian Med. Assoc. 2002, 100, 578–579. [Google Scholar]
- Sehgal, I.S.; Guleria, R.; Singh, S.; Siddiqui, M.S.; Agarwal, R. A randomised trial of Mycobacterium w in critically ill patients with COVID-19: ARMY-1. ERJ Open Res. 2021, 7, 00059-2021. [Google Scholar] [CrossRef]
- Dixit, S.; Zirpe, K.; Suryawanshi, P.; Borawake, K.; Prasad, S.; Ambapkar, S.; Ambapkar, S.; Joshi, A.; Joshi, M. Retrospective Cohort Observational Study to compare the Effect of Mycobacterium w along with Standard of Care vs Standard of Care alone in critically ill COVID-19 Patients. J. Assoc. Physicians India 2022, 70, 11–12. [Google Scholar] [CrossRef]
- Kant, S.; Sun, Y.; Pancholi, V. StkP- and PhpP-Mediated Posttranslational Modifications Modulate the S. pneumoniae Metabolism, Polysaccharide Capsule, and Virulence. Infect. Immun. 2023, 91, e0029622. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; He, Y.; Zhang, X.; Xu, J.; Luo, Y.; Wang, Y.; Franzblau, S.G.; Yang, Z.; Chan, R.J.; Liu, Y.; et al. Targeting mycobacterium protein tyrosine phosphatase B for antituberculosis agents. Proc. Natl. Acad. Sci. USA 2010, 107, 4573–4578. [Google Scholar] [CrossRef]
- Chen, D.; Liu, L.; Lu, Y.; Chen, S. Identification of fusarielin M as a novel inhibitor of Mycobacterium tuberculosis protein tyrosine phosphatase B (MptpB). Bioorg. Chem. 2021, 106, 104495. [Google Scholar] [CrossRef]
- Koliwer-Brandl, H.; Knobloch, P.; Barisch, C.; Welin, A.; Hanna, N.; Soldati, T.; Hilbi, H. Distinct Mycobacterium marinum phosphatases determine pathogen vacuole phosphoinositide pattern, phagosome maturation, and escape to the cytosol. Cell Microbiol. 2019, 21, e13008. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fernández, P.; Botella, L.; Cavet, J.S.; Domínguez, J.; Gutierrez, M.G.; Suckling, C.J.; Scott, F.J.; Tabernero, L. MptpB Inhibitor Improves the Action of Antibiotics against Mycobacterium tuberculosis and Nontuberculous Mycobacterium avium Infections. ACS Infect. Dis. 2024, 10, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, S.S.R.; Gunosewoyo, H. Combating Tuberculosis via Restoring the Host Immune Capacity by Targeting M. tb Kinases and Phosphatases. Int. J. Mol. Sci. 2024, 25, 12481. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Chao, J.D.; Av-Gay, Y. Mycobacterium tuberculosis-secreted phosphatases: From pathogenesis to targets for TB drug development. Trends Microbiol. 2013, 21, 100–109. [Google Scholar] [CrossRef]
- Koul, A.; Choidas, A.; Treder, M.; Tyagi, A.K.; Drlica, K.; Singh, Y.; Ullrich, A. Cloning and characterization of secretory tyrosine phosphatases of Mycobacterium tuberculosis. J. Bacteriol. 2000, 182, 5425–5432. [Google Scholar] [CrossRef]
- Nair, S.; Ramaswamy, P.A.; Ghosh, S.; Joshi, D.C.; Pathak, N.; Siddiqui, I.; Sharma, P.; Hasnain, S.E.; Mande, S.C.; Mukhopadhyay, S. The PPE18 of Mycobacterium tuberculosis Interacts with TLR2 and Activates IL-10 Induction in Macrophage1. J. Immunol. 2009, 183, 6269–6281. [Google Scholar] [CrossRef]
- Banerjee, S.; Nandyala, A.; Podili, R.; Katoch, V.M.; Murthy, K.J.; Hasnain, S.E. Mycobacterium tuberculosis (Mtb) isocitrate dehydrogenases show strong B cell response and distinguish vaccinated controls from TB patients. Proc. Natl. Acad. Sci. USA 2004, 101, 12652–12657. [Google Scholar] [CrossRef]
