Unsupervised Clustering of Cell Populations in Germinal Centers Using Multiplexed Immunofluorescence
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Material-Ethical Approval
2.2. Tissue Processing and Staining
2.3. Data Acquisition
2.4. Image Alignment and Registration-Cell Segmentation
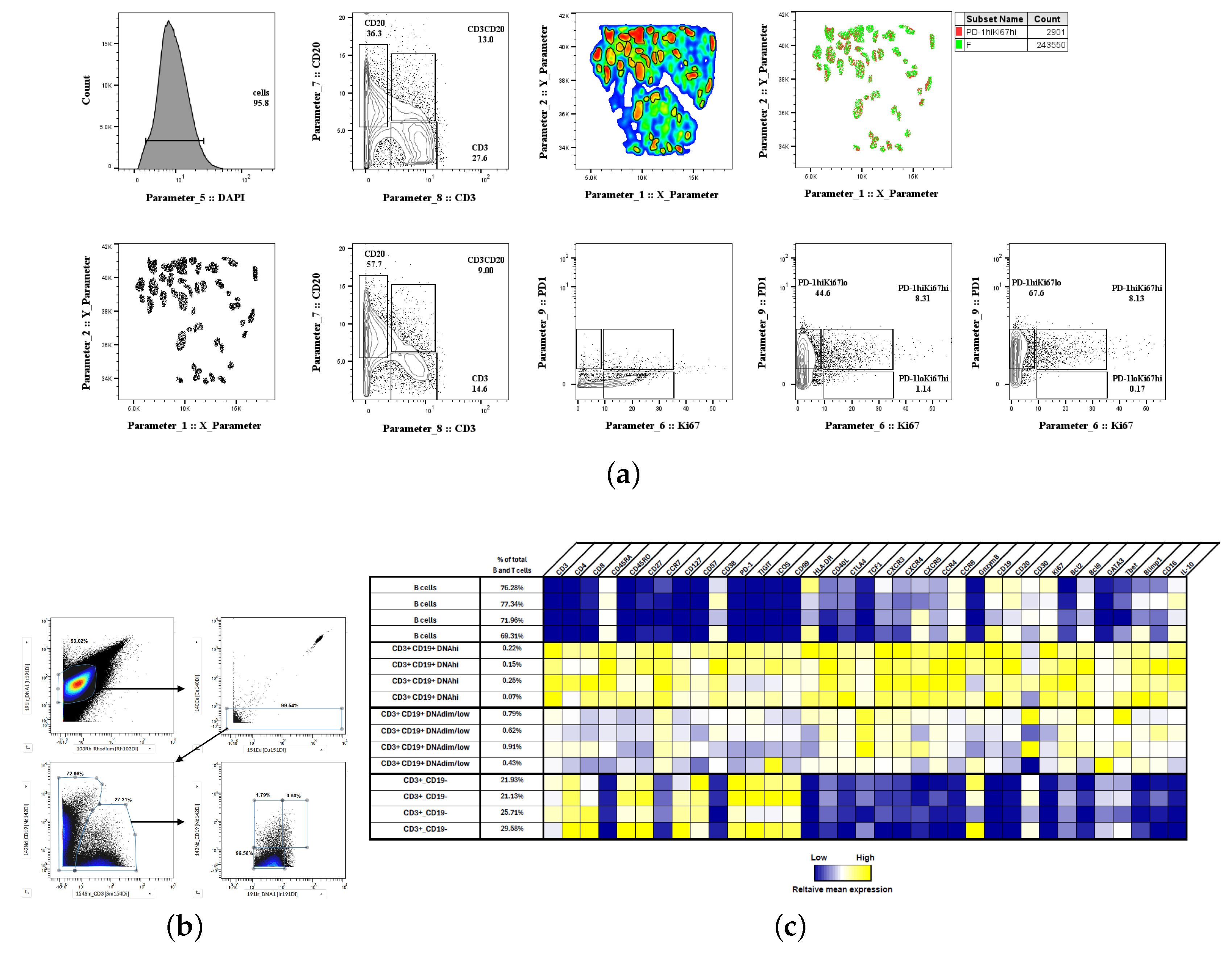
2.5. Histocytometry
2.6. Marker Spillover Correction
2.7. Cell Phenotyping with Mass Cytometry (CyTOF)
2.8. Bioinformatic Analysis (Tonsilar scRNA Data)
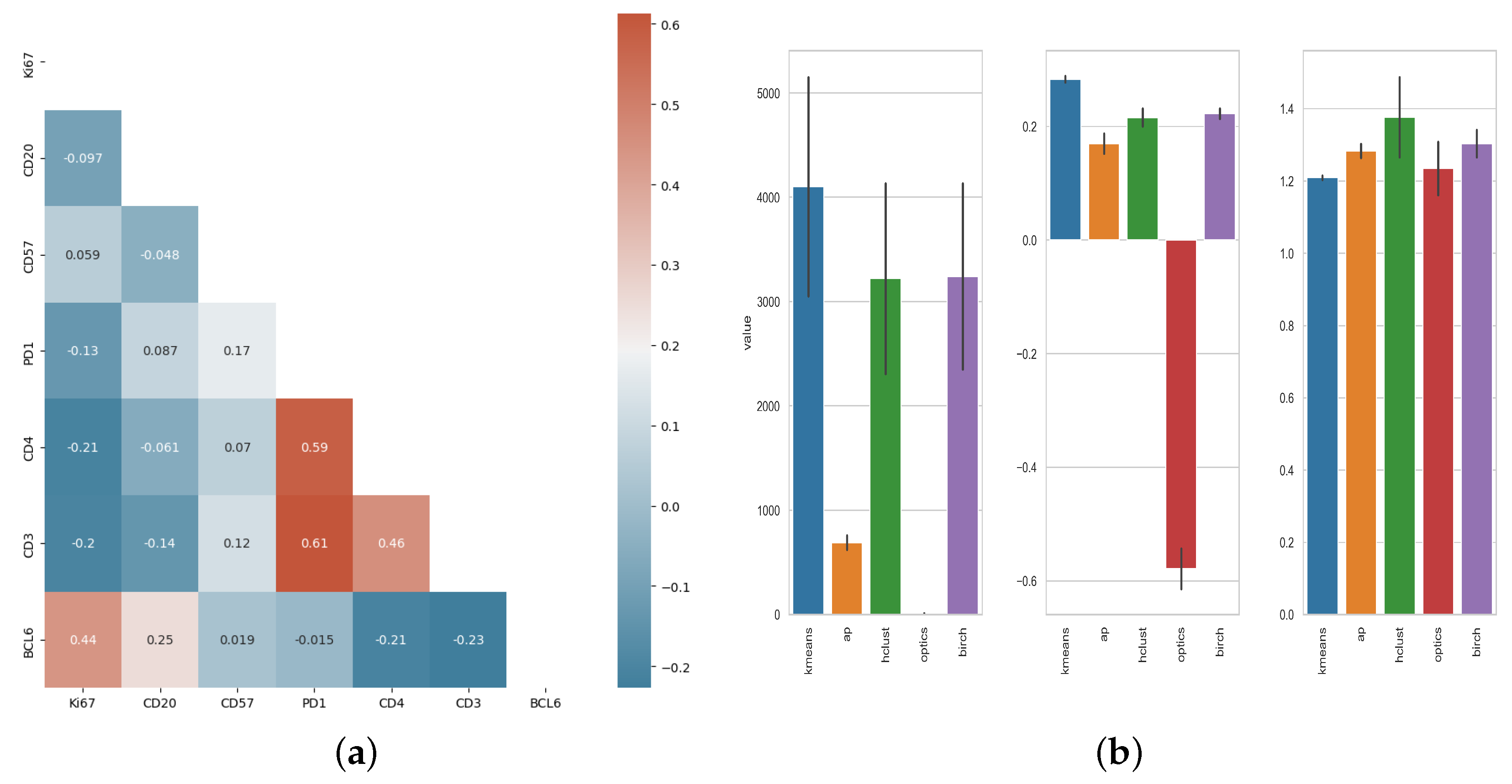
2.9. Statistical Analysis and Cell Population Clustering
3. Results
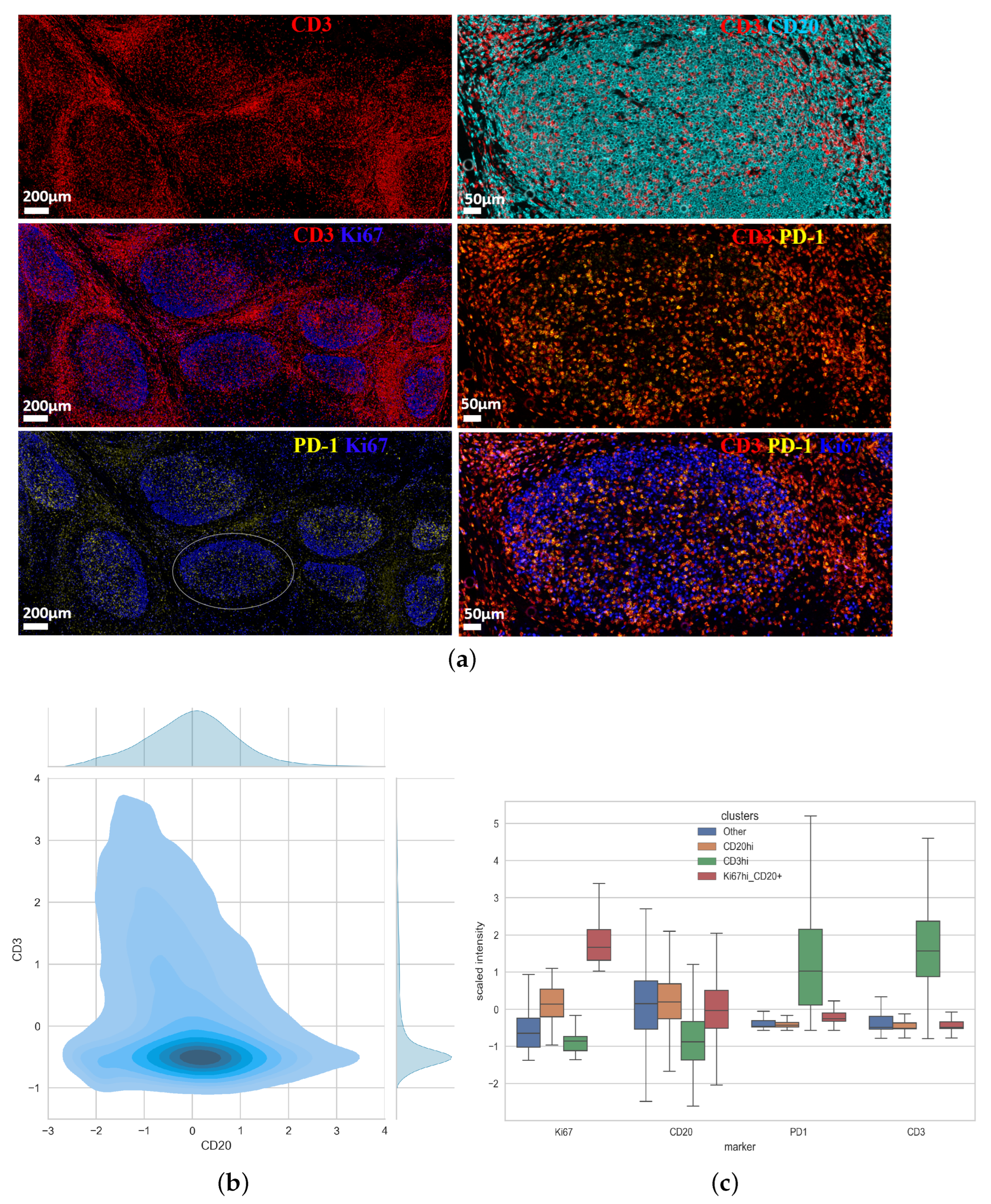
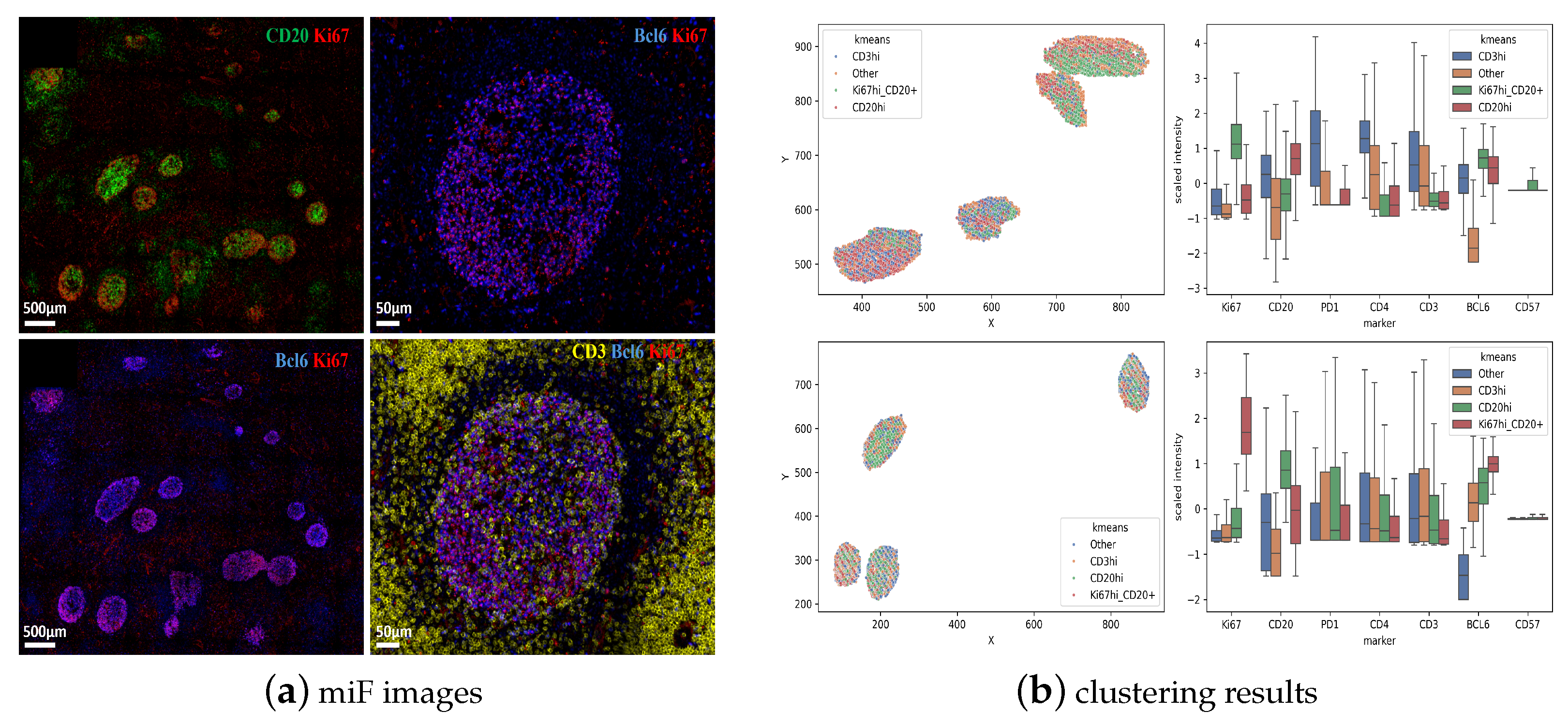
3.1. Unsupervised Clustering Analysis of Human Tonsillar Germinal Center Immune Cell Types
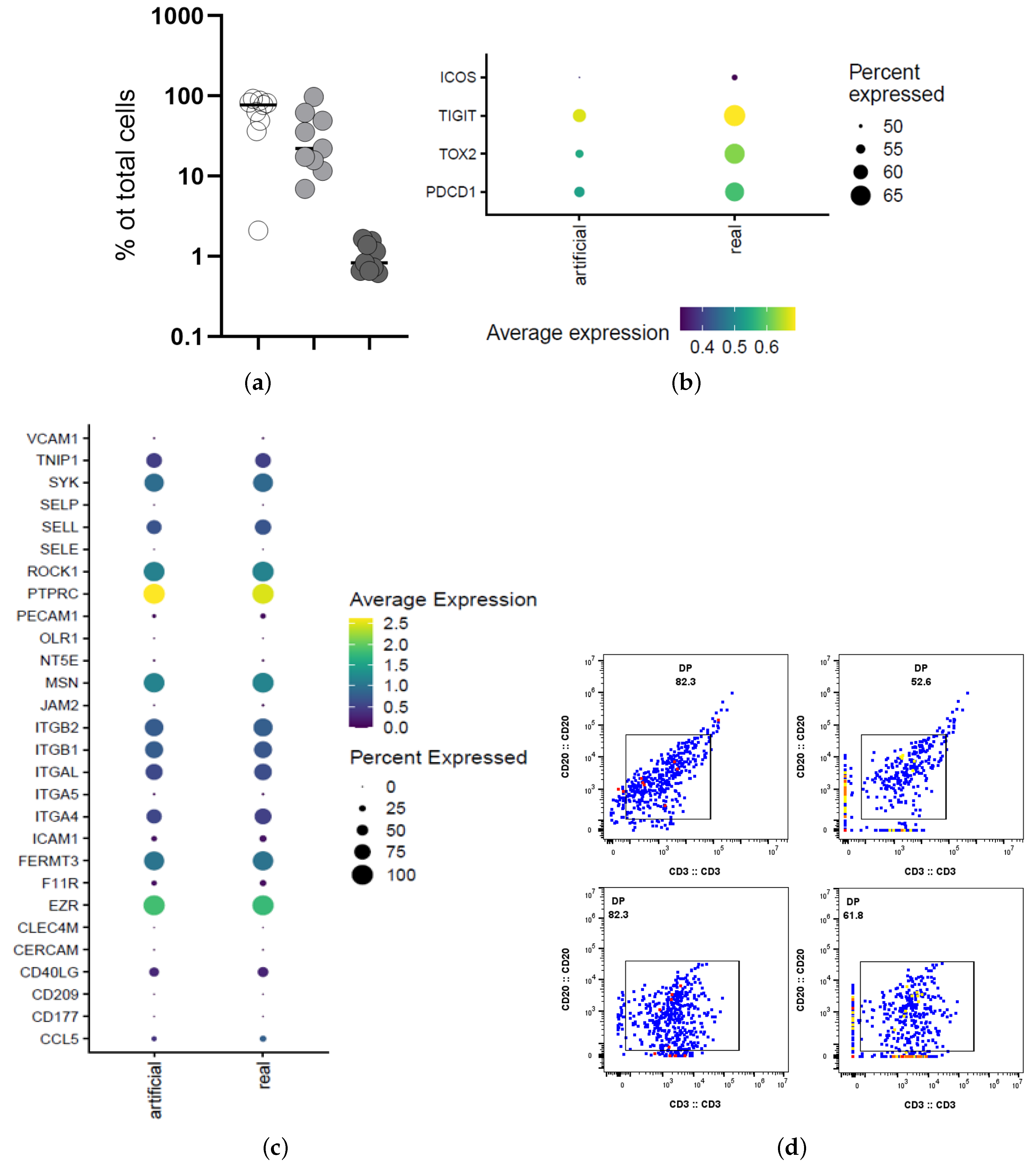
3.2. Characterization of the Follicular CD3/CD20 ‘Conjugates’
3.3. Unsupervised Clustering Analysis of Human Lymph Node Germinal Center Immune Cell Types
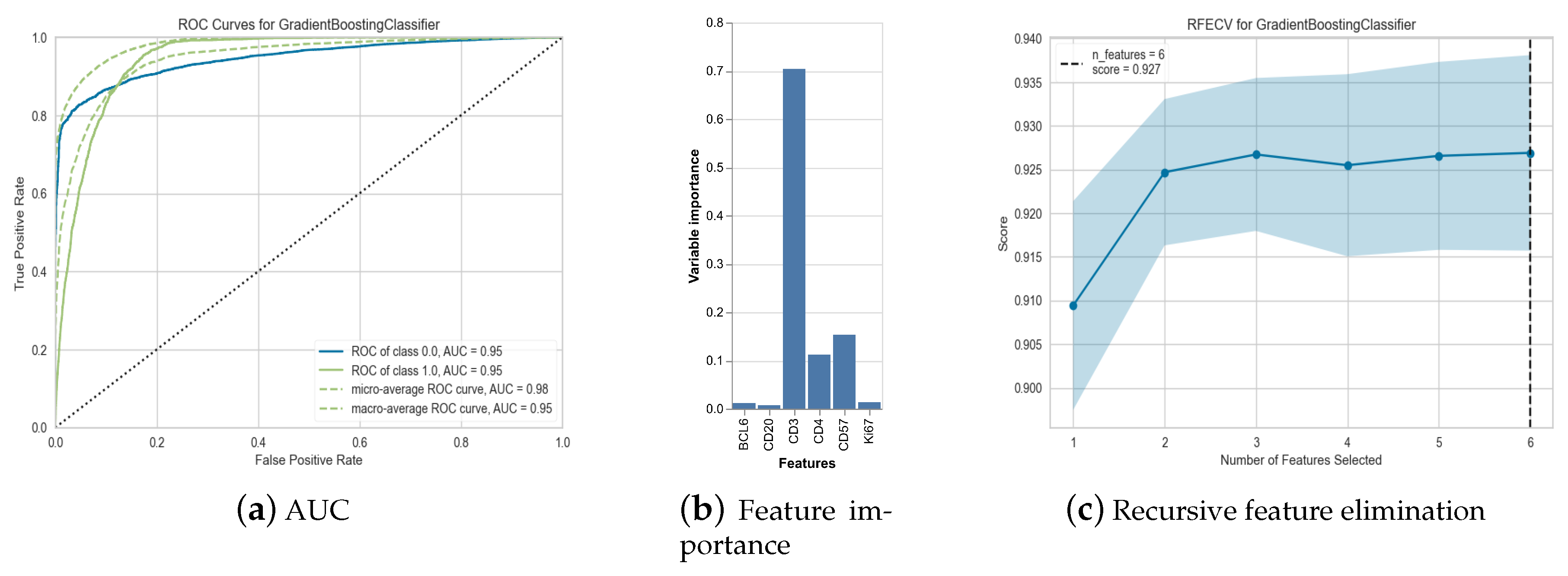
3.4. PD-1 Marker Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| mIF | multiplex immunofluorescence |
| IHC | immunohistochemistry |
| LN | lymph node |
| TS | Tonsil |
| GC | germinal center |
| LZ | Light zone |
| DZ | Dark zone |
| Tfh | T-follicular helper cells |
Appendix A
| Sample | Patient Age | Patient Gender | Localization |
|---|---|---|---|
| LN1 | 28 | Female | inguinal |
| LN2 | 22 | Female | cervical |
Appendix B
| Specificity | Clone | Cat No | Conjugated/Unconjugated | Fluorophore | Cycle |
|---|---|---|---|---|---|
| Cycling confocal mIF (8-plex) | 2 cycles | ||||
| CD3 | OTI3E10 (IgG2b) | TA506064 | Unconjugated | Alexa-546 (Secondary) | 1 |
| CD4 | Polyclonal | FAB8165N-100 | Conjugated | Alexa-700 | 1 |
| CD20 | L26 | Conjugated | eF615 | 1 | |
| Ki67 | B56 | 561281 | Conjugated | V450 | 1 |
| Bcl6 | GI191E/A8 | 760-4241 | Unconjugated | Opal-690 (Secondary) | 2 |
