Impact of Coagulase-Negative Staphylococci in Mixed Intramammary Infections with Streptococci on Milk Quality
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
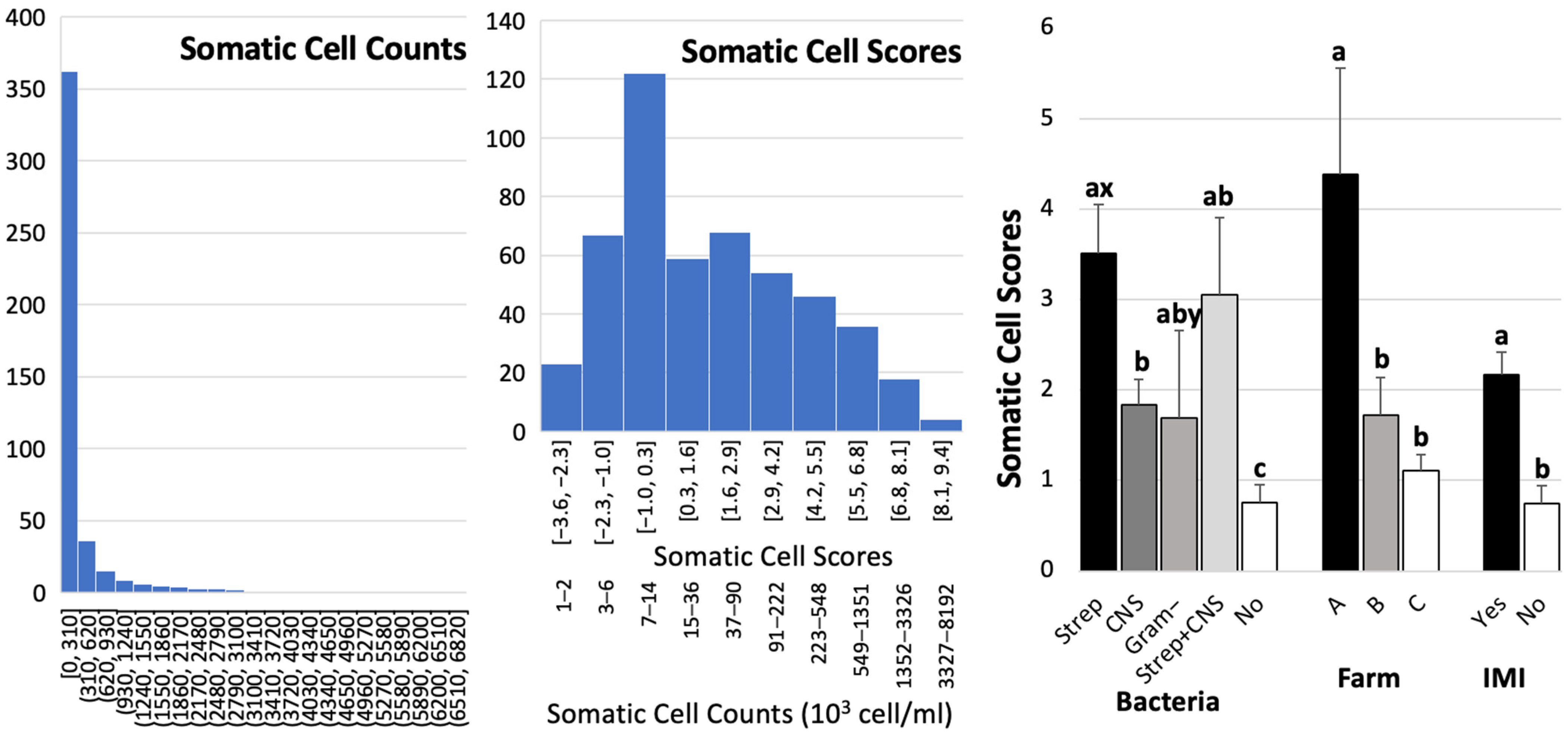
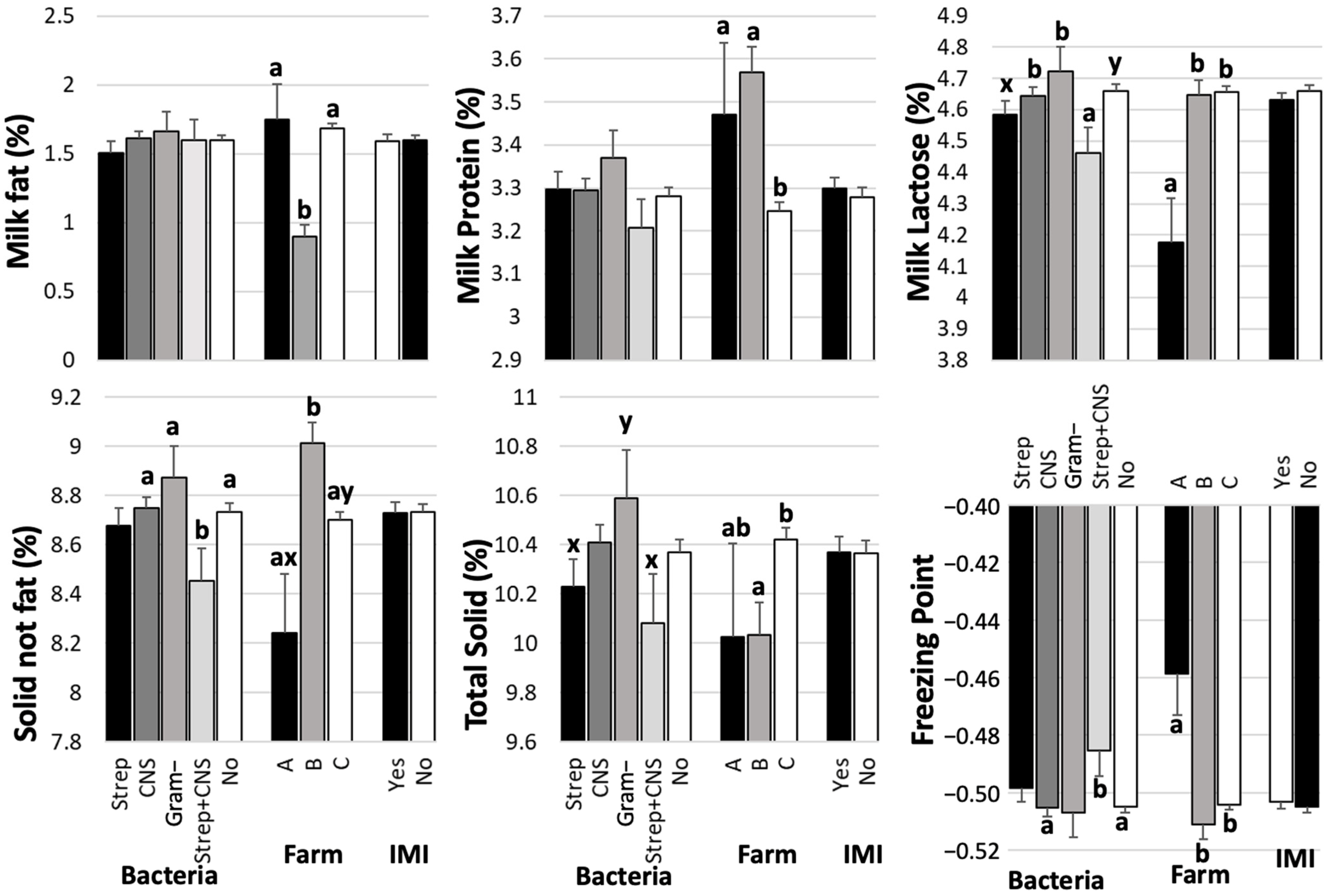
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halasa, T.; Huijps, K.; Østerås, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Seegers, H.; Fourichon, C.; Beaudeau, F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet. Res. 2003, 34, 475–491. [Google Scholar] [CrossRef]
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef]
- Bradley, A.J. Bovine mastitis: An evolving disease. Vet. J. 2002, 164, 116–128. [Google Scholar] [CrossRef]
- Zadoks, R.N.; Fitzpatrick, J.L. Changing trends in mastitis. Ir. Vet. J. 2009, 62 (Suppl. S4), S59–S70. [Google Scholar] [CrossRef]
- Keefe, G.P. Streptococcus agalactiae mastitis: A review. Can. Vet. J. 1997, 38, 429–437. [Google Scholar] [PubMed]
- Pyörälä, S.; Taponen, S. Coagulase-negative staphylococci—Emerging mastitis pathogens. Vet. Microbiol. 2009, 134, 3–8. [Google Scholar] [CrossRef]
- Tomazi, T.; Gonçalves, J.L.; Barreiro, J.R.; Arcari, M.A.; dos Santos, M.V. Bovine subclinical intramammary infection caused by coagulase-negative staphylococci increases somatic cell count but does not affect milk yield or composition. J. Dairy Sci. 2015, 98, 3071–3078. