Population Estimation and Demographic Characteristics of Free-Roaming Dogs on Kasetsart University Kamphaeng Saen Campus, Thailand: Implications for Rabies Control
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
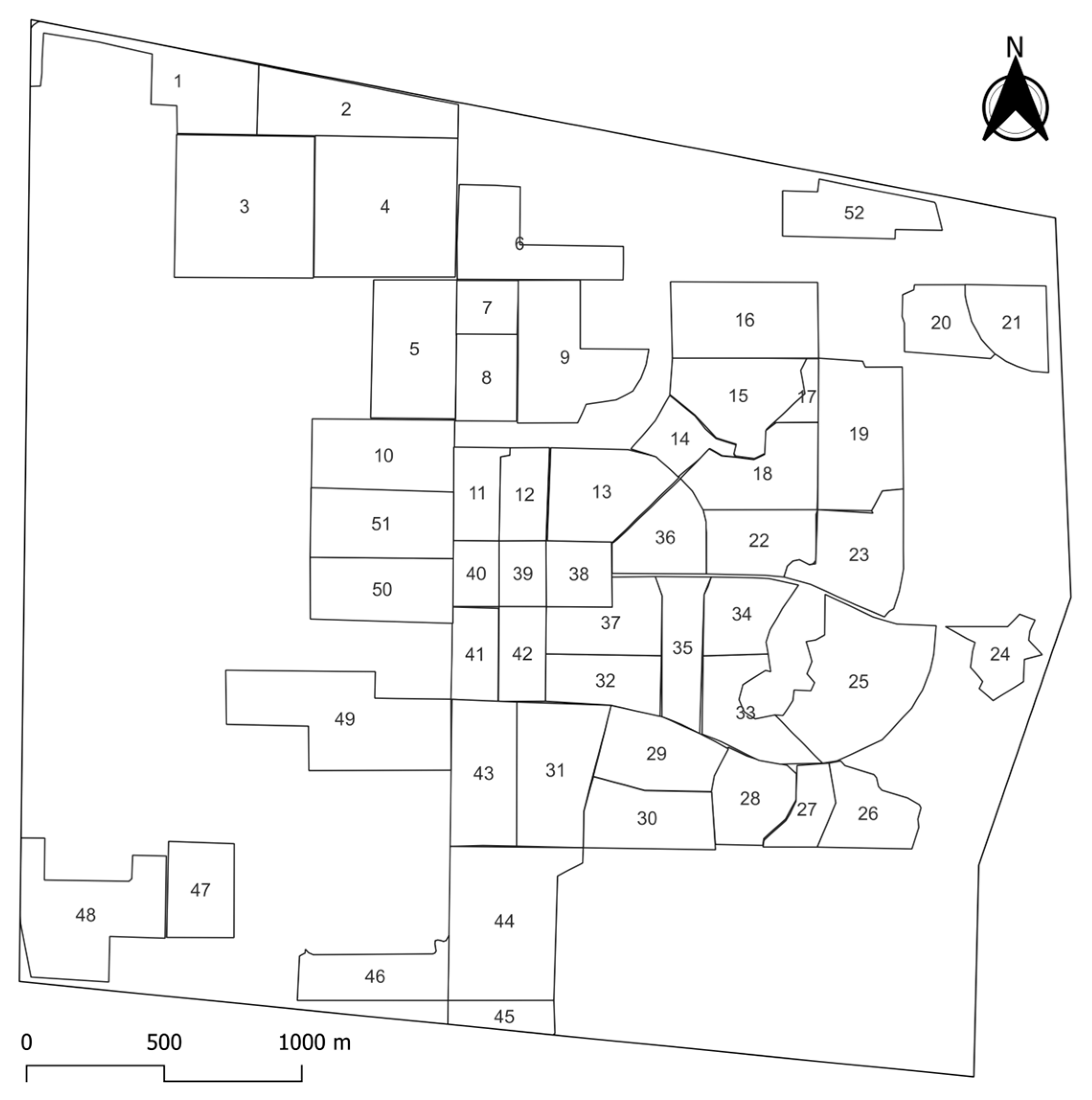
2.1. Study Site
2.2. Study Population
2.3. Data Collection
2.4. Data Analyses
2.4.1. Estimations of FRD Population Size
2.4.2. Multivariable Analyses of Factors Influencing Population Size
2.4.3. Analysis of FRD Sightings Variation
3. Results
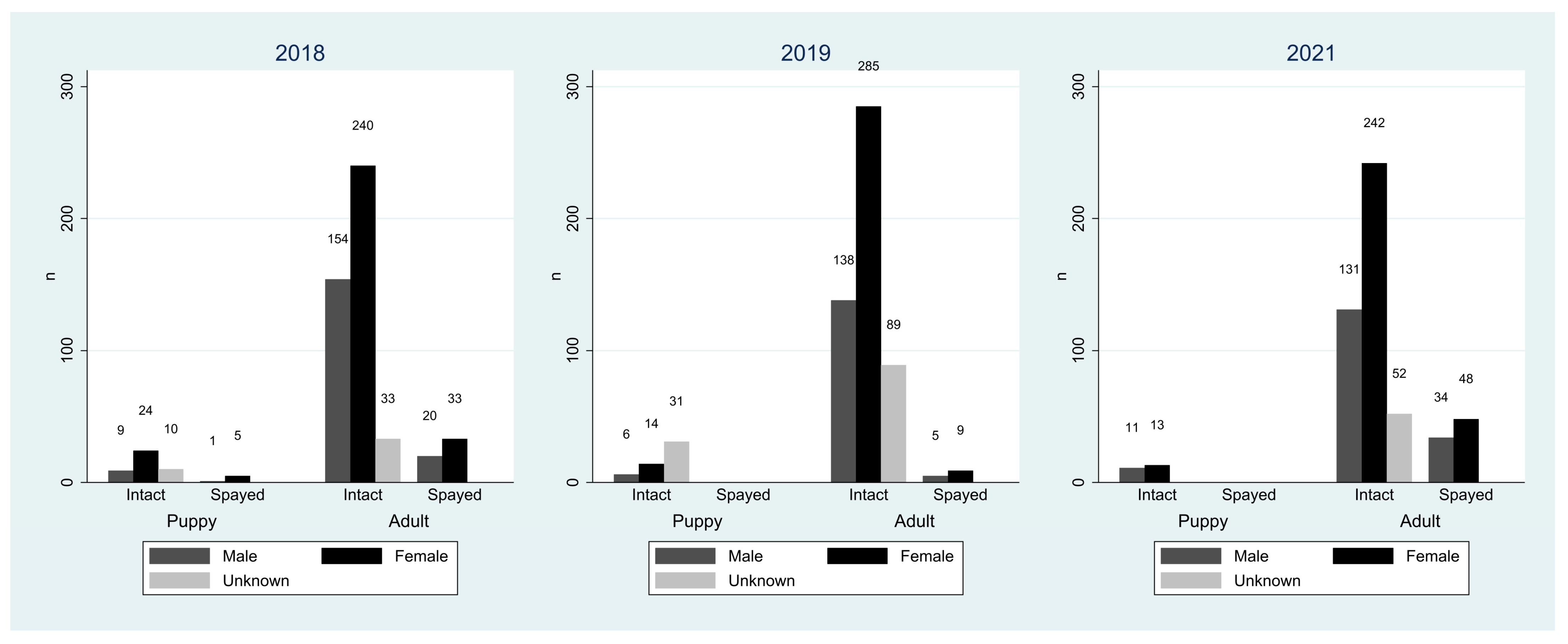
3.1. FRD Population Sizes and Demographic Characteristics
3.2. Factors Associated with Population Size
3.3. FRD Sightings Variation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FRD | Free-roaming dog |
| WHO | World Health Organization |
| DPM | Dog population management |
| WOAH | World Organisation for Animal Health |
| KU | Kasetsart University |
| KPS | Kamphaeng Saen |
| CNR | Capture–neuter–return |
| GLS | Generalized least-squares |
| WSPA | World Society for the Protection of Animals |
| ICAM | International Companion Animal Management Coalition |
References
- WSPA. Surveying roaming dog populations: Guidelines on methodology. Companion Work. Anim. Unit. 2012, 1, 1–20. [Google Scholar]
- Slater, M.R. The role of veterinary epidemiology in the study of free-roaming dogs and cats. Prev. Vet. Med. 2001, 48, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Boitani, L.; Francisci, F.; Ciucci, P.; Andreoli, G. The ecology and behavior of feral dogs: A case study from central Italy. In The Domestic Dog: Its Evolution, Behavior and Interactions with People, 2nd ed.; Serpell, J., Ed.; Cambridge University Press: Cambridge, UK, 2016; pp. 342–368. [Google Scholar]
- Smith, L.M.; Hartmann, S.; Munteanu, A.M.; Dalla Villa, P.; Quinnell, R.J.; Collins, L.M. The effectiveness of dog population management: A systematic review. Animals 2019, 9, 1020. [Google Scholar] [CrossRef]
- Jackman, J. Free-Roaming Dogs in Developing Countries: The Benefits of Capture, Neuter, and Return Programs. In The State of the Animals IV; Salem, D.J., Rowan, A.N., Eds.; Humane Society Press: Washington, DC, USA, 2007; pp. 55–78. [Google Scholar]
- Beck, A.M. The human-dog relationship: A tale of two species. In Dogs, Zoonoses, and Public Health, 2nd ed.; CABI International: Wallingford, UK, 2013; pp. 1–12. [Google Scholar]
