Comparison of Tumor Cell Responses to Different Radiotherapy Techniques: Three-Dimensional Conformal Radiotherapy (3D-CRT), Volumetric Modulated Arc Therapy (VMAT), and Helical Tomotherapy (HT)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
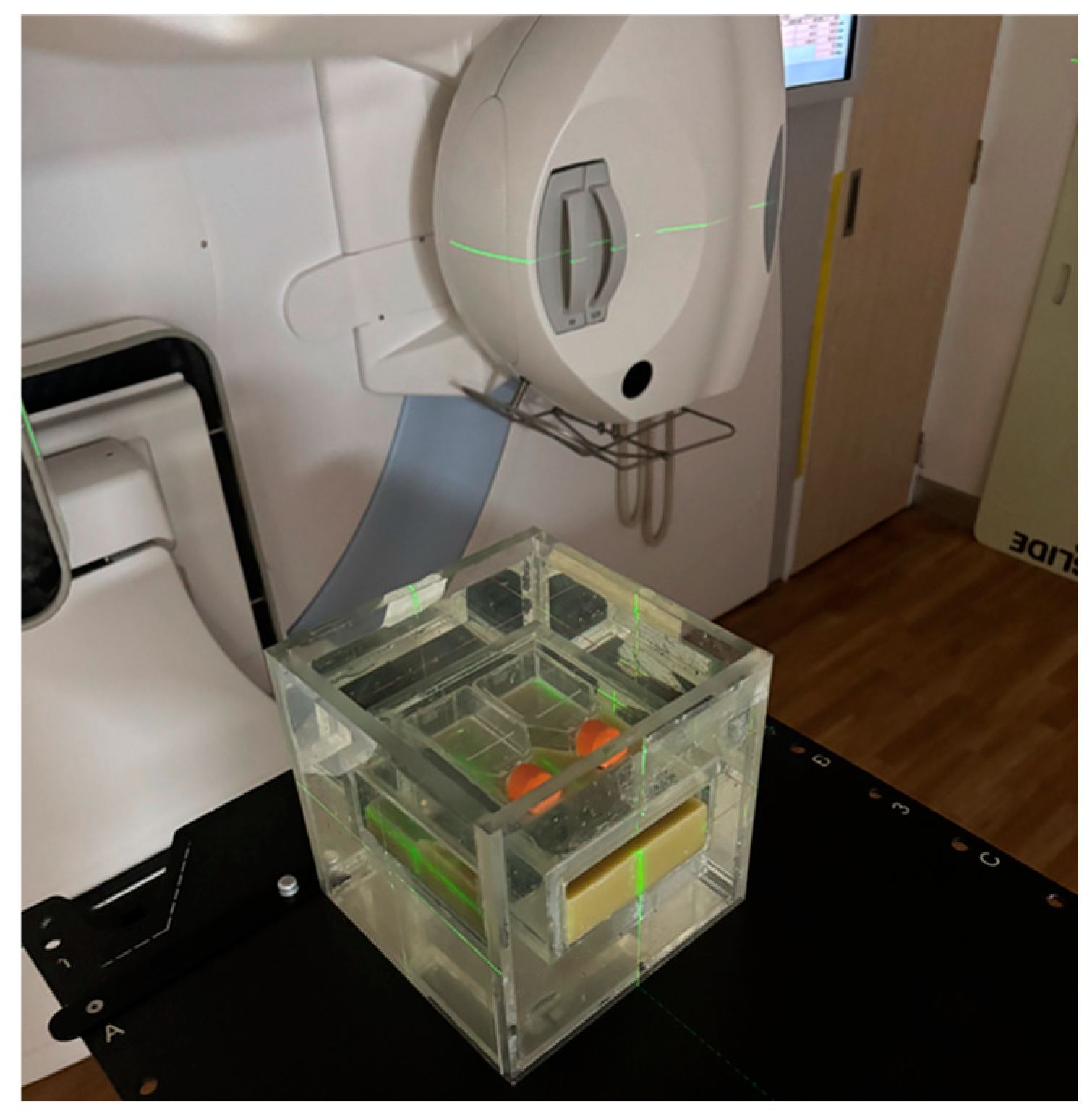
2.2. Water Phantom Design
2.3. Treatment Planning
2.4. Dose Verification
2.5. Irradiation Conditions
2.6. Clonogenic Survival Assay
2.7. Micronucleus Formation Assay
2.8. Cell Cycle Assay
2.9. Apoptosis Assay
2.10. γ-H2AX Assay
2.11. Statistical Analysis
3. Results
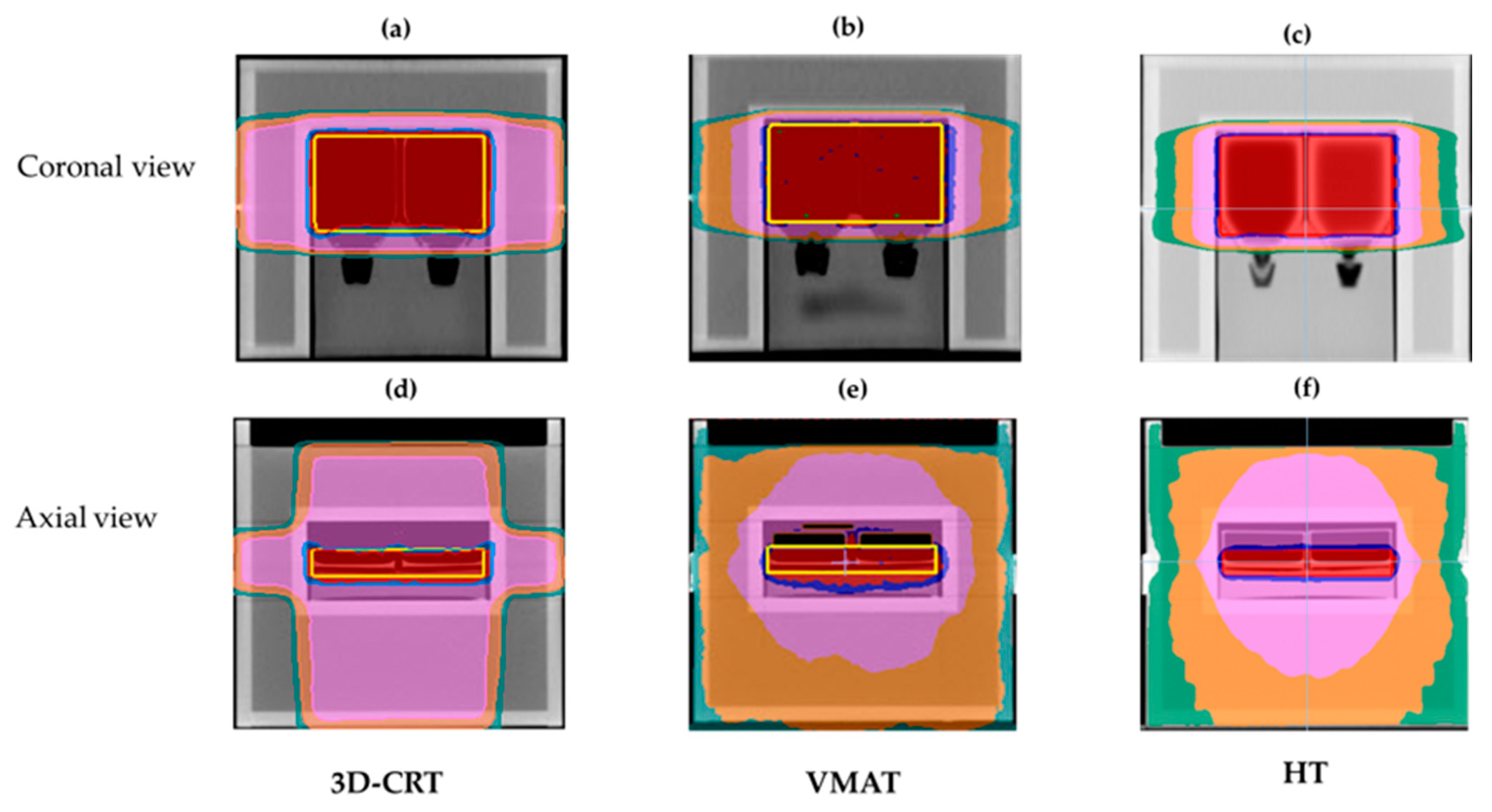
3.1. Dosimetric Results of Treatment Planning
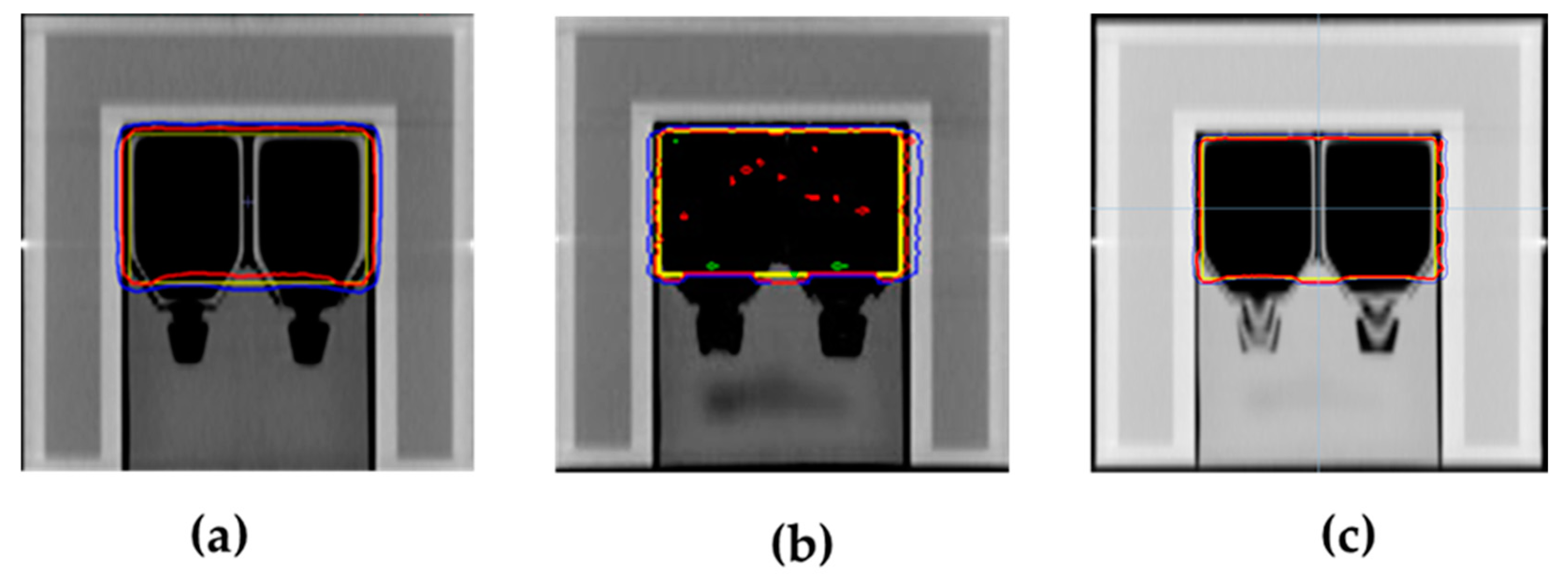
3.2. Verification Results of Dose Delivery
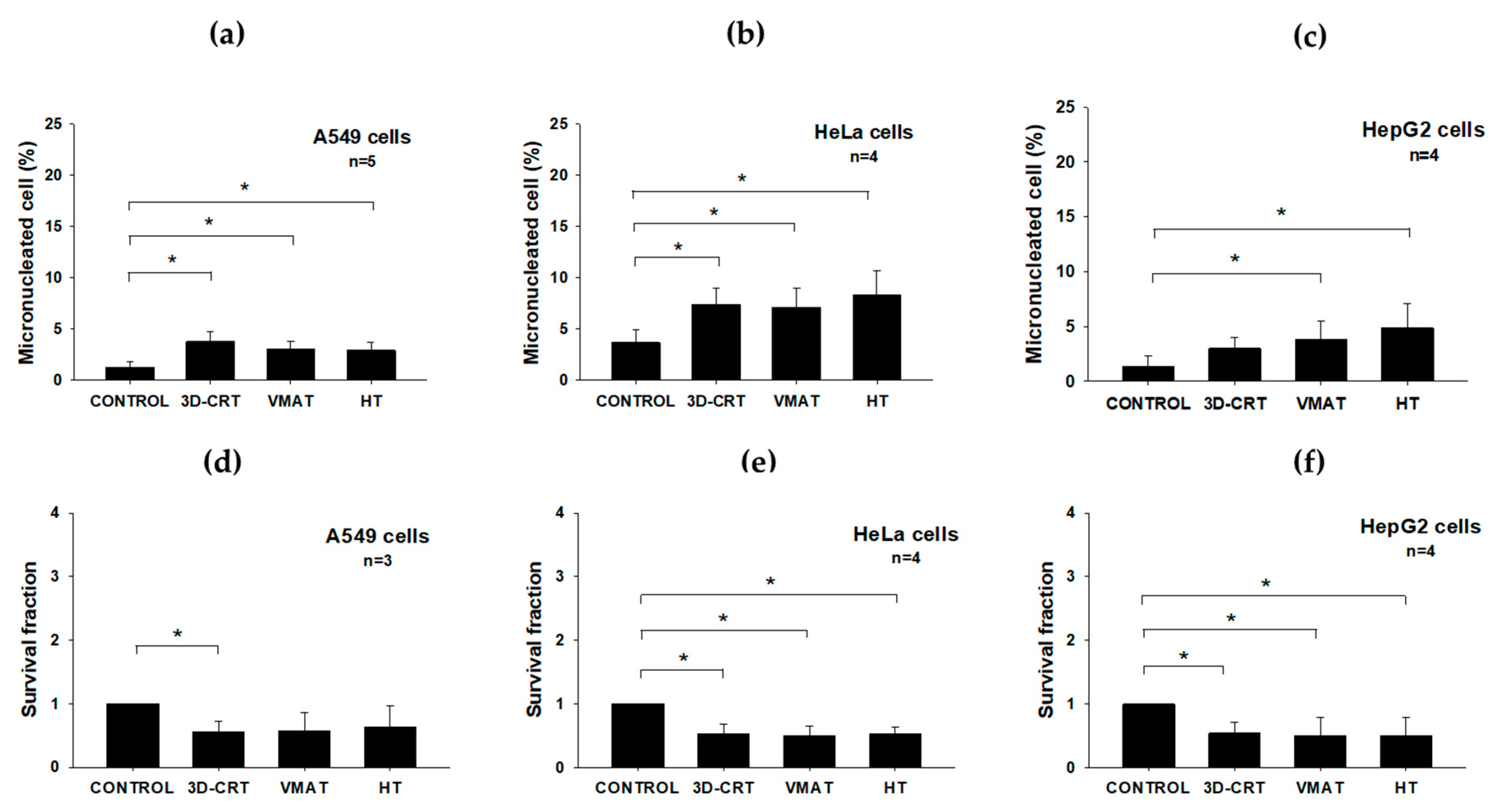
3.3. Radiobiological Impact of Various Radiotherapy Techniques on Three Cancer Cell Lines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAPM | American Association of Physicists in Medicine |
| CCC | Collapsed cone convolution |
| CT | Computed tomography |
| HI | Homogeneity index |
| HT | Helical tomotherapy |
| IC | Ionization chamber |
| ICRU | International Commission on Radiation Units and Measurements |
| MN | Micronucleus |
| MU | Monitor unit |
| OARs | Organs at risk |
| OD | Optical density |
| PBS | Phosphate buffer saline |
| PE | Plating efficiency |
| PSQA | Patient-specific quality assurance |
| PTV | Planning target volume |
| RT | Radiotherapy |
| SF | Survival fraction |
| TPS | Treatment planning system |
| VMAT | Volumetric modulated arc therapy |
| 3D-CRT | Three-dimensional conformal radiotherapy |