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, S.A.; Koop, G.; Melkie, S.T.; Getahun, C.D.; Hogeveen, H.; Lam, T.J.G.M. Prevalence of subclinical mastitis and associated risk factors at cow and herd level in dairy farms in North-West Ethiopia. Prev. Vet. Med. 2017, 145, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Persson, Y.; Nyman, A.K.J.; Grönlund-Andersson, U. Etiology and antimicrobial susceptibility of udder pathogens from cases of subclinical mastitis in dairy cows in Sweden. Acta Vet. Scand. 2011, 53, 36. [Google Scholar] [CrossRef]
- Sophorn, N.; Sambo, N.; Ohkura, S.; Nakamura, S.; Matsuyama, S.; Murase, T.; Soriya, R.; Suriyasathaporn, W. Emerging of uncommon chronic mastitis from S. gallolyticus and S. chromogenes in a Smallholder Dairy Farm in Cambodia. Transbound. Emerg. Dis. 2025, 2025, 3621605. [Google Scholar] [CrossRef]
- Chuasakhonwilai, A.; Keeklangdon, P.; Chaisri, W.; Saipinta, D.; Photiboon, K.; Kaewmuangma, D.; Anuphom, W.; Intanon, M.; Suriyasathaporn, W. Different bacterial growth of major mastitis pathogens after coculturing with Staphylococcus chromogenes and Staphylococcus hominis in milk in vitro. Front. Microbiol. 2025, 2025, 1707938. [Google Scholar] [CrossRef]
