Inhibitory Efficacy of Arthrospira platensis Extracts on Skin Pathogenic Bacteria and Skin Cancer Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Chemicals and Reagents
2.3. A. platensis Sample and Extraction
2.4. Evaluation of the Antioxidant Activities and Active Compounds
2.4.1. DPPH Radical Scavenging Assay
2.4.2. ABTS Radical Cation Decolorization Assay
2.4.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.4.4. Total Phenolic Compound Content
2.4.5. Total Flavonoid Compound Content
2.4.6. High Performance Liquid Chromatography Analysis
2.5. Antibacterial Activities
2.5.1. Bacterial Culture
2.5.2. Agar Well Diffusion Method
2.5.3. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.5.4. Inhibition of Biofilm Formation and Biofilm Eradication
2.6. Anticancer Activity
2.6.1. Cell Culture
2.6.2. Cytotoxicity on A375 Cells
2.6.3. Apoptosis on A375 Cells
2.6.4. Apoptotic Gene Expression on A375 Cells Using Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
2.7. Statistical Analysis
3. Results
3.1. Antioxidant Activities, Total Phenolic and Flavonoid Compounds of A. platensis Extracts
3.2. Identification and Quantification of Bioactive Compounds in A. platensis Extracts
3.3. Antibacterial Activities of A. platensis Extracts
3.4. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of A. platensis Extracts
3.5. Inhibition of Biofilm Formation and Biofilm Eradication of A. platensis Extracts
3.6. Cytotoxicity of A. platensis Extracts in A375 Cells
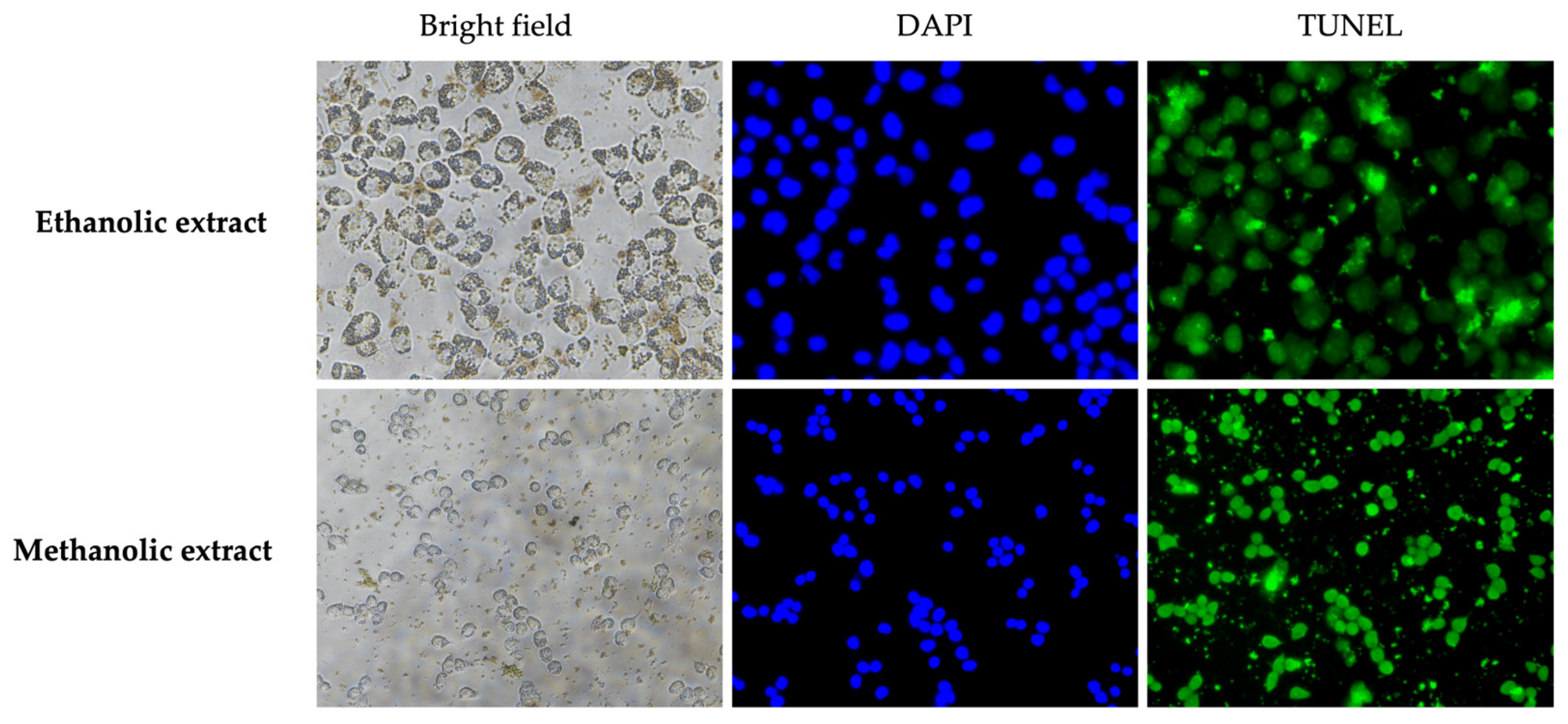
3.7. Effect of A. platensis Extracts on Apoptosis in A375 Cells
3.8. Effect of A. platensis Extracts on Apoptotic Gene Expression in A375 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2, 2-azinobis (3- ethylbenzothiazoline-6 -sulfonic acid) |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DPPH | 2, 2-diphenyl-1-picrylhydrazyl |
| FRAP | Ferric reducing antioxidant power |