- Akpinar, O.; Kapci, M.; Duman, A.; Turkdogan, K.A. Evaluation of suspected rabies animal bites and comparison of vaccination protocols. Acta Med. Mediterr. 2015, 31, 919–924. [Google Scholar]
- Sudarshan, M.; Mahendra, B.; Madhusudana, S.; Narayana, D.A.; Rahman, A.; Rao, N.; X-Meslin, F.; Lobo, D.; Ravikumar, K. An epidemiological study of animal bites in India: Results of a WHO sponsored national multi-centric rabies survey. J. Commun. Dis. 2006, 38, 32–39. [Google Scholar]
- Massei, G.; Fooks, A.; Horton, D.L.; Callaby, R.; Sharma, K.; Dhakal, I.; Dahal, U. Free-roaming dogs in Nepal: Demographics, health and public knowledge, attitudes and practices. Zoonoses Public Health 2017, 64, 29–40. [Google Scholar] [CrossRef]
- Butcher, R.L.; De Keuster, T. Dog-Associated Problems Affecting Public Health and Community Wellbeing. In Dogs, Zoonoses, and Public Health, 2nd ed.; CABI International: Wallingford, UK, 2013; pp. 24–42. [Google Scholar]
- Garde, E.; Acosta-Jamett, G.; Bronsvoort, B.M. Review of the Risks of Some Canine Zoonoses from Free-Roaming Dogs in the Post-Disaster Setting of Latin America. Animals 2013, 3, 855–865. [Google Scholar] [CrossRef]
- Kardjadj, M.; Yahiaoui, F.; Ben-Mahdi, M. Incidence of human dog-mediated zoonoses and demographic characteristics/vaccination coverage of the domestic dog population in Algeria. Rev. Sci. Tech. 2019, 38, 809–821. [Google Scholar] [CrossRef]
- Schurer, J.M.; Ndao, M.; Skinner, S.; Irvine, J.; Elmore, S.A.; Epp, T.; Jenkins, E.J. Parasitic Zoonoses: One Health Surveillance in Northern Saskatchewan. PLoS Negl. Trop. Dis. 2013, 7, e2141. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization of the United Nations; World Organisation for Animal Health; Global Alliance for Rabies Control. Zero by 30: The Global Strategic Plan to End Human Deaths from Dog-Mediated Rabies by 2030: United Against Rabies Collaboration: First Annual Progress Report: Global Strategic Plan to End Human Deaths from Dog-Mediated Rabies by 2030; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Thanapongtharm, W.; Kasemsuwan, S.; Wongphruksasoong, V.; Boonyo, K.; Pinyopummintr, T.; Wiratsudakul, A.; Gilbert, M.; Leelahapongsathon, K. Spatial Distribution and Population Estimation of Dogs in Thailand: Implications for Rabies Prevention and Control. Front. Vet. Sci. 2021, 8, 790701. [Google Scholar] [CrossRef]