References
- Insamran, W.; Sangrajrang, S. National cancer control program of Thailand. Asian Pac. J. Cancer Prev. 2020, 21, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Itahana, K. Radiation therapy and cancer control in developing countries: Can we save more lives? Int. J. Med. Sci. 2017, 14, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Chiavassa, S.; Bessieres, I.; Edouard, M.; Mathot, M.; Moignier, A. Complexity metrics for IMRT and VMAT plans: A review of current literature and applications. Br. J. Radiol. 2019, 92, 20190270. [Google Scholar] [CrossRef]
- Yang, Z.X.; Shen, J.T.; Li, Y.P.; Zhao, K.; Shi, C.H.; Xiao, Y.; Yu, J.J.; Guo, W.D.; Li, C.C.; Wang, Y.Q.; et al. Helical tomotherapy for cancer treatment: A rapid health technology assessment. J. Evid. Based Med. 2014, 79, 192–218. [Google Scholar] [CrossRef]
- Hall, E.J.; Wuu, C.S. Radiation-induced second cancers: The impact of 3D-CRT and IMRT. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 83–88. [Google Scholar] [CrossRef]
- Teoh, M.; Clark, C.H.; Wood, K.; Whitaker, S.; Nisbet, A. Volumetric modulated arc therapy: A review of current literature and clinical use in practice. Br. J. Radiol. 2011, 84, 967–996. [Google Scholar] [CrossRef]
- Lauche, O.; Kirova, Y.M.; Fenoglietto, P.; Costa, E.; Lemanski, C.; Bourgier, C.; Riou, O.; Tiberi, D.; Campana, F.; Fourquet, A.; et al. Helical tomotherapy and volumetric modulated arc therapy: New therapeutic arms in the breast cancer radiotherapy. World J. Radiol. 2016, 8, 735–742. [Google Scholar] [CrossRef]