| GAE | Gallic acid equivalent |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| HPLC | High-performance liquid chromatography |
| MRSA | Methicillin-resistant S. aureus |
| MTT | 3-(4,5-dimethylthizaol-2-yl)-2,5-diphenyl tetrazolium bromide |
| QE | Quercetin equivalent |
| RT-qPCR | Quantitative reverse transcription polymerase chain reaction |
| TEAC | Trolox equivalent antioxidant capacity |
| TPTZ | 2,4,6-tri(2-pyridyl)-s-triazine |
| Trolox | 6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick-end labeling |
References
- Hofmann, E.; Schwarz, A.; Fink, J.; Kamolz, L.-P.; Kotzbeck, P. Modelling the complexity of human skin in vitro. Biomedicines 2023, 11, 794. [Google Scholar] [CrossRef] [PubMed]
- Honari, G.; Maibach, H. Chapter 1—Skin structure and function. In Applied Dermatotoxicology; Honari, G., Maibach, H., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 1–10. [Google Scholar]
- Oh, J.; Conlan, S.; Polley, E.C.; Segre, J.A.; Kong, H.H. Shifts in human skin and nares microbiota of healthy children and adults. Genome Med. 2012, 4, 77. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.R.; James, W.D. Microbial ecology of the skin. Annu. Rev. Microbiol. 1988, 42, 441–464. [Google Scholar] [CrossRef] [PubMed]
- Giesey, R.L.; Mehrmal, S.; Uppal, P.; Delost, G. The global burden of skin and subcutaneous disease: A longitudinal analysis from the global burden of disease study from 1990–2017. SKIN J. Cutan. Med. 2021, 5, 125–136. [Google Scholar] [CrossRef]
- Linares, M.A.; Zakaria, A.; Nizran, P. Skin cancer. Prim. Care Clin. Off. Pract. 2015, 42, 645–659. [Google Scholar] [CrossRef]
- Rogers, H.W.; Weinstock, M.A.; Feldman, S.R.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef]
- National Cancer Institute. SEER Stat Fact Sheets: Melanoma of the Skin. Available online: http://seer.cancer.gov/statfacts/html/melan.html (accessed on 20 December 2024).
