Metabolic Response of Black Tiger Shrimp (Penaeus monodon) to Acute Ammonia Nitrogen Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. 96 h Acute Ammonia Nitrogen Stress
2.3. Histology Analysis
2.4. Measurement of Ammonia, Urea, and Uric Acid in the Plasma
2.5. Measurement of the Enzyme Activity in the Hepatopancreas
2.6. Statistical Analysis
3. Results
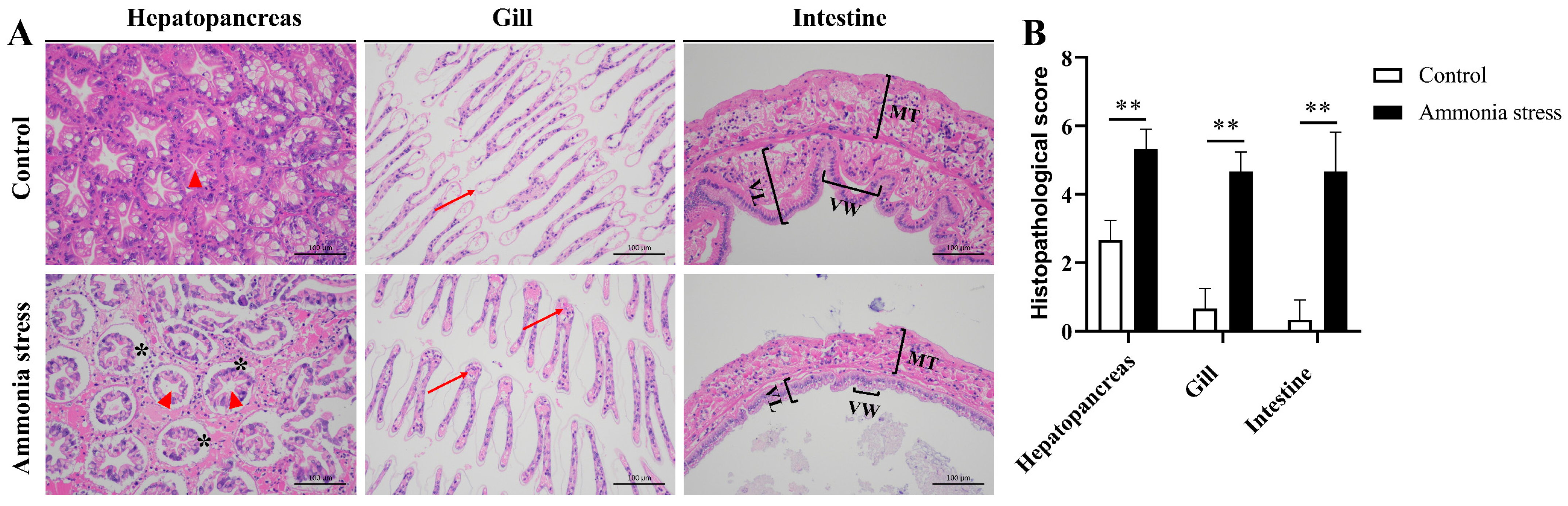
3.1. Effects of Ammonia Nitrogen Stress on Shrimp Tissues
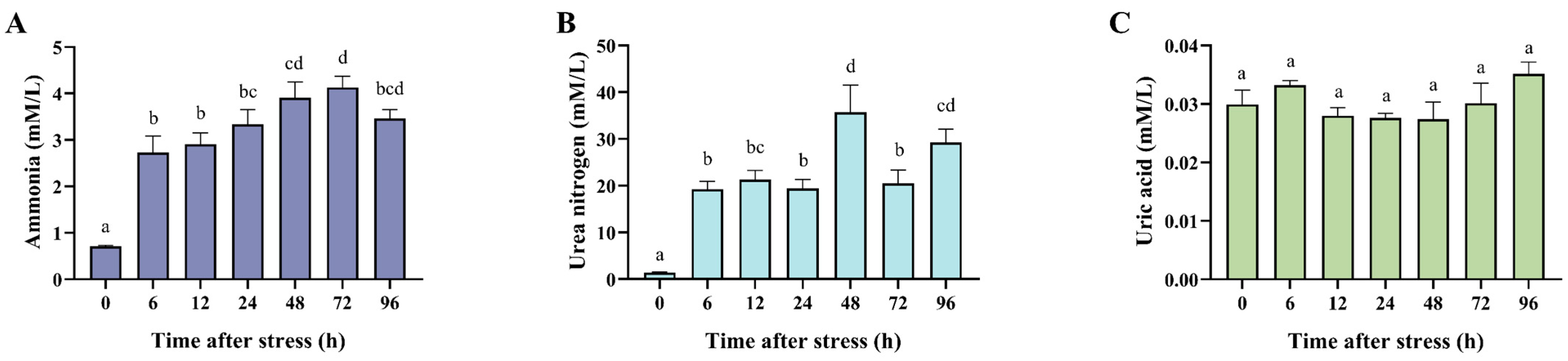
3.2. Effects of Ammonia Nitrogen Stress on the Metabolic Product in the Plasma
3.3. Effect of Ammonia Nitrogen Stress on the Activity of Metabolic-Related Enzymes
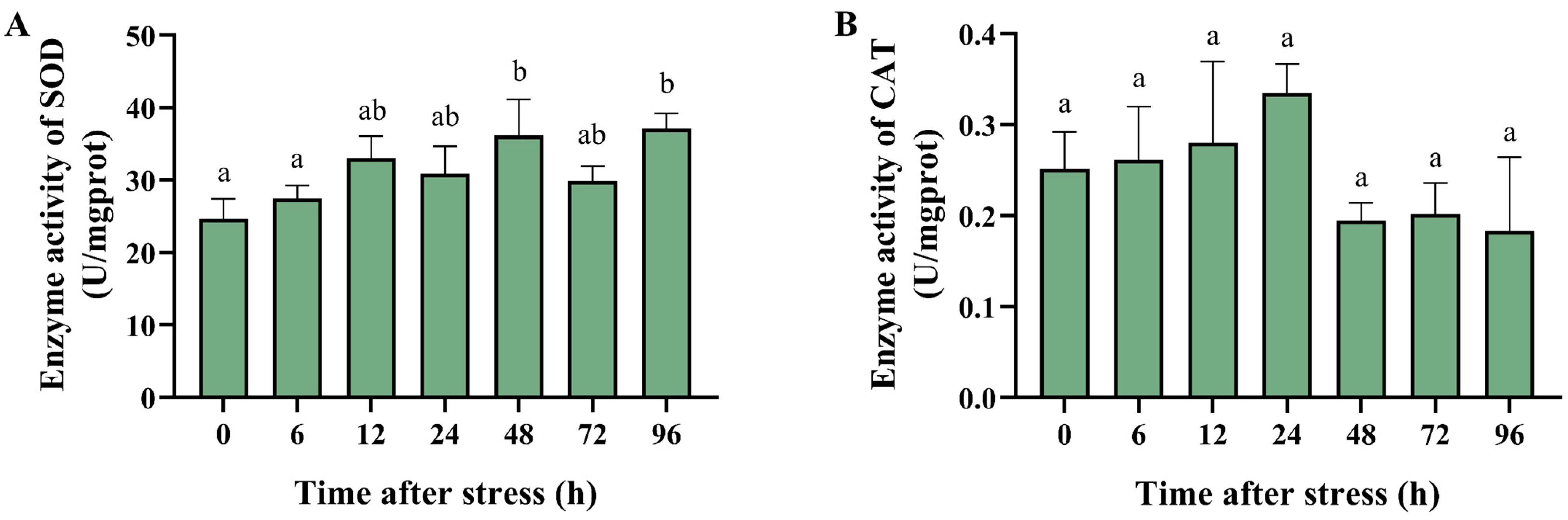
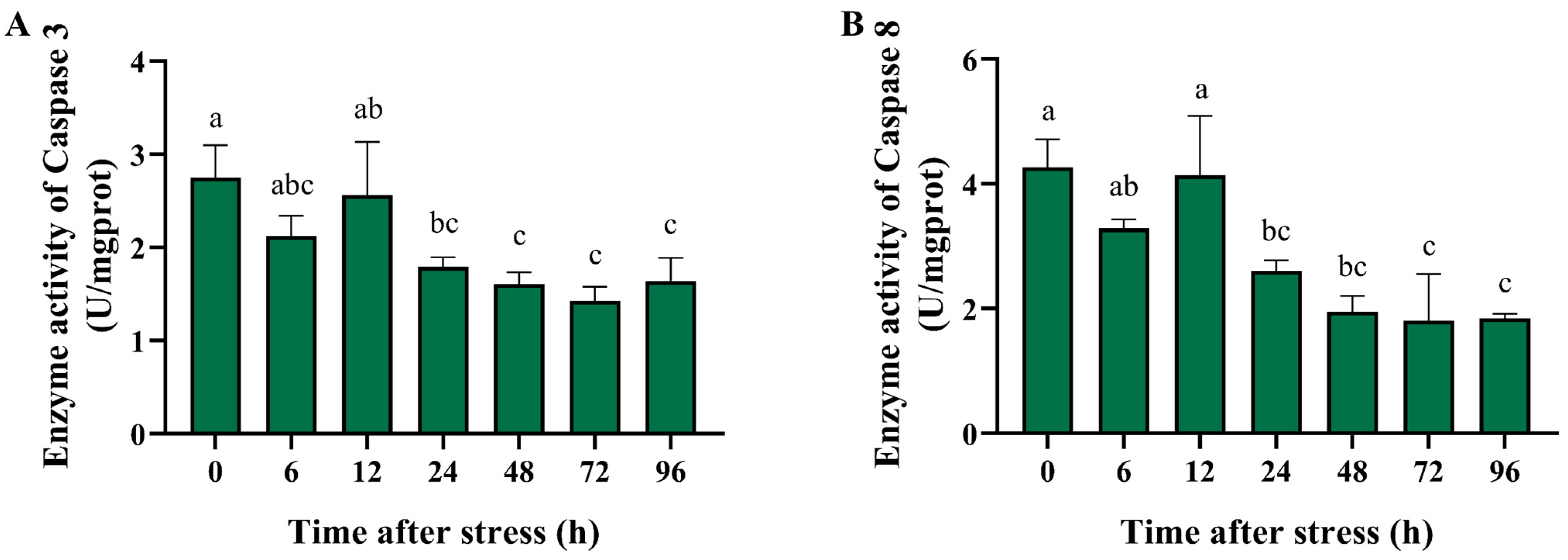
3.4. Effect of Ammonia Nitrogen Stress on the Activity of Antioxidant and Apoptosis-Related Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mengal, K.; Kor, G.; Kozák, P.; Niksirat, H. Effects of environmental factors on the cellular and molecular parameters of the immune system in decapods. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2023, 276, 111332. [Google Scholar] [CrossRef] [PubMed]
- Romano, N.; Zeng, C. Toxic Effects of Ammonia, Nitrite, and Nitrate to Decapod Crustaceans: A Review on Factors Influencing their Toxicity, Physiological Consequences, and Coping Mechanisms. Rev. Fish. Sci. 2013, 21, 1–21. [Google Scholar] [CrossRef]
- Weihrauch, D.; Morris, S.; Towle, D.W. Ammonia excretion in aquatic and terrestrial crabs. J. Exp. Biol. 2004, 207, 4491–4504. [Google Scholar] [CrossRef]
- Chen, J.C.; Lin, C.Y. Lethal effects of ammonia on Penaeus chinensis Osbeck juveniles at different salinity levels. J. Exp. Mar. Biol. Ecol. 1992, 156, 139–148. [Google Scholar] [CrossRef]
- Noor-Hamid, S.; Fortes, R.D.; Parado-Estepa, F. Effect of pH and ammonia on survival and growth of the early larval stages of Penaeus monodon Fabricius. Aquaculture 1994, 125, 67–72. [Google Scholar] [CrossRef]
- Chen, J.-C.; Chen, C.-T. Changes of osmotic and electrolyte concentrations in the haemolymph of Penaeus japonicus exposed to ambient ammonia. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1996, 114, 35–38. [Google Scholar] [CrossRef]