- Elith, C.; Dempsey, S.E.; Findlay, N.; Warren-Forward, H.M. An introduction to the intensity-modulated radiation therapy (IMRT) techniques, tomotherapy, and VMAT. J. Med. Imaging Radiat. Sci. 2011, 42, 37–43. [Google Scholar] [CrossRef]
- Alongi, F.; Giaj-Levra, N.; Fiorentino, A.; Mazzola, R.; Fersino, S.; Ricchetti, F.; Ruggieri, R. Low-dose bath with volumetric modulated arc therapy in breast cancer: “Much ado about nothing?”. Tumori 2016, 102, 335–336. [Google Scholar] [CrossRef]
- Palumbo, I.; Marcantonini, M.; Scialpi, M.; Bini, V.D.I.; Benedetto, M.; Nucciarelli, S.; Fulcheri, C.; Perrucci, E.; Aristei, C. Heart and coronary artery dose sparing in left-sided breast cancer: 3D-conformal radiotherapy vs. helical tomotherapy. In Vivo 2023, 37, 2760–2767. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.X.; Xu, S.P.; Dai, X.K.; Ju, Z.J.; Gong, H.S.; Xie, C.B.; Yin, L.M.; Yang, J. Clinical dosimetric study of three radiotherapy techniques for postoperative breast cancer: Helical Tomotherapy, IMRT, and 3D-CRT. Technol. Cancer Res. Treat. 2011, 10, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Altinok, P.; Tekçe, E.; Karaköse, F.; Berk, K.; Kalafat, Ü.; Kiziltan, H.S.; Akgün, Z.; Mayadagli, A. Dosimetric comparison of modern radiotherapy techniques for gastric cancer after total gastrectomy. J. Cancer Res. Ther. 2020, 16 (Suppl. S1), S133–S137. [Google Scholar] [CrossRef] [PubMed]
- Myers, P.A.; Mavroidis, P.; Papanikolaou, N.; Stathakis, S. Comparing conformal, arc radiotherapy and helical tomotherapy in craniospinal irradiation planning. J. Appl. Clin. Med. Phys. 2014, 15, 4724. [Google Scholar] [CrossRef]