| PD-1 | Polyclonal | FAB7115G | Conjugated | Alexa-488 | 1 |
| CD57 | QA17A04 | 393326 | Conjugated | BV421 | 1, 2 |
| Mouse IgG2b | Polyclonal | A-21143 | Conjugated | Alexa-546 | 1 |
| Mouse IgG (OmniMap) | Polyclonal | 760-4310 | Conjugated | HRP substrate: Opal-690) | 2 |
| SYTO45 | 10297192 | 1, 2 | |||
| Polaris mIF (5-plex) | Order 1-cycle | ||||
| PD-1 | NAT105 | ACI 3137 AK, CK | Unconjugated | Opal 620 | 1 |
| CD3 | OTI3E10 | TA506064 | Unconjugated | Opal 570 | 2 |
| CD20 | L26 | NCL-L-CD20-L26 | Unconjugated | Opal 520 | 3 |
| Ki67 | MIB-1 | M7240 | Unconjugated | Opal 480 | 4 |
| DAPI | 5 |
| Antibody | Metal | Clone | Company | Cat. N. | Amount |
|---|---|---|---|---|---|
| Cell Viability | 103Rh | Cell-ID | Standard Biotools | 201103A | 0.6 L |
| CD8 | 1123ln | RPA-T8 | Biolegend | 301018 | 0.6 L |
| CD4 | 115ln | RPA-T4 | Biolegend | 300515 | 0.6 L |
| CCR6 | 141Pr | 11A49 | Standard Biotools | 3141014A | 1 L |
| CD19 | 142Nd | HIB19 | Standard Biotools | 3142001B | 1.6 L |
| CCR6 | 144Nd | G034E3 | Standard Biotools | 3141003A | 1.88 L |
| CD20 | 147Sm | 2H7 | Standard Biotools | 3147001B | 0.67 L |
| ICOS | 148Nd | C398.4A | Standard Biotools | 3148019B | 1 L |
| CCR4 | 149Sm | L291H4 | Standard Biotools | 3149029A | 1.5 L |
| CD40L | 152Sm | TRAP1 | BD Biosciences | 555698 | 3 L |
| TIGIT | 153Eu | MBSA43 | Standard Biotools | 3153019B | 2 L |
| CD3 | 154SM | UCHT1 | Standard Biotools | 3154003B | 0.5 L |
| CD27 | 155Gd | L128 | Standard Biotools | 3155001B | 1 L |
| CXCR3 | 156Gd | G025H7 | Standard Biotools | 3156004B | 0.8 L |
| CCR7 | 159Td | G043H7 | Standard Biotools | 3159003B | 1 L |
| CD30 | 162Dy | BY88 | Biolegend | 333902 | 3 L |
| CXCR5 | 164Dy | RF8B2 | Standard Biotools | 3164029B | 1.2 L |
| CD45RO | 165Ho | UCHL1 | Standard Biotools | 3165011B | 0.7 L |