- Duthie, G.G.; Duthie, S.J.; Kyle, J.A. Plant polyphenols in cancer and heart disease; implications as nutritional antioxidants. Nutr. Res. Rev. 2000, 13, 79–106. [Google Scholar] [CrossRef]
- Linos, E.; Katz, K.A.; Colditz, G.A. Skin cancer-the importance of prevention. JAMA Intern. Med. 2016, 176, 1435–1436. [Google Scholar] [CrossRef]
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the food and functional food industries. Food. Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef]
- Chang, M.; Liu, K. Arthrospira platensis as future food: A review on functional ingredients, bioactivities and application in the food industry. Int. J. Food. Sci. Technol. 2024, 59, 1197–1212. [Google Scholar] [CrossRef]
- Pyne, P.K.; Bhattacharjee, P.; Srivastav, P.P. Microalgae (Spirulina platensis) and its bioactive molecules: Review. Indian J. Nutri. 2017, 4, 160–165. [Google Scholar]
- Karkos, P.D.; Leong, S.C.; Karkos, C.D.; Sivaji, N.; Assimakopoulos, D.A. Spirulina in clinical practice: Evidence-based human applications. Evid. Based Complement. Altern. Med. 2011, 2011, 531053. [Google Scholar] [CrossRef]
- Thevarajah, B.; Nishshanka, G.K.S.H.; Premaratne, M.; Nimarshana, P.H.V.; Nagarajan, D.; Chan, J.-S.; Ariyadasa, T.U. Large-scale production of Spirulina -based proteins and c-phycocyanin: A biorefinery approach. Biochem. Eng. J. 2022, 185, 108541. [Google Scholar] [CrossRef]
- de Marco Castro, E.; Shannon, E.; Abu-Ghannam, N. Effect of fermentation on enhancing the nutraceutical properties of Arthrospira platensis (Spirulina). Fermentation 2019, 5, 28–43. [Google Scholar] [CrossRef]
- Bellahcen, T.O.; AAmiri, A.; Touam, I.; Hmimid, F.; Amrani, A.E.; Cherif, A.; Cherki, M. Evaluation of Moroccan microalgae: Spirulina platensis as a potential source of natural antioxidants. J. Complement. Integr. Med. 2020, 17, 20190036. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, K.; Ghasemi, Y.; Ebrahimzadeh, M.A. Antioxidant activity, phenol and flavonoid content of 13 citrus species peels and tissues. Pak. J. Pharm. Sci. 2009, 22, 277–281. [Google Scholar]
- Alrubaie, G.; Zaki, N.H.; Al-Hashimi, A.; Khuyon, A. Antibacterial effect of Spirulina platensis extracts on the viability of bacterial species isolated form acne patients in Baghdad. Ann. Rom. Soc. Cell Biol. 2021, 25, 3851–3859. [Google Scholar]
- Al-Ghanayem, A.A. Effect of methanol extracts of Arthrospira platensis on survival and increased disease resistance in Litopenaeus vannamei against vibriosis. J. Pure Appl. Microbiol. 2023, 17, 2140–2148. [Google Scholar] [CrossRef]
- Dhale, R.P.; Ghorpade, M.V.; Dharmadhikari, C.A. Comparison of various methods used to detect biofilm production of Candida species. J. Clin. Diagn. Res. 2014, 8, DC18–DC20. [Google Scholar] [CrossRef]
- Haney, E.F.; Trimble, M.J.; Cheng, J.T.; Vallé, Q.; Hancock, R.E.W. Critical assessment of methods to quantify biofilm growth and evaluate antibiofilm activity of host defence peptides. Biomolecules 2018, 8, 29. [Google Scholar] [CrossRef]
- Haney, E.F.; Trimble, M.J.; Hancock, R.E.W. Microtiter plate assays to assess antibiofilm activity against bacteria. Nat. Protoc. 2021, 16, 2615–2632. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Su, J.; Li, B.; Chen, T.; Wong, Y.S. Synergistic apoptosis-inducing effects on A375 human melanoma cells of natural borneol and curcumin. PLoS ONE 2014, 9, e101277. [Google Scholar] [CrossRef]
- Lee, J.; Park, A.; Kim, M.J.; Lim, H.J.; Rha, Y.A.; Kang, H.G. Spirulina extract enhanced a protective effect in type 1 diabetes by anti-apoptosis and anti-ROS production. Nutrients 2017, 9, 1363. [Google Scholar] [CrossRef] [PubMed]