- Rao, M.; Yang, W.; Chen, F.; Sheng, K.; Ye, J.; Mehta, V.; Shepard, D.; Cao, D. Comparison of Elekta VMAT with helical tomotherapy and fixed field IMRT: Plan quality, delivery efficiency and accuracy. Med. Phys. 2010, 37, 1350–1359. [Google Scholar] [CrossRef]
- Liu, X.; Huang, E.; Wang, Y.; He, Y.; Luo, H.; Zhong, M.; Qiu, D.; Li, C.; Yang, H.; He, G.; et al. Dosimetric comparison of helical tomotherapy, VMAT, fixed-field IMRT and 3D-conformal radiotherapy for stage I-II nasal natural killer T-cell lymphoma. Radiat. Oncol. 2017, 12, 76. [Google Scholar] [CrossRef]
- Göksel, E.O.; Tezcanli, E.; Arifoğlu, A.; Küçücük, H.; Şenkesen, Ö.; Abacıoğlu, U.; Aslay, I.; Şengöz, M. Dosimetric evaluation of VMAT and helical tomotherapy techniques comparing conventional volumes with clinical target volumes based on new ESTRO ACROP post-mastectomy with immediate implant reconstruction contouring guidelines. Radiat. Oncol. 2022, 17, 168. [Google Scholar] [CrossRef]
- Paganetti, H. Changes in tumor cell response due to prolonged dose delivery times in fractionated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 892–900. [Google Scholar] [CrossRef]
- Wang, J.Z.; Li, X.A.; D’Souza, W.D.; Stewart, R.D. Impact of prolonged fraction delivery times on tumor control: A note of caution for intensity-modulated radiation therapy (IMRT). Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 543–552. [Google Scholar] [CrossRef]
- Nikolakopoulou, A.; Peppa, V.; Alexiou, A.; Pissakas, G.; Terzoudi, G.; Karaiskos, P. Comparison and evaluation of different radiotherapy techniques using biodosimetry based on cytogenetics. Cancers 2022, 14, 146. [Google Scholar] [CrossRef]