| CD38 | 167Er | HIT2 | Standard Biotools | 3167001B | 0.6 L |
| CD45RA | 170Er | HI100 | Standard Biotools | 3171001B | 1.6 L |
| HLA-DR | 174Yb | L243 | Standard Biotools | 3174001B | 0.7 L |
| CD279/PD-1 | 175Lu | EH12.2H7 | Standard Biotools | 3175008B | 1 L |
| CD127 | 176Yb | A019D5 | Standard Biotools | 3176004B | 0.7 L |
| CTLA4 | 174Pt | Ipilimumab | BMS | ACB3065 | 2.5 L |
| CD57 | 198Pt | NK-1 | BD Biosciences | 555618 | 1 L |
| CD16 | 209Bi | 3G8 | Standard Biotools | 3209002B | 1.5 L |
| Anti-PE | 145Nd | PE001 | Standard Biotools | 3145006B | 2 L |
| GATA-3 | 146Nd | TWAJ | ThermoFisher | 14-9966-82 | 1 L |
| Tbet | 161Dy | 4B10 | Standard Biotools | 3161015B | 2 L |
| Bcl6 | 163Dy | K112-91 | Standard Biotools | 3163012B | 2 L |
| Ki67 | 168Tm | Ki-67 | Standard Biotools | 3168001B | 1.4 L |
| Blimp1 | 169Tm | 646702 | Bio-techne | MAB36081 | 3 L |
| Granzyme B | 171Yb | GB11 | Standard Biotools | 3171002B | 2 L |
| Bcl2 | 173Yb | 100 | Biolegend | 658702 | 2 L |
References
- Tan, W.C.C.; Nerurkar, S.N.; Cai, H.Y.; Ng, H.H.M.; Wu, D.; Wee, Y.T.F.; Lim, J.C.T.; Yeong, J.; Lim, T.K.H. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun. 2020, 40, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Kuswanto, W.; Nolan, G.; Lu, G. Highly multiplexed spatial profiling with CODEX: Bioinformatic analysis and application in human disease. Semin. Immunopathol. 2022, 45, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Harms, P.W.; Frankel, T.L.; Moutafi, M.; Rao, A.; Rimm, D.L.; Taube, J.M.; Thomas, D.; Chan, M.P.; Pantanowitz, L. Multiplex Immunohistochemistry and Immunofluorescence: A Practical Update for Pathologists. Mod. Pathol. 2023, 36, 100197. [Google Scholar] [CrossRef]
- Hickey, J.W.; Tan, Y.; Nolan, G.P.; Goltsev, Y. Strategies for Accurate Cell Type Identification in CODEX Multiplexed Imaging Data. Front. Immunol. 2021, 12, 727626. [Google Scholar] [CrossRef] [PubMed]