- Chen, J.C.; Lin, C.Y. Oxygen consumption and ammonia-N excretion of Penaeus chinensis juveniles exposed to ambient ammonia at different salinity levels. Comp. Biochem. Physiol. Part C Comp. Pharmacol. Toxicol. 1992, 102, 287–291. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Chen, J.C. Hemocyanin oxygen affinity, and the fractionation of oxyhemocyanin and deoxyhemocyanin for Penaeus monodon exposed to elevated nitrite. Aquat. Toxicol. 1999, 45, 35–46. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, F.; Huang, J.; Yang, L.; Jiang, S.; Yang, Q.; He, J.; Jiang, S. Transcriptome reveals involvement of immune defense, oxidative imbalance, and apoptosis in ammonia-stress response of the black tiger shrimp (Penaeus monodon). Fish Shellfish Immunol. 2018, 83, 162–170. [Google Scholar] [CrossRef]
- Liu, S.; Pan, L.; Liu, M.; Yang, L. Effects of ammonia exposure on nitrogen metabolism in gills and hemolymph of the swimming crab Portunus trituberculatus. Aquaculture 2014, 432, 351–359. [Google Scholar] [CrossRef]
- Ren, Q.; Pan, L.; Zhao, Q.; Si, L. Ammonia and urea excretion in the swimming crab Portunus trituberculatus exposed to elevated ambient ammonia-N. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 187, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liu, Q.; Kan, D.; Zhao, W.; Guo, H.; Lv, L. Effects of ammonia-N exposure on the growth, metabolizing enzymes, and metabolome of Macrobrachium rosenbergii. Ecotoxicol. Environ. Saf. 2020, 189, 110046. [Google Scholar] [CrossRef]
- Li, C.; Zhao, T.; Ren, L.; Cui, D.; Zhan, Y.; Chang, Y. Characterization of a novel glutamate dehydrogenase gene and its response to heat stress in the sea urchin Strongylocentrotus intermedius. Aquac. Rep. 2023, 28, 101446. [Google Scholar] [CrossRef]
- Linton, S.; Greenaway, P. Urate deposits in the gecarcinid land crab Gecarcoidea natalis are synthesised de novo from excess dietary nitrogen. J. Exp. Biol. 1997, 200, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- Pahlich, E.; Joy, K.W. Glutamate Dehydrogenase from Pea Roots: Purification and Properties of the Enzyme. Can. J. Biochem. 1971, 49, 127. [Google Scholar] [CrossRef]