- International Commission on Radiation Units and Measurements (ICRU). Prescribing, Recording, and Reporting Photon-Beam Intensity-Modulated Radiation Therapy (IMRT); ICRU Report 83; ICRU: Bethesda, MD, USA, 2010. [Google Scholar]
- American Association of Physicists in Medicine (AAPM). Task Group 119: IMRT Commissioning Tests; Report No. 119; AAPM: College Park, MD, USA, 2009. [Google Scholar]
- French, M.; Moeley, A.A. Cytokinesis-block micronucleus method in human lymphocytes: Effect of in vivo aging and low-dose x-irradiation. Mutat. Res. 1986, 161, 193–198. [Google Scholar]
- Prabhu, K.S.; Kuttikrishnan, S.; Ahmad, N.; Habeeba, U.; Mariyam, Z.; Suleman, M.; Bhat, A.A.; Uddin, S. H2AX: A key player in DNA damage response and a promising target for cancer therapy. Biomed. Pharmacother. 2024, 175, 116663. [Google Scholar] [CrossRef] [PubMed]
- Sancar, A.; Lindsey-Boltz, L.A.; Unsal-Kaçmaz, K.; Linn, S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004, 73, 39–85. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Keyomarsi, K. Role of cell cycle in mediating sensitivity to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 928–942. [Google Scholar] [CrossRef]
- Kinhikar, R.A.; Ghadi, Y.G.; Sahoo, P.; Laskar, S.G.; Deshpande, D.D.; Shrivastava, S.K.; Agarwal, J. Dosimetric comparison of three-dimensional conformal radiotherapy, intensity modulated radiotherapy, and helical tomotherapy for lung stereotactic body radiotherapy. J. Med. Phys. 2015, 40, 190–197. [Google Scholar] [CrossRef]