- Lacinski, R.A.; Dziadowicz, S.A.; Melemai, V.K.; Fitzpatrick, B.; Pisquiy, J.J.; Heim, T.; Lohse, I.; Schoedel, K.E.; Llosa, N.J.; Weiss, K.R.; et al. Spatial multiplexed immunofluorescence analysis reveals coordinated cellular networks associated with overall survival in metastatic osteosarcoma. Bone Res. 2024, 12, 55. [Google Scholar] [CrossRef]
- Cyster, J.G.; Allen, C.D.C. B Cell Responses: Cell Interaction Dynamics and Decisions. Cell 2019, 177, 524–540. [Google Scholar] [CrossRef]
- Choi, J.; Crotty, S.; Choi, Y.S. Cytokines in Follicular Helper T Cell Biology in Physiologic and Pathologic Conditions. Immune Network 2024, 24, e8. [Google Scholar] [CrossRef]
- Shane, C. Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar]
- Küppers, R. Mechanisms of B-cell lymphoma pathogenesis. Nat. Rev. Cancer 2005, 5, 251–262. [Google Scholar] [CrossRef]
- Estes, J.D.; Kityo, C.; Ssali, F.; Swainson, L.; Makamdop, K.N.; Prete, G.Q.D.; Deeks, S.G.; Luciw, P.A.; Chipman, J.G.; Beilman, G.J.; et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nat. Med. 2017, 23, 1271–1276. [Google Scholar] [CrossRef]
- Radtke, A.J.; Kandov, E.; Lowekamp, B.; Speranza, E.; Chu, C.J.; Gola, A.; Thakur, N.; Shih, R.; Yao, L.; Yaniv, Z.R.; et al. IBEX: A versatile multiplex optical imaging approach for deep phenotyping and spatial analysis of cells in complex tissues. Proc. Natl. Acad. Sci. USA 2020, 117, 33455–33465. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, S.; Ioannidou, K.; Mora, B.B.; Orfanakis, M.; Brenna, C.; Muller, Y.D.; Estrada, P.M.D.R.; Sharma, A.A.; Pantaleo, G.; de Leval, L.; et al. Cellular and molecular determinants mediating the dysregulated germinal center immune dynamics in systemic lupus erythematosus. Front. Immunol. 2025, 16, 1530327. [Google Scholar] [CrossRef] [PubMed]