- Michele, R. Nitrogen excretion in marine and fresh-water crustacea. Biol. Rev. 1987, 62, 1–24. [Google Scholar]
- Chen, J.C.; Lin, C.Y. Responses of oxygen consumption, Ammonia-N excretion and Urea-N excretion of Penaeus chinensis exposed to ambient ammonia at different salinity and pH levels. Aquaculture 1995, 136, 243–255. [Google Scholar] [CrossRef]
- Lee, W.C.; Chen, J.C. Nitrogenous excretion and arginase specific activity of kuruma shrimp Marsupenaeus japonicus exposed to elevated ambient nitrite. J. Exp. Mar. Biol. Ecol. 2004, 308, 103–111. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, L.; Wei, C.; Tong, R.; Li, Y.; Ding, M.; Wang, H. Crustacean hyperglycemic hormone (CHH) regulates the ammonia excretion and metabolism in white shrimp, Litopenaeus vannamei under ammonia-N stress. Sci. Total Environ. 2020, 723, 138128. [Google Scholar] [CrossRef]
- Sekine, M.; Okamoto, K.; Pai, E.F.; Nagata, K.; Ichida, K.; Hille, R.; Nishino, T. Allopurinol and oxypurinol differ in their strength and mechanisms of inhibition of xanthine oxidoreductase. J. Biol. Chem. 2023, 299, 105189. [Google Scholar] [CrossRef]
- Cancio, I.; Cajaraville, M.P. Seasonal variation of xanthine oxidoreductase activity in the digestive gland cells of the mussel Mytilus galloprovincialis: A biochemical, histochemical and immunochemical study. Biol. Cell 1999, 91, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Ge, W.; Xu, X.; Xu, X.; Sun, Q.; Xu, X.; Zhang, J. A crucial role of adenosine deaminase in regulating gluconeogenesis in mice. J. Biol. Chem. 2024, 300, 107425. [Google Scholar] [CrossRef]
- Murthy, C.R.; Rama Rao, K.V.; Bai, G.; Norenberg, M.D. Ammonia-induced production of free radicals in primary cultures of rat astrocytes. J. Neurosci. Res. 2001, 66, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidant defence mechanisms: From the beginning to the end (of the beginning). Free Radic. Res. 1999, 31, 261–272. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Jiang, S.; Li, Y.; Yang, Q.; Jiang, S.; Yang, L.; Huang, J.; Zhou, F. Comprehensive expression analysis of the beta integrin from Penaeus monodon indicating its participation in innate immunity and ammonia nitrogen stress response. Fish Shellfish Immunol. 2020, 98, 887–898. [Google Scholar] [CrossRef]