- Komol, P.; Sommanosak, S.; Jaroensrisuwat, P.; Wiratsudakul, A.; Leelahapongsathon, K. The Spread of Rabies Among Dogs in Pranburi District, Thailand: A Metapopulation Modeling Approach. Front. Vet. Sci. 2020, 7, 570504. [Google Scholar] [CrossRef] [PubMed]
- International Companion Animal Management Coalition (ICAM). Humane Dog Population Management Guidance; International Companion Animal Management Coalition: London, UK, 2019. [Google Scholar]
- Taylor, L.H.; Wallace, R.M.; Balaram, D.; Lindenmayer, J.M.; Eckery, D.C.; Mutonono-Watkiss, B.; Parravani, E.; Nel, L.H. The Role of Dog Population Management in Rabies Elimination—A Review of Current Approaches and Future Opportunities. Front. Vet. Sci. 2017, 4, 109. [Google Scholar] [CrossRef]
- Massei, G. Fertility control in dogs. In Dogs, Zoonoses and Public Health, 2nd ed.; Macpherson, C.N., Meslin, F.-X., Wandeler, A.I., Eds.; CABI International: Wallingford, UK, 2013; pp. 259–270. [Google Scholar]
- OIE. Dog Population Management. In Terrestrial Animal Health Code; World Organisation for Animal Health: Paris, France, 2022. [Google Scholar]
- Tenzin, T.; Ahmed, R.; Debnath, N.C.; Ahmed, G.; Yamage, M. Free-Roaming Dog Population Estimation and Status of the Dog Population Management and Rabies Control Program in Dhaka City, Bangladesh. PLoS Negl. Trop. Dis. 2015, 9, e0003784. [Google Scholar] [CrossRef]
- World Health Organization Regional Office for South-East Asia. Strategic Framework for Elimination of Human Rabies Transmitted by Dogs in the South-East Asia Region; WHO Regional Office for South-East Asia: New Delhi, India, 2012. [Google Scholar]
- Amaral, A.C.; Ward, M.P.; da Costa Freitas, J. Estimation of roaming dog populations in Timor Leste. Prev. Vet. Med. 2014, 113, 608–613. [Google Scholar] [CrossRef]
- Serafini, C.; Rosa, G.; Guimaraes, A.; De Morais, H.; Biondo, A. Survey of owned feline and canine populations in apartments from a neighbourhood in Curitiba, Brazil. Zoonoses Public Health 2008, 55, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Google. KPS Dogs Census. Available online: https://www.google.com/maps/d/ (accessed on 1 August 2024).