- Sangboonruang, S.; Kitidee, K.; Chantawannakul, P.; Tragoolpua, K.; Tragoolpua, Y. Melittin from Apis florea venom as a promising therapeutic agent for skin cancer treatment. Antibiotics 2020, 9, 517. [Google Scholar] [CrossRef]
- Gentscheva, G.; Nikolova, K.; Panayotova, V.; Peycheva, K.; Makedonski, L.; Slavov, P.; Radusheva, P.; Petrova, P.; Yotkovska, I. Application of Arthrospira platensis for medicinal purposes and the food industry: A review of the literature. Life 2023, 13, 845. [Google Scholar] [CrossRef] [PubMed]
- Spínola, M.P.; Mendes, A.R.; Prates, J.A.M. Chemical composition, bioactivities, and applications of Spirulina (Limnospira platensis) in food, feed, and medicine. Foods 2024, 13, 3656. [Google Scholar] [CrossRef]
- Riyadi, P.H.; Susanto, E.; Anggo, A.D.; Arifin, M.H.; Rizki, L. Effect of methanol solvent concentration on the extraction of bioactive compounds using ultrasonic-assisted extraction (UAE) from Spirulina platensis. Food Res. 2023, 7, 59–66. [Google Scholar] [CrossRef]
- Fayyad, R.J.; Ali, A.N.M.; Dwaish, A.S.; Khayoon, A. Anticancer activity of spirulina platensis methanolic extracts against L20B and MCF7 human cancer cell lines. Plant Arch. 2019, 19, 1419–1426. [Google Scholar]
- Rahim, A.; Cakir, C.; Ozturk, M.; Sahin, B.; Soulaimani, A.; Sibaoueih, M.; Nasser, B.; Eddoha, R.; Essamadi, A.; Amiri, B.E. Chemical characterization and nutritional value of Spirulina platensis cultivated in natural conditions of Chichaoua region (Morocco). S. Afr. J. Bot. 2021, 141, 235–242. [Google Scholar] [CrossRef]
- Habibi, R.; Tadayoni, M.; Mohammadpour, H. Evaluation of the functional properties of ethanolic and polysaccharide extracts of Spirulina platensis. Bioact. Carbohydr. Diet. Fibre. 2024, 31, 100401. [Google Scholar] [CrossRef]
- Gabr, G.A.; El-Sayed, S.M.; Hikal, M.S. Antioxidant activities of phycocyanin: A bioactive compound from Spirulina platensis. J. Pharm. Res. Int. 2020, 32, 73–85. [Google Scholar] [CrossRef]
- Gentscheva, G.; Milkova-Tomova, I.; Pehlivanov, I.; Gugleva, V.; Nikolova, K.; Petkova, N.; Andonova, V.; Buhalova, D.; Pisanova, E. Chemical Characterization of Selected Algae and Cyanobacteria from Bulgaria as Sources of Compounds with Antioxidant Activity. Appl. Sci. 2022, 12, 9935. [Google Scholar] [CrossRef]
- Hassanpour, S.H.; Doroudi, A. Review of the antioxidant potential of flavonoids as a subgroup of polyphenols and partial substitute for synthetic antioxidants. Avicenna J. Phytomed. 2023, 13, 354–376. [Google Scholar] [PubMed]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.K.; Rocha, J.M. Recent developments in polyphenol applications on human health: A review with current knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef]
- Andrianto, D.; Safithri, M.; Tarman, K. Total phenolic content and proliferation activity of Spirulina extract in lymphocyte cell. BIO Web Conf. 2024, 92, 02009. [Google Scholar] [CrossRef]
- Gheda, S.; Abd El-Zaher, E.H.F.; Abou-Zeid, A.M.; Bedair, N.A.; Pereira, L. Potential Activity of Arthrospira platensis as Antioxidant, Cytotoxic and Antifungal against Some Skin Diseases: Topical Cream Application. Mar. Drugs 2023, 21, 160. [Google Scholar] [CrossRef]
- Gheda, S.; Abo-Shady, A.; Abdel-Karim, O.; Ismail, G. Antioxidant and antihyperglycemic activity of Arthrospira platensis (Spirulina platensis) methanolic extract: In vitro and in vivo study. Egypt. J. Bot. 2021, 61, 71–93. [Google Scholar] [CrossRef]
- AL Ubeed, H.M.S.; Bhuyan, D.J.; Alsherbiny, M.A.; Basu, A.; Vuong, Q.V. A comprehensive review on the techniques for extraction of bioactive compounds from medicinal cannabis. Molecules 2022, 27, 604. [Google Scholar] [CrossRef]
- Chaves, B.J.; Tadi, P. Gentamicin. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557550/ (accessed on 20 December 2024).