- Joiner, M.C.; Marples, B.; Lambin, P.; Short, S.C.; Turesson, I. Low-dose hypersensitivity: Current status and possible mechanisms. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 379–389. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.; Liu, J.; Gao, S.; Liu, B.; Yan, J. Dosimetric comparison of helical tomotherapy and conventional Linac-based X-knife stereotactic body radiation therapy for primary lung cancer or pulmonary metastases. J. Thorac. Dis. 2018, 10, 999–1006. [Google Scholar] [CrossRef]
- Autsavapromporn, N.; Kobayashi, A.; Liu, C.; Duangya, A.; Oikawa, M.; Tengku Ahmad, T.A.; Konishi, T. Primary and secondary bystander effects of proton microbeam irradiation on human lung cancer cells under hypoxic conditions. Biology 2023, 12, 1485. [Google Scholar] [CrossRef]
- Serrano-Mendioroz, I.; Garate-Soraluze, E.; Rodriguez-Ruiz, M.E. A simple method to assess clonogenic survival of irradiated cancer cells. Methods Cell Biol. 2023, 174, 127–136. [Google Scholar]
- Oktaria, S.; Lerch, M.L.F.; Rosenfeld, A.B.; Tehei, M.; Corde, S. In vitro investigation of the dose-rate effect on the biological effectiveness of megavoltage X-ray radiation doses. Appl. Radiat. Isot. 2017, 28, 114–119. [Google Scholar] [CrossRef]
- Yang, L.; Yang, X.; Tang, Y.; Zhang, D.; Zhu, L.; Wang, S.; Wang, B.; Ma, T. Inhibition of DNA-PK activity sensitizes A549 cells to X-ray irradiation by inducing the ATM-dependent DNA damage response. Mol. Med. Rep. 2018, 17, 7545–7552. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J. The bystander effect. Health Phys. 2003, 85, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Chakraborty, A. Radiation-induced bystander phenomenon: Insight and implications in radiotherapy. Int. J. Radiat. Biol. 2019, 95, 243263. [Google Scholar] [CrossRef] [PubMed]