- Moysi, E.; Estrada, P.M.D.R.; Torres-Ruiz, F.; Reyes-Terán, G.; Koup, R.A.; Petrovas, C. In Situ Characterization of Human Lymphoid Tissue Immune Cells by Multispectral Confocal Imaging and Quantitative Image Analysis; Implications for HIV Reservoir Characterization. Front. Immunol. 2021, 12, 683396. [Google Scholar] [CrossRef]
- Gerner, M.Y.; Kastenmuller, W.; Ifrim, I.; Kabat, J.; Germain, R.N. Histo-cytometry: A method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 2012, 37, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhu, B.; Rovira-Clave, X.; Chen, H.; Markovic, M.; Chan, C.N.; Su, T.H.; McIlwain, D.R.; Estes, J.D.; Keren, L.; et al. Adjacent Cell Marker Lateral Spillover Compensation and Reinforcement for Multiplexed Images. Front. Immunol. 2021, 12, 652631. [Google Scholar] [CrossRef]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. ilastik: Interactive machine learning for (bio)image analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef]
- Massoni-Badosa, R.; Aguilar-Fernández, S.; Nieto, J.C.; Soler-Vila, P.; Elosua-Bayes, M.; Marchese, D.; Kulis, M.; Vilas-Zornoza, A.; Bühler, M.M.; Rashmi, S.; et al. An atlas of cells in the human tonsil. Immunity 2024, 57, 379–399. [Google Scholar] [CrossRef]
- Germain, P.L.; Lun, A.; Meixide, C.G.; Macnair, W.; Robinson, M.D. Doublet identification in single-cell sequencing data using scDblFinder. f1000research 2022, 10, 979. [Google Scholar] [CrossRef]
- Hao, Y.; Stuart, T.; Kowalski, M.H.; Choudhary, S.; Hoffman, P.; Hartman, A.; Srivastava, A.; Molla, G.; Madad, S.; Fernandez-Granda, C.; et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 2024, 42, 293–304. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Ali, M. PyCaret: An Open Source, Low-Code Machine Learning Library in Python; Version 1.0.0; PyCaret: Online, 2020; Available online: https://www.pycaret.org (accessed on 2 May 2025).