- Patel, S.; Preuss, C.V.; Bernice, F. Vancomycin. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459263/ (accessed on 20 December 2024).
- Wali, N.M.; Abdljbaar, A.S. Effect of ethanol and alkaloid extract of Spirulina platensis against dermatophyte fungi. Plant. Arch. 2020, 20, 2736–2743. [Google Scholar]
- Hossain, T.J. Methods for screening and evaluation of antimicrobial activity: A review of protocols, advantages, and limitations. Eur. J. Microbiol. Immunol. 2024, 14, 97–115. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.E.; El-Saadony, M.T.; Shehata, A.M.; Saad, A.M.; Aldhumri, S.A.; Ouda, S.M.; Mesalam, N.M. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2022, 29, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- LewisOscar, F.; Nithya, C.; Bakkiyaraj, D.; Arunkumar, M.; Alharbi, N.S.; Thajuddin, N. Biofilm inhibitory effect of Spirulina platensis extracts on bacteria of clinical significance. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 537–544. [Google Scholar] [CrossRef]
- Mirani, Z.A.; Fatima, A.; Urooj, S.; Aziz, M.; Khan, M.N.; Abbas, T. Relationship of cell surface hydrophobicity with biofilm formation and growth rate: A study on Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli. Iran J. Basic Med. Sci. 2018, 21, 760–769. [Google Scholar]
- Mirani, Z.A.; Naz, S.; Khan, F.; Aziz, M.; Asadullah; Khan, M.N.; Khan, S.I. Antibacterial fatty acids destabilize hydrophobic and multicellular aggregates of biofilm in S. aureus. J. Antibiot. 2017, 70, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, G.M.; van der Mei, H.C.; Busscher, H.J. Bacterial adhesion to surface hydrophilic and hydrophobic contact lenses. Biomaterials 2001, 22, 3217–3224. [Google Scholar] [CrossRef]
- Djalil, A.D.; Nurmareta, Y.; Pribadi, U.; Rahayu, W.S.; Utami, P.I.; Suwandri. Bioactivity of acetone extract from Spirulina platensis. IOP Conf. Ser. Mater. Sci. Eng. 2018, 288, 012002. [Google Scholar] [CrossRef]
- Hernández, F.Y.F.; Khandual, S.; López, I.G.R. Cytotoxic effect of Spirulina platensis extracts on human acute leukemia Kasumi-1 and chronic myelogenous leukemia K-562 cell lines. Asian Pac. J. Trop. Biomed. 2017, 7, 14–19. [Google Scholar] [CrossRef]
- Al-saily, H.M.N.; Hassan, W.S.; Chabuk, H.A.H. Anti-oxidant and cytotoxic activity of Spirulina platensis ethanolic extract against Caco-2 and HepG2 cancer cell lines. J. Appl. Nat. Sci. 2024, 16, 325–333. [Google Scholar] [CrossRef]
- Akbarizare, M.; Ofoghi, H.; Hadizadeh, M.; Moazami, N. In vitro assessment of the cytotoxic effects of secondary metabolites from Spirulina platensis on hepatocellular carcinoma. Egypt. Liver J. 2020, 10, 11. [Google Scholar] [CrossRef]
- Mapoung, S.; Arjsri, P.; Thippraphan, P.; Semmarath, W.; Yodkeeree, S.; Chiewchanvit, S.; Piyamongkol, W.; Limtrakul, P. Photochemoprotective effects of Spirulina platensis extract against UVB irradiated human skin fibroblasts. S. Afr. J. Bot. 2020, 130, 198–207. [Google Scholar] [CrossRef]