- Goodman, Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 2007, 47, 598–607. [Google Scholar] [CrossRef]
- Zilberg, D.; Munday, B.L. Pathology of experimental amoebic gill disease in Atlantic salmon, Salmo salar L., and the effect of pre-maintenance of fish in sea water on the infection. J. Fish Dis. 2010, 23, 401–407. [Google Scholar] [CrossRef]
- Ringø, E.; Salinas, I.; Olsen, R.E.; Nyhaug, A.; Myklebust, R.; Mayhew, T.M. Histological changes in intestine of Atlantic salmon (Salmo salar L.) following in vitro exposure to pathogenic and probiotic bacterial strains. Cell Tissue Res. 2007, 328, 109–116. [Google Scholar] [CrossRef]
- Alwael, H.; Alharthi, A.S.; Dabi, M.M.; Oubaha, M.; El-Shahawi, M.S. A highly sensitive electrochemical sensing probe incorporating classical Berthelot’s reaction and glassy carbon electrode for measuring ultra-trace levels of ammonia/ NH4+ in water. Electrochem. Commun. 2024, 162, 107686. [Google Scholar] [CrossRef]
- Melara, F.; da Silva, L.K.; Mandelli, N.A.; Krein, D.D.C.; Chiomento, J.L.T.; Dettmer, A.; Piccin, J.S. Effect on N release by urea coating with chitosan, starch and urease inhibitor. Int. J. Biol. Macromol. 2025, 303, 140603. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Miao, S.; Yu, W.; Jiang, E.-Y.; Gong, M.; Liu, G.; Luo, X.; Zhang, M.-Z. Visual detection of uric acid in serum through catalytic oxidation by a novel cellulose membrane biosensor with schiff base immobilized uricase. Biosens. Bioelectron. 2025, 268, 116912. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Li, Y.; Yu, X.; Zhang, N.; Liao, Q.; Pan, L. The mechanism of reactive oxygen species generation, DNA damage and apoptosis in hemocytes of Litopenaeus vannamei under ammonia nitrogen exposure. Aquat. Toxicol. 2024, 272, 106958. [Google Scholar] [CrossRef]