- Pandey, S.; Tripathi, D.; Khubaib, M.; Kumar, A.; Sheikh, J.A.; Sumanlatha, G.; Ehtesham, N.Z.; Hasnain, S.E. Mycobacterium tuberculosis Peptidyl-Prolyl Isomerases Are Immunogenic, Alter Cytokine Profile and Aid in Intracellular Survival. Front. Cell Infect. Microbiol. 2017, 7, 38. [Google Scholar] [CrossRef]
- Garg, R.; Tripathi, D.; Kant, S.; Chandra, H.; Bhatnagar, R.; Banerjee, N. The Conserved Hypothetical Protein Rv0574c Is Required for Cell Wall Integrity, Stress Tolerance, and Virulence of Mycobacterium tuberculosis. Infect. Immun. 2015, 83, 120–129. [Google Scholar] [CrossRef]
- Khawary, M.; Rakshit, R.; Bahl, A.; Juneja, P.; Kant, S.; Pandey, S.; Tripathi, D. M.tb-Rv2462c of Mycobacterium tuberculosis Shows Chaperone-like Activity and Plays a Role in Stress Adaptation and Immunomodulation. Biology 2022, 12, 69. [Google Scholar] [CrossRef]
- Beresford, N.J.; Saville, C.; Bennett, H.J.; Roberts, I.S.; Tabernero, L. A new family of phosphoinositide phosphatases in microorganisms: Identification and biochemical analysis. BMC Genom. 2010, 11, 457. [Google Scholar] [CrossRef] [PubMed]
- Grundner, C.; Ng, H.L.; Alber, T. Mycobacterium tuberculosis protein tyrosine phosphatase PtpB structure reveals a diverged fold and a buried active site. Structure 2005, 13, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, G.; De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 2006, 443, 651–657. [Google Scholar] [CrossRef]
- Beresford, N.; Patel, S.; Armstrong, J.; Szöor, B.; Fordham-Skelton, A.P.; Tabernero, L. MptpB, a virulence factor from Mycobacterium tuberculosis, exhibits triple-specificity phosphatase activity. Biochem. J. 2007, 406, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, A.V.; Kutuzov, M.A. Protozoan protein tyrosine phosphatases. Int. J. Parasitol. 2008, 38, 1279–1295. [Google Scholar] [CrossRef]
- Cosentino-Gomes, D.; Meyer-Fernandes, J.R. Ecto-phosphatases in protozoan parasites: Possible roles in nutrition, growth and ROS sensing. J. Bioenerg. Biomembr. 2011, 43, 89–92. [Google Scholar] [CrossRef]
- Brenchley, R.; Tariq, H.; McElhinney, H.; Szöor, B.; Huxley-Jones, J.; Stevens, R.; Matthews, K.; Tabernero, L. The TriTryp phosphatome: Analysis of the protein phosphatase catalytic domains. BMC Genom. 2007, 8, 434. [Google Scholar] [CrossRef]
- Wilkes, J.M.; Doerig, C. The protein-phosphatome of the human malaria parasite Plasmodium falciparum. BMC Genom. 2008, 9, 412. [Google Scholar] [CrossRef]
- Chatterjee, A.; Pandey, S.; Singh, P.K.; Pathak, N.P.; Rai, N.; Ramachandran, R.; Tripathi, R.P.; Srivastava, K.K. Biochemical and functional characterizations of tyrosine phosphatases from pathogenic and nonpathogenic mycobacteria: Indication of phenyl cyclopropyl methyl-/phenyl butenyl azoles as tyrosine phosphatase inhibitors. Appl. Microbiol. Biotechnol. 2015, 99, 7539–7548. [Google Scholar] [CrossRef]
- Prisic, S.; Husson, R.N. Mycobacterium tuberculosis Serine/Threonine Protein Kinases. Microbiol. Spectr. 2014, 2, 681–708. [Google Scholar] [CrossRef]
- Hogan, M.; Bahta, M.; Tsuji, K.; Nguyen, T.X.; Cherry, S.; Lountos, G.T.; Tropea, J.E.; Zhao, B.M.; Zhao, X.Z.; Waugh, D.S.; et al. Targeting Protein-Protein Interactions of Tyrosine Phosphatases with Microarrayed Fragment Libraries Displayed on Phosphopeptide Substrate Scaffolds. ACS Comb. Sci. 2019, 21, 158–170. [Google Scholar] [CrossRef]