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M.L. seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Satyanarayan, A.; Moritz, D.; Wongsuphasawat, K.; Heer, J. Vega-Lite: A Grammar of Interactive Graphics. IEEE Trans. Vis. Comput. Graph. 2017, 23, 341–350. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.I. A Unified Approach to Interpreting Model Predictions. In Advances in Neural Information Processing Systems 30 (NeurIPS 2017); Guyon, I., Luxburg, U.V., Bengio, S., Wallach, H., Fergus, R., Vishwanathan, S., Garnett, R., Eds.; Curran Associates, Inc.: Red Hook, NY, USA, 2017; pp. 4765–4774. [Google Scholar]
- Orfanakis, M.; Molyvdas, A.; Petrovas, C. In Situ Characterization of Human Follicular Helper CD4 T Cells. In Intracellular Pathogens: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2024; pp. 281–293. [Google Scholar]
- Kamphorst, A.O.; Ahmed, R. Manipulating the PD-1 pathway to improve immunity. Curr. Opin. Immunol. 2013, 25, 381–388. [Google Scholar] [CrossRef]
- Yu, D.; Vinuesa, C.G. The elusive identity of T follicular helper cells. Trends Immunol. 2010, 31, 377–383. [Google Scholar] [CrossRef]
- Mesin, L.; Ersching, J.; Victora, G.D. Germinal Center B Cell Dynamics. Immunity 2016, 45, 471–482. [Google Scholar] [CrossRef]
- Li, W.; Germain, R.N.; Gerner, M.Y. High-dimensional cell-level analysis of tissues with Ce3D multiplex volume imaging. Nat. Protoc. 2019, 14, 1708–1733. [Google Scholar] [CrossRef]
- Wang, V.G.; Liu, Z.; Martinek, J.; Foroughi Pour, A.; Zhou, J.; Boruchov, H.; Ray, K.; Palucka, K.; Chuang, J.H. Computational immune synapse analysis reveals T-cell interactions in distinct tumor microenvironments. Commun. Biol. 2024, 7, 1201. [Google Scholar] [CrossRef]