| Parameter | 3D-CRT | Dual arc VMAT | HT |
|---|---|---|---|
| D95% (Gy) | 1.98 | 2.01 | 2.00 |
| D2% (Gy) | 2.10 | 2.08 | 2.08 |
| Dmean (Gy) | 2.05 | 2.02 | 2.05 |
| HI | 0.08 | 0.35 | 0.02 |
| Beam on Time(s) | 27.3 | 125.8 | 103.2 |
| Monitor Unit (MU) rate | 272.6 | 520.7 | 1782.8 |
| Technique | (%) Dose Difference | |
|---|---|---|
| Point Dose Measurement (IC) | EBT3 Film | |
| 3D-CRT | −1.97 | 0 |
| Dual arc VMAT | 2.49 | 0 |
| HT | 0.67 | 3.1 |
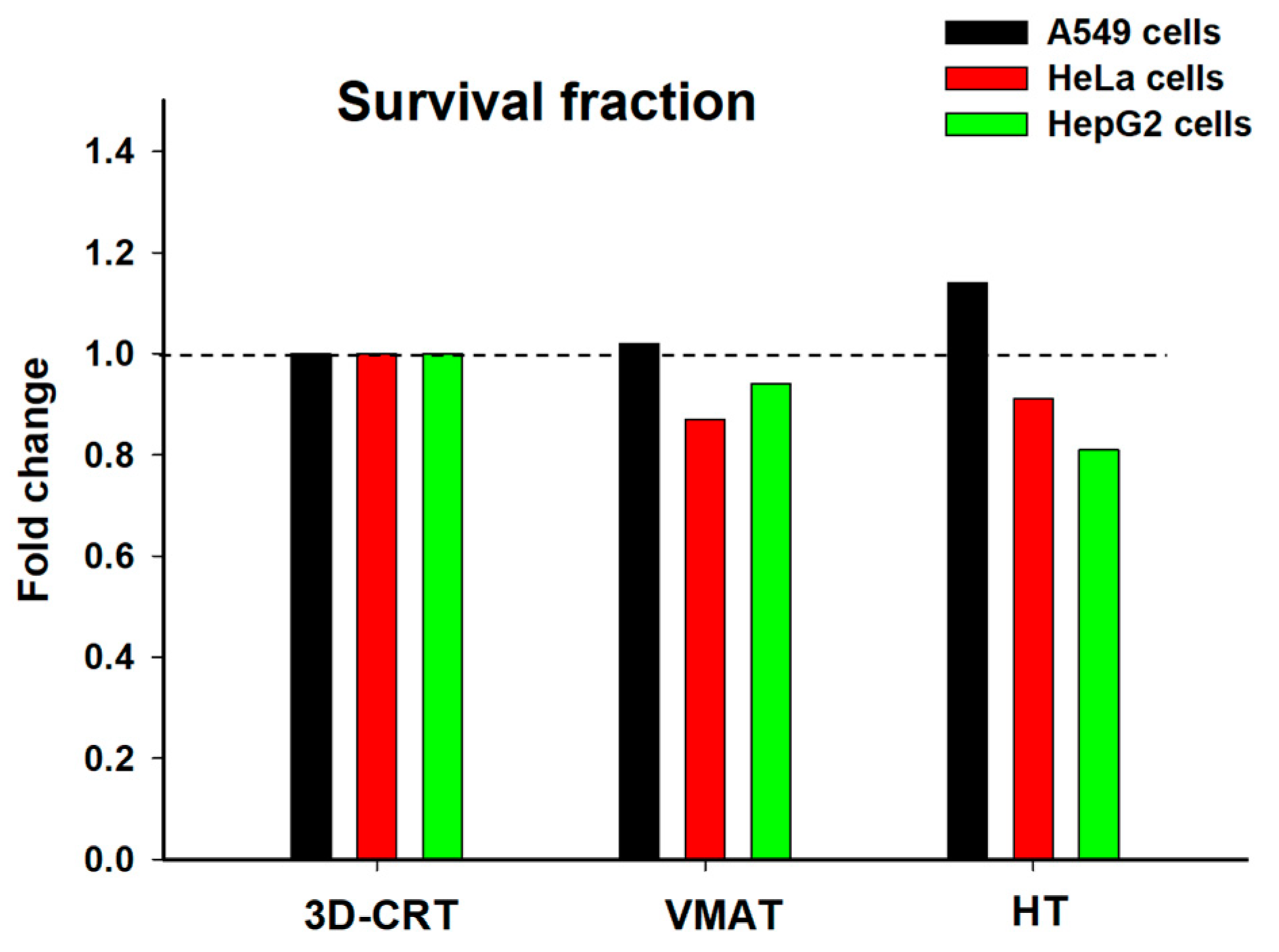
| Cell Line | Technique | % γ-H2AX Foci/Cell | % Apoptosis | % Micronculeated Cell | Survival Fraction |
|---|---|---|---|---|---|
| A549 | Control | 9.37 ± 4.54 | 6.42 ± 2.34 | 1.28 ± 0.53 | 1.00 ± 0.00 |
| 3D-CRT | 26.88 ± 6.08 | 12.91 ± 7.16 | 3.76 ± 0.98 | 0.56 ± 0.17 | |
| VMAT | 24.37 ± 7.54 | 10.72 ± 6.03 | 3.00 ± 0.83 | 0.57 ± 0.30 | |
| HT | 23.94 ± 7.73 | 9.49 ± 5.30 | 2.94 ± 0.77 | 0.64 ± 0.33 | |
| HeLa | Control | 16.86 ± 8.83 | 5.02 ± 1.70 | 3.68 ± 1.29 | 1.00 ± 0.00 |
| 3D-CRT | 28.91 ± 17.03 | 9.04 ± 1.82 | 7.38 ± 1.64 | 0.52 ± 0.16 | |
| VMAT | 23.95 ± 13.92 | 8.19 ± 2.45 | 7.10 ± 1.91 | 0.45 ± 0.11 | |
| HT | 17.42 ± 6.55 | 10.05 ± 3.57 | 8.28 ± 2.36 | 0.48 ± 0.04 | |
| HepG2 | Control | 11.23 ± 3.41 | 8.42 ± 0.14 | 1.40 ± 0.91 | 1.00 ± 0.00 |
| 3D-CRT | 24.92 ± 7.16 | 10.60 ± 3.18 | 3.00 ± 0.99 | 0.54 ± 0.17 | |
| VMAT | 20.17 ± 2.81 | 11.54 ± 3.83 | 3.85 ± 1.66 | 0.51 ± 0.28 | |