- Chwil, M.; Mihelič, R.; Matraszek-Gawron, R.; Terlecka, P.; Skoczylas, M.M.; Terlecki, K. Comprehensive Review of the Latest Investigations of the Health-Enhancing Effects of Selected Properties of Arthrospira and Spirulina Microalgae on Skin. Pharmaceuticals 2024, 17, 1321. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, D.; Zhuang, J.; Zhang, F.; Xu, C. Caspase-8 and Caspase-9 functioned differently at different stages of the cyclic stretch-induced apoptosis in human periodontal ligament cells. PLoS ONE 2016, 11, e0168268. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2). J. Nutr. 2006, 136, 2715–2721. [Google Scholar] [CrossRef]
- Jafarbeigloo, H.R.G.; Sheibani, S.; Bazmandeh, A.Z. Flavonoids kaempferol (KAE) and quercetine (QUE) inhibited proliferation of human leukemia THP-1 cells by up regulation of pro-apoptotic protein Bax and caspase 3/8 expression and down regulation of anti-apoptotic proteins Bcl-2, Bcl-xl and Mcl-1 expression. Ann. Cancer Res. Ther. 2021, 29, 41–46. [Google Scholar]
- Al-Jabery, R.N.; Auda, M.A.; Al-Rekabi, H.Y. Anticancer potential of phenolic extract from Spirulina platensis against esophagus cancer cells. J. Adv. Med. Biomed. Res. 2023, 31, 499–506. [Google Scholar] [CrossRef]
- Tin, M.M.Y.; Cho, C.H.; Chan, K.; James, A.E.; Ko, J.K.S. Astragalus saponins induce growth inhibition and apoptosis in human colon cancer cells and tumor xenograft. Carcinogenesis 2007, 28, 1347–1355. [Google Scholar] [CrossRef]
- Kamran, S.; Sinniah, A.; Abdulghani, M.A.M.; Alshawsh, M.A. Therapeutic potential of certain terpenoids as anticancer agents: A scoping review. Cancers 2022, 14, 1100. [Google Scholar] [CrossRef] [PubMed]
- Asoudeh-Fard, A.; Najafipour, R.; Salehi, M.; Mahmoudi, M.; Salahshourifar, I.; Eghdami, A.; Parsaei, A.; Piri, H. Apoptotic effect of phycocyanin on HT-29 colon cancer through activation of caspase enzymes and p53 cell signaling pathway. Iran. J. Toxicol. 2024, 18, 39–44. [Google Scholar] [CrossRef]
- Roy, K.R.; Arunasree, K.M.; Reddy, N.P.; Dheeraj, B.; Reddy, G.V.; Reddanna, P. Alteration of mitochondrial membrane potential by Spirulina platensis C-phycocyanin induces apoptosis in the doxorubicinresistant human hepatocellular-carcinoma cell line HepG2. Biotechnol. Appl. Biochem. 2007, 47, 159–167. [Google Scholar] [CrossRef]
- Dai, J.; Shen, J.; Pan, W.; Shen, S.; Das, U.N. Effects of Polyunsaturated Fatty Acids on the Growth of Gastric Cancer Cells In Vitro. Lipids Health Dis. 2013, 12, 71. [Google Scholar] [CrossRef] [PubMed]







| Genes | Forward Primer Sequences 5′-3′ | Reverse Primer Sequences 3′-5′ |
|---|---|---|
| Caspase-3 | TGTTTGTGTGCTTCTGAGCC | TCAAGCTTGTCGGCATACTG |
| Caspase-8 | GTGGAGGAAAGCAATCTGTC | TATTAGCCCTGCCTGGTGTCT |
| Caspase-9 | GACTCCCTCGAGTCTCCAGAT | GACTCCCTCGAGTCTCCAGAT |
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC |
| A. platensis | DPPH (mg GAE/g Extract) | ABTS (mg TEAC/g Extract) | FRAP (mg TEAC/g Extract) |