- Tanguy, A.; Boutet, I.; Moraga, D. Molecular characterization of the glutamine synthetase gene in the Pacific oyster Crassostrea gigas: Expression study in response to xenobiotic exposure and developmental stage. Biochim. Biophys. Acta (BBA)–Gene Struct. Expr. 2005, 1681, 116–125. [Google Scholar] [CrossRef]
- Wang, Y.; Li, E.; Yu, N.; Wang, X.; Cai, C.; Tang, B.; Chen, L.; Van Wormhoudt, A. Characterization and expression of glutamate dehydrogenase in response to acute salinity stress in the Chinese mitten crab, Eriocheir sinensis. PLoS ONE 2012, 7, e37316. [Google Scholar] [CrossRef]
- Hlordzi, V.; Wang, J.; Kuebutornye, F.K.A.; Yang, X.; Tan, B.; Li, T.; Cui, Z.; Lv, S.; Lao, T.; Chi, S. Hydrolysed fish protein powder is better at the growth performance, hepatopancreas and intestinal development of Pacific white shrimp (Litopenaeus vannamei). Aquac. Rep. 2022, 23, 101025. [Google Scholar] [CrossRef]
- Otto-Ślusarczyk, D.; Graboń, W.; Mielczarek-Puta, M. Aspartate aminotransferase--key enzyme in the human systemic metabolism. Postep. Hig. Med. Dosw. 2016, 70, 219–230. [Google Scholar] [CrossRef]
- Fried, R.; Fried, L.W. Xanthine Oxidase (Xanthine Dehydrogenase). In Methods of Enzymatic Analysis; Academic Press: Cambridge, MA, USA, 1974; pp. 644–649. [Google Scholar]
- Senger, M.R.; Rosemberg, D.B.; Seibt, K.J.; Dias, R.D.; Bogo, M.R.; Bonan, C.D. Influence of mercury chloride on adenosine deaminase activity and gene expression in zebrafish (Danio rerio) brain. NeuroToxicology 2010, 31, 291–296. [Google Scholar] [CrossRef]
- Štefanić, Z.; Mikleušević, G.; Luić, M.; Bzowska, A.; Leščić Ašler, I. Structural characterization of purine nucleoside phosphorylase from human pathogen Helicobacter pylori. Int. J. Biol. Macromol. 2017, 101, 518–526. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Shi, W.; Zhang, Y.; Chang, G.; Wu, N.; Xue, C.; Li, J. RNA-seq analysis uncovers effects of ammonia on metabolism, oxidant-antioxidant equilibrium and apoptosis in the red swamp crayfish (Procambarus clarkii). Aquac. Rep. 2020, 18, 100459. [Google Scholar] [CrossRef]