- Chaisri, W.; Intanon, M.; Saipinta, D.; Srithanasuwan, A.; Pangprasit, N.; Jaraja, W.; Chuasakhonwilai, A.; Suriyasathaporn, W. Variation in interleukin-4, -6, and -10 in mastitis milk: Associations with infections, pathogens, somatic cell counts, and oxidative stress. Vet. Sci. 2024, 11, 350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koop, G.; van Werven, T.; Schuiling, H.J.; Nielen, M. The effect of subclinical mastitis on milk yield in dairy goats. J. Dairy Sci. 2010, 93, 5809–5817. [Google Scholar] [CrossRef]
- De Buck, J.; Ha, V.; Naushad, S.; Nobrega, D.B.; Luby, C.; Middleton, J.R.; De Vliegher, S.; Barkema, H.W. Non-aureus staphylococci and bovine udder health: Current understanding and knowledge gaps. Front. Vet. Sci. 2021, 8, 658031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- Lam, T.; Olde Riekerink, R.; Sampimon, O.; Smith, H. Mastitis diagnostics and performance monitoring: A practical approach. Ir. Vet. J. 2009, 62, S34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leelahapongsathon, K.; Piroon, T.; Chaisri, W.; Suriyasathaporn, W. Factors in dry period associated with intramammary infection and subsequent clinical mastitis in early postpartum cows. Asian-Australas. J. Anim. Sci. 2016, 4, 580–585. [Google Scholar] [CrossRef] [PubMed]
- NMC. National Mastitis Council Recommended Milking Procedures. 2013. Available online: https://www.nmconline.org/wp-content/uploads/2016/09/Recommended-Milking-Procedures.pdf (accessed on 1 January 2025).