- Padhan, K.; Moysi, E.; Noto, A.; Chassiakos, A.; Ghneim, K.; Perra, M.M.; Shah, S.; Papaioannou, V.; Fabozzi, G.; Ambrozak, D.R.; et al. Acquisition of optimal TFH cell function is defined by specific molecular, positional, and TCR dynamic signatures. Proc. Natl. Acad. Sci. USA 2021, 118, e2016855118. [Google Scholar] [CrossRef]
- Samer, S.; Chowdhury, A.; Salinas, T.R.W.; Estrada, P.M.D.R.; Reuter, M.; Tharp, G.; Bosinger, S.; Cervasi, B.; Auger, J.; Gill, K.; et al. Lymph-Node-Based CD3+ CD20+ Cells Emerge from Membrane Exchange between T Follicular Helper Cells and B Cells and Increase Their Frequency following Simian Immunodeficiency Virus Infection. J. Virol. 2023, 97, e0176022. [Google Scholar] [CrossRef]
- Allen, C.D.C.; Okada, T.; Tang, H.L.; Cyster, J.G. Imaging of Germinal Center Selection Events during Affinity Maturation. Science 2007, 315, 528–531. [Google Scholar] [CrossRef]
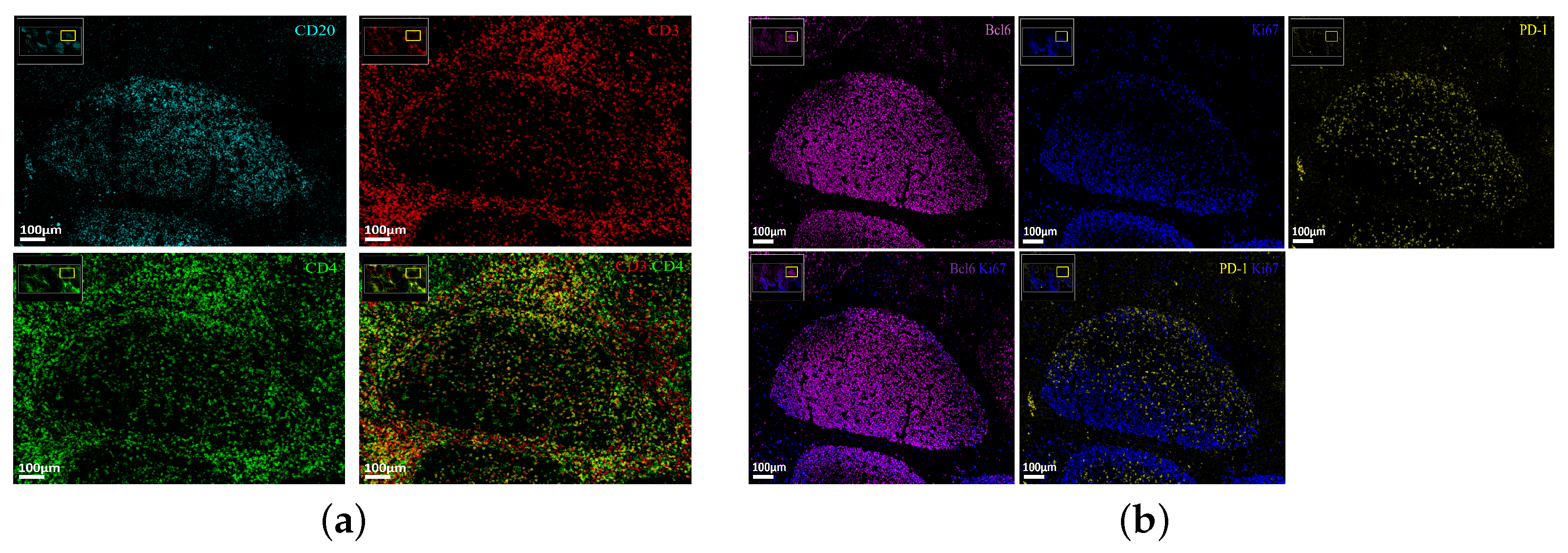
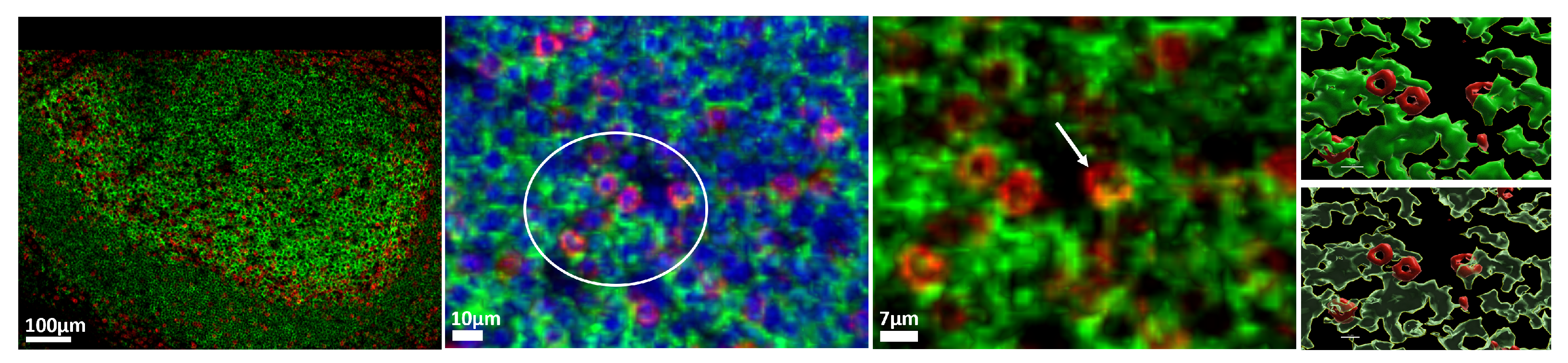
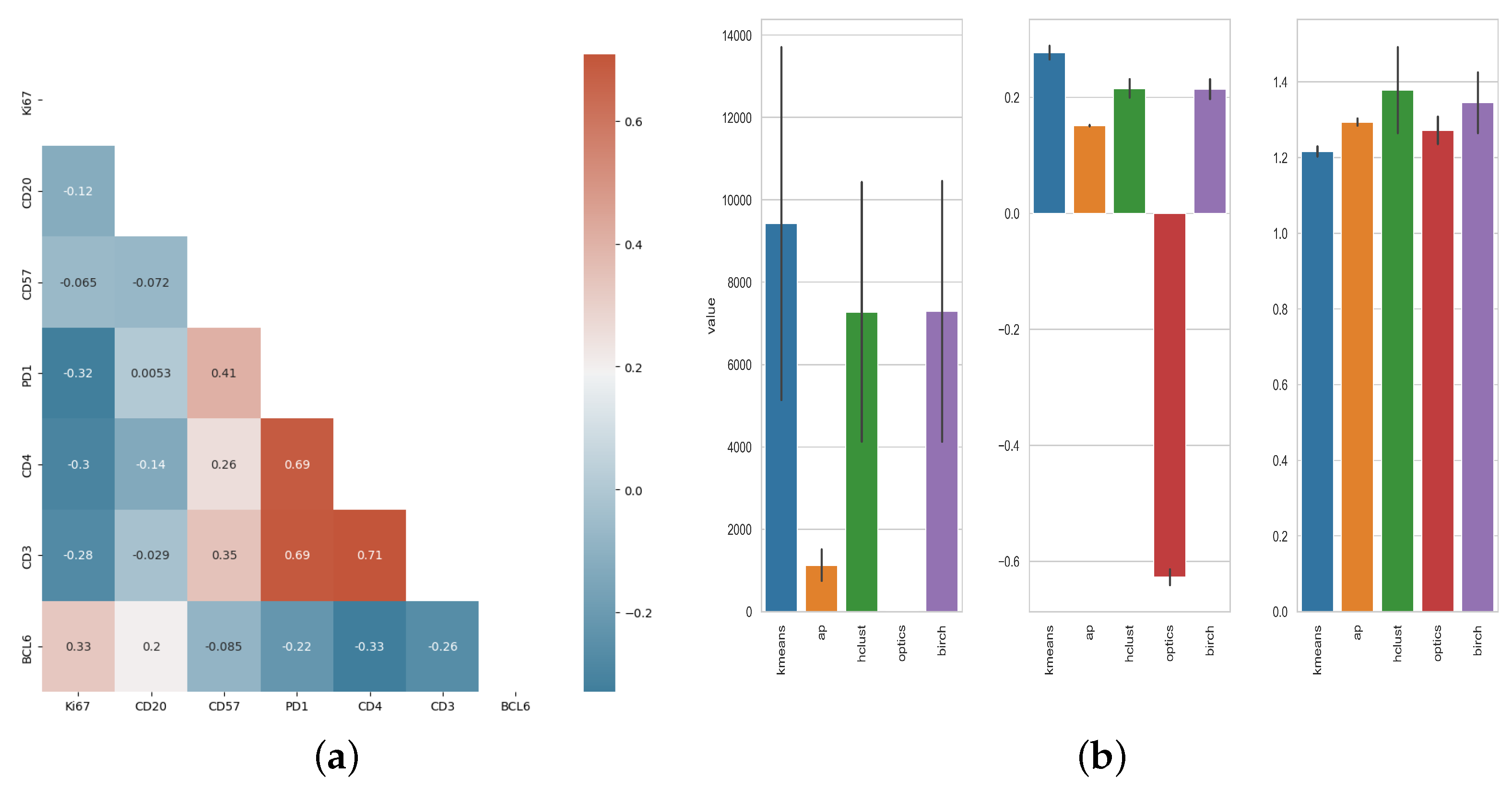
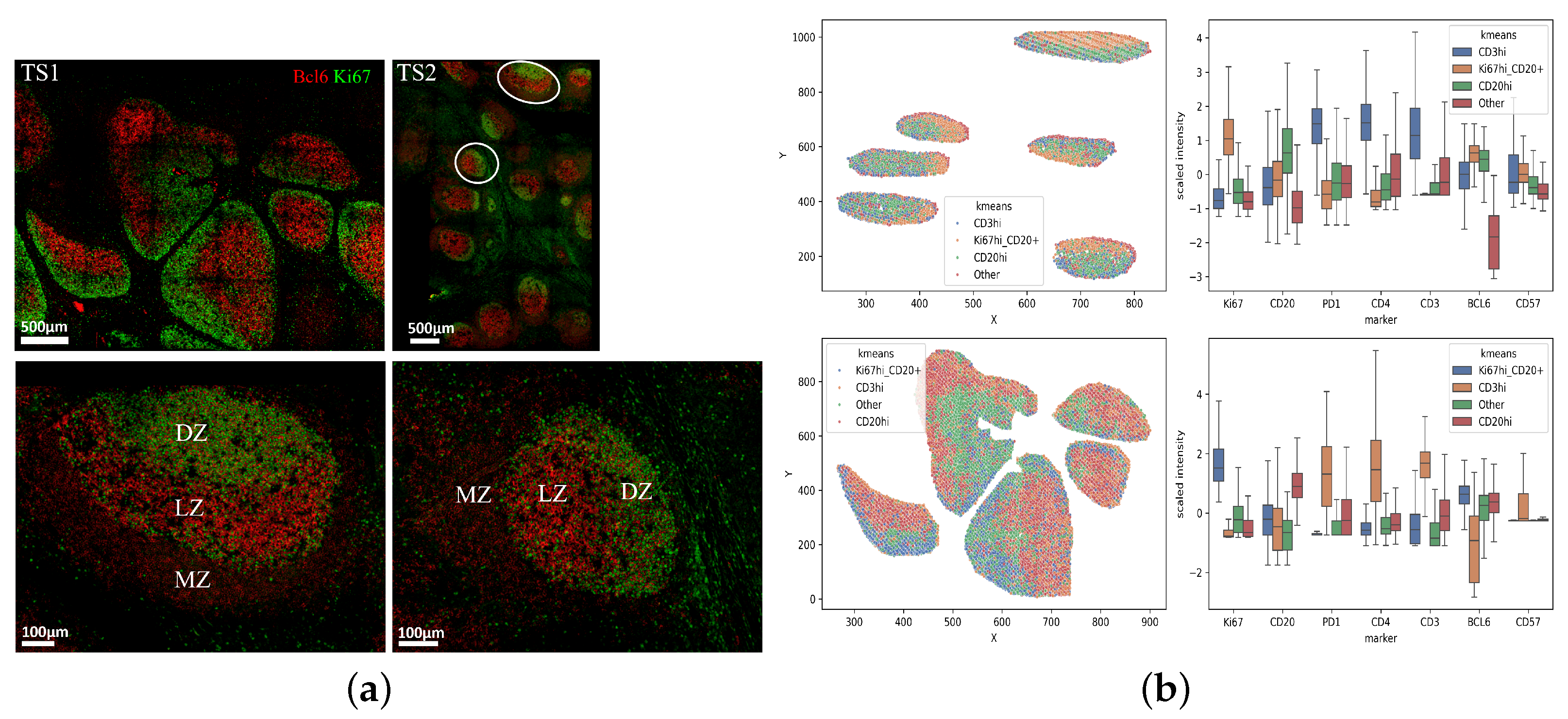
| Sample | Num. GC 1 | Clusters | Number of Cells |
|---|---|---|---|
| TS1 | 6 | Ki67highCD20+ | 3841 |
| CD20high | 3662 | ||
| CD3high | 2239 | ||
| Other | 1756 | ||
| TS2 | 5 | CD20high | 13,419 |
| Other | 10,244 | ||
| Ki67highCD20+ | 6831 | ||
| CD3high | 6545 |
| Sample | Num. GC 1 | Clusters | Number of Cells |
|---|---|---|---|
| LN1 | 4 | CD20high | 2260 |
| Ki67highCD20+ | 1943 | ||
| CD3high | 1347 | ||
| LN2 | 4 | CD20high | 2395 |
| Other | 1660 | ||
| CD3high | 1519 | ||
| Ki67highCD20+ | 1153 |
| Model | Accuracy | AUC | Recall | Prec. |
|---|---|---|---|---|
| Gradient Boosting Classifier | 0.9269 | 0.9467 | 0.5297 | 0.6873 |
| Light Gradient Boosting Machine | 0.9263 | 0.9456 | 0.5251 | 0.6843 |