| HT | 23.76 ± 11.01 | 12.31 ± 2.44 | 4.85 ± 2.21 | 0.44 ± 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasetthamrongrat, P.; Phumsankhot, R.; Duangya, A.; Watcharawipha, A.; Nobnop, W.; Autsavapromporn, N. Comparison of Tumor Cell Responses to Different Radiotherapy Techniques: Three-Dimensional Conformal Radiotherapy (3D-CRT), Volumetric Modulated Arc Therapy (VMAT), and Helical Tomotherapy (HT). Biology 2025, 14, 529. https://doi.org/10.3390/biology14050529
Kasetthamrongrat P, Phumsankhot R, Duangya A, Watcharawipha A, Nobnop W, Autsavapromporn N. Comparison of Tumor Cell Responses to Different Radiotherapy Techniques: Three-Dimensional Conformal Radiotherapy (3D-CRT), Volumetric Modulated Arc Therapy (VMAT), and Helical Tomotherapy (HT). Biology. 2025; 14(5):529. https://doi.org/10.3390/biology14050529
Chicago/Turabian StyleKasetthamrongrat, Phanwadee, Rinwarat Phumsankhot, Aphidet Duangya, Anirut Watcharawipha, Wannapha Nobnop, and Narongchai Autsavapromporn. 2025. "Comparison of Tumor Cell Responses to Different Radiotherapy Techniques: Three-Dimensional Conformal Radiotherapy (3D-CRT), Volumetric Modulated Arc Therapy (VMAT), and Helical Tomotherapy (HT)" Biology 14, no. 5: 529. https://doi.org/10.3390/biology14050529
APA StyleKasetthamrongrat, P., Phumsankhot, R., Duangya, A., Watcharawipha, A., Nobnop, W., & Autsavapromporn, N. (2025). Comparison of Tumor Cell Responses to Different Radiotherapy Techniques: Three-Dimensional Conformal Radiotherapy (3D-CRT), Volumetric Modulated Arc Therapy (VMAT), and Helical Tomotherapy (HT). Biology, 14(5), 529. https://doi.org/10.3390/biology14050529