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley: Reading, MA, USA, 1977. [Google Scholar]
- Constable, P.D.; Hinchcliff, K.W.; Done, S.H.; Grünberg, W. Diseases of the Mammary Gland. In Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs, and Goats, 11th ed.; Elsevier: St. Louis, MO, USA, 2017; pp. 495–560. [Google Scholar]
- Zalewska, M.; Brzozowska, P.; Rzewuska, M.; Kawecka-Grochocka, E.; Urbańska, D.M.; Sakowski, T.; Bagnicka, E. The quality and technological parameters of milk obtained from dairy cows with subclinical mastitis. J. Dairy Sci. 2025, 108, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Berglund, I.; Pettersson, G.; Ostensson, K.; Svennersten-Sjaunja, K. Quarter milking for improved detection of increased SCC. Reprod. Domest. Anim. 2007, 42, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Antanaitis, R.; Juozaitienė, V.; Jonike, V.; Baumgartner, W.; Paulauskas, A. Milk lactose as a biomarker of subclinical mastitis in dairy cows. Animal 2021, 11, 1736. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suriyasathaporn, W. Milk Quality and Antimicrobial Resistance against Mastitis Pathogens after Changing from a Conventional to an Experimentally Organic Dairy Farm. Asian-Australas. J. Anim. Sci. 2010, 23, 659–664. [Google Scholar] [CrossRef]
- Litwinczuk, Z.; Barlowska, J.; Chabuz, W.; Brodziak, A. Nutritional value and technological suitability of milk from cows of three Polish breeds included in the genetic resources conservation programme. Ann. Anim. Sci. 2012, 12, 423. [Google Scholar] [CrossRef]
- Watters, R.D.; Schuring, N.; Erb, H.N.; Schukken, Y.H. The effect of premilking udder preparation on Holstein cows milked 3 times daily. J. Dairy Sci. 2012, 95, 1170–1176. [Google Scholar] [CrossRef]
- Rico, D.E.; Marshall, E.R.; Choi, J.; Kaylegian, K.E.; Dechow, C.D.; Harvatine, K.J. Within-milking variation in milk composition and fatty acid profile of Holstein dairy cows. J. Dairy Sci. 2014, 97, 4259–4268. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kuki, C.; Oyama, S.; Kumura, H. Pro-inflammatory cytokine TNF-α is a key inhibitory factor for lactose synthesis pathway in lactating mammary epithelial cells. Exp. Cell Res. 2016, 340, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Matsunaga, K.; Tsugami, Y.; Wakasa, H.; Takanori Nishimura, T. IL-1β is a key inflammatory cytokine that weakens lactation-specific tight junctions of mammary epithelial cells. Exp. Cell Res. 2021, 409, 112938. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Streicher, K.L. The role of oxidative stress in the pathogenesis of mastitis. J. Dairy Sci. 2002, 85, E102–E114. [Google Scholar]
- Suriyasathaporn, W.; Kongkaew, A.; Intanon, M.; Srithanasuwan, A.; Saipinta, D.; Pangprasit, N.; Thongtharb, A.; Chuasakhonwilai, A.; Chaisri, W. Non-aureus staphylococci cause the spontaneous cure or persistent infection of major bovine mastitis pathogens in the murine mammary glands. Animal 2024, 14, 3526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bezerra, A.B.; de Leon, C.M.C.G.; Givisiez, P.E.N.; Silva, N.M.V.; Santos Filho, L.; Pereira, W.E.; Filho, E.C.P.; Azevedo, P.S.; Oliveira, C.J.B. Pathogen-specific changes in composition and quality traits of milk from goats affected by subclinical intramammary infections. J. Dairy Res. 2021, 88, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Suriyasathaporn, W.; Schukken, Y.H.; Nielen, M.; Brand, A. Low somatic cell count: A risk factor for subsequent clinical mastitis in a dairy herd. J. Dairy Sci. 2000, 83, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Kehrli, M.E.; Shuster, D.E. Factors affecting milk somatic cells and their role in health of the bovine mammary gland. J. Dairy Sci. 1994, 77, 619–627. [Google Scholar] [CrossRef]
- Khatun, M.; Bruckmaier, R.M.; Thomson, P.C.; House, J.; García, S.C. Suitability of somatic cell count, electrical conductivity, and lactate dehydrogenase activity in foremilk before versus after alveolar milk ejection for mastitis detection. J. Dairy Sci. 2019, 102, 9200–9212. [Google Scholar] [CrossRef]