|---|---|---|---|
| Ethanolic extract | 8.96 ± 0.84 a | 53.03 ± 4.21 a | 48.06 ± 0.78 a |
| Methanolic extract | 3.50 ± 0.23 b | 43.44 ± 2.36 b | 19.70 ± 0.38 b |
| A. platensis | Total Phenolic (mg GAE/g Extract) | Total Flavonoid (mg QE/g Extract) |
|---|---|---|
| Ethanolic extract | 38.79 ± 1.61 a | 27.50 ± 0.53 a |
| Methanolic extract | 23.71 ± 0.93 b | 25.85 ± 0.51 b |
| A. platensis | Gallic Acid (mg/g Extract) | Quercetin Extracts Identified by HPLC (mg/g Extract) |
|---|---|---|
| Ethanolic extract | 20.50 ± 0.03 a | 0.09 ± 0.01 |
| Methanolic extract | 21.84 ± 0.77 a | Undetected |
| A. platensis | Inhibition Zone Diameter (mm) | |||||
|---|---|---|---|---|---|---|
| Skin Pathogenic Bacteria | ||||||
| S. aureus | S. epidermidis | MRSA | M. luteus | P. aeruginosa | C. acnes | |
| Ethanolic extract | 11.33 ± 1.04 bc | 12.40 ± 0.96 c | 0 a | 12.50 ± 0.50 c | 0 a | 9.67 ± 0.58 b |
| Methanolic extract | 10.77 ± 0.25 bc | 0 a | 0 a | 10.83 ± 0.58 bc | 0 a | 11.00 ± 0.50 bc |
| Gentamicin (1 mg/mL) | 28.33 ± 0.76 d | 29.50 ± 1.73 d | ND | 37.50 ± 2.18 g | 32.17 ± 0.58 e | 35.67 ± 1.89 f |
| Vancomycin (1 mg/mL) | ND | ND | 28.67 ± 1.04 d | ND | ND | ND |
| DMSO | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a |
| A. platensis | MIC and MBC (mg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin Pathogenic Bacteria | ||||||||||||
| S. aureus | S. epidermidis | MRSA | M. luteus | P. aeruginosa | C. acnes | |||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Ethanolic extract | 125 | 125 | 125 | 125 | 31.25 | 125 | 31.25 | 125 | 125 | 125 | 125 | 125 |
| Methanolic extract | 125 | 125 | 62.5 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 |
| Gentamicin (1 mg/mL) | 0.0039 | 0.0039 | 0.0156 | 0.0156 | ND | ND | 0.0039 | 0.0039 | 0.0039 | 0.0039 | 0.0625 | 0.0625 |
| Vancomycin (1 mg/mL) | ND | ND | ND | ND | 0.0312 | 0.0312 | ND | ND | ND | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rungjiraphirat, R.; Cheepchirasuk, N.; Suriyaprom, S.; Tragoolpua, Y. Inhibitory Efficacy of Arthrospira platensis Extracts on Skin Pathogenic Bacteria and Skin Cancer Cells. Biology 2025, 14, 502. https://doi.org/10.3390/biology14050502
Rungjiraphirat R, Cheepchirasuk N, Suriyaprom S, Tragoolpua Y. Inhibitory Efficacy of Arthrospira platensis Extracts on Skin Pathogenic Bacteria and Skin Cancer Cells. Biology. 2025; 14(5):502. https://doi.org/10.3390/biology14050502
Chicago/Turabian StyleRungjiraphirat, Ranchana, Nitsanat Cheepchirasuk, Sureeporn Suriyaprom, and Yingmanee Tragoolpua. 2025. "Inhibitory Efficacy of Arthrospira platensis Extracts on Skin Pathogenic Bacteria and Skin Cancer Cells" Biology 14, no. 5: 502. https://doi.org/10.3390/biology14050502
APA StyleRungjiraphirat, R., Cheepchirasuk, N., Suriyaprom, S., & Tragoolpua, Y. (2025). Inhibitory Efficacy of Arthrospira platensis Extracts on Skin Pathogenic Bacteria and Skin Cancer Cells. Biology, 14(5), 502. https://doi.org/10.3390/biology14050502