- Flynn, E.M.; Hanson, J.A.; Alber, T.; Yang, H. Dynamic active-site protection by the M. tuberculosis protein tyrosine phosphatase PtpB lid domain. J. Am. Chem. Soc. 2010, 132, 4772–4780. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Kant, S.; Khawary, M.; Tripathi, D. Macrophages in Microbial Pathogenesis: Commonalities of Defense Evasion Mechanisms. Infect. Immun. 2022, 90, e0029121. [Google Scholar] [CrossRef] [PubMed]
- Bahl, A.; Pandey, S.; Rakshit, R.; Kant, S.; Tripathi, D. Infection-induced trained immunity: A twist in paradigm of innate host defense and generation of immunological memory. Infect. Immun. 2025, 93, e0047224. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wu, X.; Jin, C.; Li, F.; Xiong, S.; Dong, Y. MptpB Promotes Mycobacteria Survival by Inhibiting the Expression of Inflammatory Mediators and Cell Apoptosis in Macrophages. Front. Cell Infect. Microbiol. 2018, 8, 171. [Google Scholar] [CrossRef]
- Chai, Q.; Wang, L.; Liu, C.H.; Ge, B. New insights into the evasion of host innate immunity by Mycobacterium tuberculosis. Cell. Mol. Immunol. 2020, 17, 901–913. [Google Scholar] [CrossRef]
- Augenstreich, J.; Briken, V. Host Cell Targets of Released Lipid and Secreted Protein Effectors of Mycobacterium tuberculosis. Front. Cell Infect. Microbiol. 2020, 10, 595029. [Google Scholar] [CrossRef]
- Pal, R.; Bisht, M.K.; Mukhopadhyay, S. Secretory proteins of Mycobacterium tuberculosis and their roles in modulation of host immune responses: Focus on therapeutic targets. Febs J. 2022, 289, 4146–4171. [Google Scholar] [CrossRef]
- Salmeen, A.; Andersen, J.N.; Myers, M.P.; Meng, T.C.; Hinks, J.A.; Tonks, N.K.; Barford, D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 2003, 423, 769–773. [Google Scholar] [CrossRef]
- Beresford, N.J.; Mulhearn, D.; Szczepankiewicz, B.; Liu, G.; Johnson, M.E.; Fordham-Skelton, A.; Abad-Zapatero, C.; Cavet, J.S.; Tabernero, L. Inhibition of MptpB phosphatase from Mycobacterium tuberculosis impairs mycobacterial survival in macrophages. J. Antimicrob. Chemother. 2009, 63, 928–936. [Google Scholar] [CrossRef]
- Ruddraraju, K.V.; Aggarwal, D.; Zhang, Z.Y. Therapeutic Targeting of Protein Tyrosine Phosphatases from Mycobacterium tuberculosis. Microorganisms 2020, 9, 14. [Google Scholar] [CrossRef]
- Grundner, C.; Perrin, D.; Hooft van Huijsduijnen, R.; Swinnen, D.; Gonzalez, J.; Gee, C.L.; Wells, T.N.; Alber, T. Structural basis for selective inhibition of Mycobacterium tuberculosis protein tyrosine phosphatase PtpB. Structure 2007, 15, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.K.; Sodhi, A.; Biswas, S.K.; Dahiya, Y.; Dhillon, M.K. Mycobacterium indicus pranii mediates macrophage activation through TLR2 and NOD2 in a MyD88 dependent manner. Vaccine 2012, 30, 5748–5754. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Yu, S.; Zhong, Y.; Lu, Z.; Qiu, C.; Yu, Y.; Zhang, X.; Zhang, Y.; Lei, Z.; Qiang, L.; et al. A bacterial phospholipid phosphatase inhibits host pyroptosis by hijacking ubiquitin. Science 2022, 378, eabq0132. [Google Scholar] [CrossRef]
- Mascarello, A.; Mori, M.; Chiaradia-Delatorre, L.D.; Menegatti, A.C.; Delle Monache, F.; Ferrari, F.; Yunes, R.A.; Nunes, R.J.; Terenzi, H.; Botta, B.; et al. Discovery of Mycobacterium tuberculosis protein tyrosine phosphatase B (PtpB) inhibitors from natural products. PLoS ONE 2013, 8, e77081. [Google Scholar] [CrossRef]