- Rosemberg, D.B.; Rico, E.P.; Guidoti, M.R.; Dias, R.D.; Souza, D.O.; Bonan, C.D.; Bogo, M.R. Adenosine deaminase-related genes: Molecular identification, tissue expression pattern and truncated alternative splice isoform in adult zebrafish (Danio rerio). Life Sci. 2007, 81, 1526–1534. [Google Scholar] [CrossRef]
- Liguo, Q.; Xiang, S.; Simeng, Y.; Qian, H.; Xiaoping, D.; Hailong, Z. Changes of Ammonia-Metabolizing Enzyme Activity and Gene Expression of Two Strains in Shrimp Litopenaeus vannamei Under Ammonia Stress. Front. Physiol. 2018, 9, 211. [Google Scholar]
- Zhao, M.; Yao, D.; Li, S.; Zhang, Y.; Aweya, J.J. Effects of ammonia on shrimp physiology and immunity: A review. Rev. Aquac. 2020, 12, 2194–2211. [Google Scholar] [CrossRef]
- Lin, L.; Zhuo, H.; Zhang, Y.; Li, J.; Zhou, X.; Wu, G.; Guo, C.; Liu, J. Effects of ammonia exposure and post-exposure recovery in pacific white shrimp, Litopenaeus vannamei: Histological, physiological and molecular responses. Aquat. Toxicol. 2024, 277, 107133. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Liu, Q.; Wang, Y.; Zhang, J.; Xiong, D. Impairment of the intestine barrier function in Litopenaeus vannamei exposed to ammonia and nitrite stress. Fish Shellfish Immunol. 2018, 78, 279–288. [Google Scholar] [CrossRef]
- Duan, Y.; Zhong, G.; Nan, Y.; Yang, Y.; Xiao, M.; Li, H. Effects of Nitrite Stress on the Antioxidant, Immunity, Energy Metabolism, and Microbial Community Status in the Intestine of Litopenaeus vannamei. Antioxidants 2024, 13, 1318. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.C.; Corsi, D.; Cavi, N.; Bruni, N.; Dosio, F. Superoxide Dismutase Administration: A Review of Proposed Human Uses. Molecules 2021, 26, 1844. [Google Scholar] [CrossRef]
- Batista, J.J.; Silva, F.C.O.; de Araújo, M.I.F.; de Almeida Moura Nunes, P.H.; de Oliveira Ferreira, S.A.; da Silva, L.A.; de Siqueira Patriota, L.L.; Napoleão, T.H.; Paiva, P.M.G.; de Carvalho, J.M.; et al. Parkia pendula polysaccharides have no acute toxicity and prevent ethanol-induced gastric ulcers via downregulation of TBARS, IL-6, and TNF-α and upregulation of SOD, CAT, and IL-10. Int. J. Biol. Macromol. 2025, 309, 142702. [Google Scholar] [CrossRef]