| Ada Boost Classifier | 0.9242 | 0.9425 | 0.5070 | 0.6787 |
| Extreme Gradient Boosting | 0.9236 | 0.9410 | 0.5157 | 0.6622 |
| Extra Trees Classifier | 0.9264 | 0.9405 | 0.4923 | 0.6991 |
| Ridge Classifier | 0.9208 | 0.9403 | 0.3400 | 0.7293 |
| Linear Discriminant Analysis | 0.9224 | 0.9403 | 0.5828 | 0.6307 |
| SVM-Linear Kernel | 0.9218 | 0.9395 | 0.4521 | 0.6958 |
| Logistic Regression | 0.9244 | 0.9394 | 0.4816 | 0.6825 |
| Random Forest Classifier | 0.9258 | 0.9390 | 0.4999 | 0.6919 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgermeister, S.; Orfanakis, M.; Georgakis, S.; Brenna, C.; Lindsay, H.; Fenwick, C.; Pantaleo, G.; Gottardo, R.; Petrovas, C. Unsupervised Clustering of Cell Populations in Germinal Centers Using Multiplexed Immunofluorescence. Biology 2025, 14, 530. https://doi.org/10.3390/biology14050530
Burgermeister S, Orfanakis M, Georgakis S, Brenna C, Lindsay H, Fenwick C, Pantaleo G, Gottardo R, Petrovas C. Unsupervised Clustering of Cell Populations in Germinal Centers Using Multiplexed Immunofluorescence. Biology. 2025; 14(5):530. https://doi.org/10.3390/biology14050530
Chicago/Turabian StyleBurgermeister, Simon, Michail Orfanakis, Spiros Georgakis, Cloe Brenna, Helen Lindsay, Craig Fenwick, Giuseppe Pantaleo, Raphael Gottardo, and Constantinos Petrovas. 2025. "Unsupervised Clustering of Cell Populations in Germinal Centers Using Multiplexed Immunofluorescence" Biology 14, no. 5: 530. https://doi.org/10.3390/biology14050530
APA StyleBurgermeister, S., Orfanakis, M., Georgakis, S., Brenna, C., Lindsay, H., Fenwick, C., Pantaleo, G., Gottardo, R., & Petrovas, C. (2025). Unsupervised Clustering of Cell Populations in Germinal Centers Using Multiplexed Immunofluorescence. Biology, 14(5), 530. https://doi.org/10.3390/biology14050530