- Kant, S.; Pancholi, V. Novel Tyrosine Kinase-Mediated Phosphorylation With Dual Specificity Plays a Key Role in the Modulation of Streptococcus pyogenes Physiology and Virulence. Front. Microbiol. 2021, 12, 689246. [Google Scholar] [CrossRef]
- Chai, Q.; Zhang, Y.; Liu, C.H. Mycobacterium tuberculosis: An Adaptable Pathogen Associated With Multiple Human Diseases. Front. Cell Infect. Microbiol. 2018, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Bahl, A.; Rakshit, R.; Pandey, S.; Tripathi, D. Genome wide screening to discover novel toxin-antitoxin modules in Mycobacterium indicus pranii; perspective on gene acquisition during mycobacterial evolution. Biotechnol. Appl. Biochem. 2025, 72, 116–137. [Google Scholar] [CrossRef]
- Vickers, C.F.; Silva, A.P.G.; Chakraborty, A.; Fernandez, P.; Kurepina, N.; Saville, C.; Naranjo, Y.; Pons, M.; Schnettger, L.S.; Gutierrez, M.G.; et al. Structure-Based Design of MptpB Inhibitors That Reduce Multidrug-Resistant Mycobacterium tuberculosis Survival and Infection Burden in Vivo. J. Med. Chem. 2018, 61, 8337–8352. [Google Scholar] [CrossRef]
- Dogra, S.; Jain, S.; Sharma, A.; Chhabra, S.; Narang, T. Mycobacterium Indicus Pranii (MIP) Vaccine: Pharmacology, Indication, Dosing Schedules, Administration, and Side Effects in Clinical Practice. Indian Dermatol. Online J. 2023, 14, 753–761. [Google Scholar] [CrossRef]
- Zhu, J.; Fan, J.; Xia, Y.; Wang, H.; Li, Y.; Feng, Z.; Fu, C. Potential therapeutic targets of macrophages in inhibiting immune damage and fibrotic processes in musculoskeletal diseases. Front. Immunol. 2023, 14, 1219487. [Google Scholar] [CrossRef] [PubMed]
| Sr No. | Primer | Sequence (5′-3′) |
|---|---|---|
| 1 | MIP_07528 FP | AAAGGATCCATGACTGAGGCGTTGCGA |
| 2 | MIP_07528 RP | AAAAAGCTTTCAGGCGAGCAGCGCCTC |
| 3 | Rv0153c FP | AAAGGATCCATGGCTGTCCGTGAACTGC |
| 4 | Rv0153 RP | AAAAAGCTTTCATCCGAGCAGCACCCCGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raunak, R.; Rakshit, R.; Bahl, A.; Sinha, S.; Pandey, S.; Kant, S.; Tripathi, D. Functional Characterization of MIP_07528 of Mycobacterium indicus pranii for Tyrosine Phosphatase Activity Displays Sensitivity to Oxidative Inactivation and Plays a Role in Immunomodulation. Biology 2025, 14, 565. https://doi.org/10.3390/biology14050565
Raunak R, Rakshit R, Bahl A, Sinha S, Pandey S, Kant S, Tripathi D. Functional Characterization of MIP_07528 of Mycobacterium indicus pranii for Tyrosine Phosphatase Activity Displays Sensitivity to Oxidative Inactivation and Plays a Role in Immunomodulation. Biology. 2025; 14(5):565. https://doi.org/10.3390/biology14050565
Chicago/Turabian StyleRaunak, Raunak, Roopshali Rakshit, Aayush Bahl, Soumya Sinha, Saurabh Pandey, Sashi Kant, and Deeksha Tripathi. 2025. "Functional Characterization of MIP_07528 of Mycobacterium indicus pranii for Tyrosine Phosphatase Activity Displays Sensitivity to Oxidative Inactivation and Plays a Role in Immunomodulation" Biology 14, no. 5: 565. https://doi.org/10.3390/biology14050565
APA StyleRaunak, R., Rakshit, R., Bahl, A., Sinha, S., Pandey, S., Kant, S., & Tripathi, D. (2025). Functional Characterization of MIP_07528 of Mycobacterium indicus pranii for Tyrosine Phosphatase Activity Displays Sensitivity to Oxidative Inactivation and Plays a Role in Immunomodulation. Biology, 14(5), 565. https://doi.org/10.3390/biology14050565