- Fatehi, F.; Zali, A.; Honarvar, M.; Dehghan-banadaky, M.; Young, A.J.; Ghiasvand, M.; Eftekhari, M. Review of the relationship between milk urea nitrogen and days in milk, parity, and monthly temperature mean in Iranian Holstein cows. J. Dairy Sci. 2011, 95, 5156–5163. [Google Scholar] [CrossRef]
- Park, C.S.; Jacobson, N.L. The mammary gland and lactation. In Dukes’ Physiology of Domestic Animals, 11th ed.; Swenson, M.J., Reece, W.O., Eds.; Cornell University: Ithaca, NY, USA, 1993; pp. 711–727. [Google Scholar]
- Mayberrya, D.; Asha, A.; Prestwidgea, D.; Goddea, M.; Hendersona, B.; Duncanb, A.; Blummel, M.; Reddy, Y.R.; Herrero, M. Yield gap analyses to estimate attainable bovine milk yields and evaluate options to increase production in Ethiopia and India. Agric. Syst. 2017, 115, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mukasafari, M.A.; Mpatswenumugabo, J.P.; Ndahetuye, J.B. Management factors affecting milk yield, composition, and quality on smallholder dairy farms. Trop. Anim. Health Prod. 2025, 57, 4. [Google Scholar] [CrossRef] [PubMed]
- Kala, S.; Pytlewski, J. The effect of parity and lactation stage on the milk composition, and somatic cell count of Holstein-Friesian Cows. J. Anim. Feed Sci 2018, 27, 305–312. [Google Scholar]
- Walen, A. Milk Composition and Properties. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, J., McSweeney, P., Eds.; Academic Press: London, UK, 2022; pp. 609–617. [Google Scholar]
- Henno, M.; Ots, M.; Joudu, I.; Kaart, T.; Kärt, O. Factors affecting the freezing point stability of milk from individual cows. Int. Dairy J. 2008, 18, 210–215. [Google Scholar] [CrossRef]
- Szklo, M.; Nieto, F.J. Epidemiology: Beyond the Basics, 4th ed.; Jones & Bartlett Learning: Burlington, MA, USA, 2019. [Google Scholar]

| Fat | Protein | Lactose | SNF | TS | FP | |
|---|---|---|---|---|---|---|
| Protein | 0.016 | |||||
| Lactose | −0.107 ** | 0.024 | ||||
| SNF | −0.080 * | 0.704 *** | 0.693 *** | |||
| TS | 0.732 *** | 0.515 *** | 0.353 *** | 0.616 *** | ||
| FP | 0.107 ** | −0.264 *** | −0.852 *** | −0.842 *** | −0.481 *** | |
| SCS | 0.120 ** | −0.178 *** | −0.520 *** | −0.291 *** | −0.083 | 0.500 *** |
| Bacteria Group | Farm | Total | ||
|---|---|---|---|---|
| A | B | C | ||
| Streptococci | 0 (0) | 12 (13.64) | 23 (6.55) | 35 (7.78) |
| Coagulase-negative Staphylococci (CNS) | 8 (72.73) | 19 (21.59) | 88 (25.07) | 115 (25.56) |
| Gram-bacteria | 0 (0) | 1 (1.14) | 8 (2.28) | 9 (2.00) |
| Streptococci-CNS (Mixed) | 2 (18.18) | 8 (9.09) | 1 (0.28) | 11 (2.44) |
| None detected (None) | 1 (9.09) | 47 (54.55) | 225 (65.81) | 280 (62.22) |
| Total | 11 | 88 | 351 | 450 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, S.; Nouv, S.; Dim, K.; Na, S.; Sokhom, P.; Matsuyama, S.; Murase, T.; Ohkura, S.; Suriyasathaporn, W. Impact of Coagulase-Negative Staphylococci in Mixed Intramammary Infections with Streptococci on Milk Quality. Biology 2025, 14, 1672. https://doi.org/10.3390/biology14121672
Nakamura S, Nouv S, Dim K, Na S, Sokhom P, Matsuyama S, Murase T, Ohkura S, Suriyasathaporn W. Impact of Coagulase-Negative Staphylococci in Mixed Intramammary Infections with Streptococci on Milk Quality. Biology. 2025; 14(12):1672. https://doi.org/10.3390/biology14121672
Chicago/Turabian StyleNakamura, Sho, Sophorn Nouv, Kanan Dim, Sambo Na, Panhavatey Sokhom, Shuichi Matsuyama, Tetsuma Murase, Satoshi Ohkura, and Witaya Suriyasathaporn. 2025. "Impact of Coagulase-Negative Staphylococci in Mixed Intramammary Infections with Streptococci on Milk Quality" Biology 14, no. 12: 1672. https://doi.org/10.3390/biology14121672
APA StyleNakamura, S., Nouv, S., Dim, K., Na, S., Sokhom, P., Matsuyama, S., Murase, T., Ohkura, S., & Suriyasathaporn, W. (2025). Impact of Coagulase-Negative Staphylococci in Mixed Intramammary Infections with Streptococci on Milk Quality. Biology, 14(12), 1672. https://doi.org/10.3390/biology14121672