- Ou, H.; Liang, J.; Liu, J. Effects of acute ammonia exposure on oxidative stress, endoplasmic reticulum stress and apoptosis in the kuruma shrimp (Marsupenaeus japonicus). Aquac. Rep. 2022, 27, 101383. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Y.; Wu, Z.; Hergazy, A.; Lan, J.; Zhao, L.; Liu, X.; Chen, N.; Lin, L. Transcriptomic analysis of liver from grass carp (Ctenopharyngodon idellus) exposed to high environmental ammonia reveals the activation of antioxidant and apoptosis pathways. Fish Shellfish Immunol. 2017, 63, 444–451. [Google Scholar] [CrossRef]
- Cheng, C.H.; Yang, F.F.; Ling, R.Z.; Liao, S.A.; Miao, Y.T.; Ye, C.X.; Wang, A.L. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat. Toxicol. 2015, 164, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Liu, R.; Zhao, D.; Wang, L.; Sun, M.; Wang, M.; Song, L. Ammonia exposure induces oxidative stress, endoplasmic reticulum stress and apoptosis in hepatopancreas of pacific white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2016, 54, 523–528. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Jiang, S.; Jiang, S.; Li, Y.; Yang, Q.; Yang, L.; Huang, J.; Shi, J.; Li, P.; Diao, H.; et al. Metabolic Response of Black Tiger Shrimp (Penaeus monodon) to Acute Ammonia Nitrogen Stress. Biology 2025, 14, 501. https://doi.org/10.3390/biology14050501
Ding Y, Jiang S, Jiang S, Li Y, Yang Q, Yang L, Huang J, Shi J, Li P, Diao H, et al. Metabolic Response of Black Tiger Shrimp (Penaeus monodon) to Acute Ammonia Nitrogen Stress. Biology. 2025; 14(5):501. https://doi.org/10.3390/biology14050501
Chicago/Turabian StyleDing, Yangyang, Shigui Jiang, Song Jiang, Yundong Li, Qibin Yang, Lishi Yang, Jianhua Huang, Jianzhi Shi, Pengying Li, Hongshan Diao, and et al. 2025. "Metabolic Response of Black Tiger Shrimp (Penaeus monodon) to Acute Ammonia Nitrogen Stress" Biology 14, no. 5: 501. https://doi.org/10.3390/biology14050501
APA StyleDing, Y., Jiang, S., Jiang, S., Li, Y., Yang, Q., Yang, L., Huang, J., Shi, J., Li, P., Diao, H., & Zhou, F. (2025). Metabolic Response of Black Tiger Shrimp (Penaeus monodon) to Acute Ammonia Nitrogen Stress. Biology, 14(5), 501. https://doi.org/10.3390/biology14050501