- Phokham, K.; Therakul, N.; Eurwilaichit, A.; Boonkasemsanti, A.; Leelahapongsathon, K.; Kasemsuwan, S. A comparison of Roaming-dog population estimation method in Kasetsart University, Kamphaeng Saen Campus, Nakhon Pathom. In Proceedings of the 29th Case Study Conference of 6th Year Students., Faculty of Veterinary Medicine, Kasetsart University, Nakhon Pathom, Thailand, 14–15 March 2020. [Google Scholar]
- Centre for Genomic Pathogen Surveillance. Epicollect5. Available online: https://five.epicollect.net (accessed on 11 January 2022).
- Microsoft Excel, Microsoft Excel 365; Microsoft Corporation: Redmond, WA, USA, 2025.
- StataCorp. Stata Statistical Software: Release 17, StataCorp LLC: College Station, TX, USA, 2021.
- Pinheiro, J.; Bates, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models, R package version 3.1-167; R Package Version: Lexington, MA, USA, 2025.
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Maruyama, N.; Nakama, S. Block count method for estimating serow populations. Jap. J. Ecol. 1983, 33, 243–251. [Google Scholar]
- Corlatti, L.; Fattorini, L.; Nelli, L. The use of block counts, mark-resight and distance sampling to estimate population size of a mountain-dwelling ungulate. Popul. Ecol. 2015, 57, 419. [Google Scholar] [CrossRef]
- Thapa, K.; Jackson, R.; Gurung, L.; Acharya, H.; Gurung, R. Applying the double observer methodology for assessing blue sheep population size in Nar Phu valley, Annapurna Conservation Area, Nepal. Wildl. Biol. 2021, 2021, wlb-00877. [Google Scholar] [CrossRef]
- Goda, R.; Ando, M.; Sato, H.; Shibata, E.I. Application of fecal pellet group count to sika deer (Cervus nippon) population monitoring on Mt. Ohdaigahara, central Japan. Mammal Study 2008, 33, 93–97. [Google Scholar] [CrossRef]
- Mizuki, I.; Itô, H.; Yamasaki, M.; Fukumoto, S.; Okamoto, Y.; Katsuki, M.; Fukushima, K.; Sakai, M.; Sakaguchi, S.; Fujiki, D. Seasonal and annual fluctuations of deer populations estimated by a Bayesian state–space model. PLoS ONE 2020, 15, e0225872. [Google Scholar] [CrossRef]
- Borkowski, J.; Palmer, S.C.; Borowski, Z. Drive counts as a method of estimating ungulate density in forests: Mission impossible? Acta Theriol. 2011, 56, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Shamsaddini, S.; Ahmadi Gohari, M.; Kamyabi, H.; Nasibi, S.; Derakhshani, A.; Mohammadi, M.A.; Mousavi, S.M.; Baneshi, M.R.; Hiby, E.; Harandi, M.F. Dynamic modeling of female neutering interventions for free-roaming dog population management in an urban setting of southeastern Iran. Sci. Rep. 2022, 12, 4781. [Google Scholar] [CrossRef] [PubMed]
- Pérez, G.E.; Conte, A.; Garde, E.J.; Messori, S.; Vanderstichel, R.; Serpell, J. Movement and home range of owned free-roaming male dogs in Puerto Natales, Chile. Appl. Anim. Behav. Sci. 2018, 205, 74–82. [Google Scholar] [CrossRef]
- Dürr, S.; Dhand, N.K.; Bombara, C.; Molloy, S.; Ward, M.P. What influences the home range size of free-roaming domestic dogs? Epidemiol. Infect. 2017, 145, 1339–1350. [Google Scholar] [CrossRef]
- Kittisiam, T.; Phimpraphai, W.; Kasemsuwan, S.; Thakur, K.K. Analyses of Contact Networks of Community Dogs on a University Campus in Nakhon Pathom, Thailand. Vet. Sci. 2021, 8, 299. [Google Scholar] [CrossRef]
- Ivanter, E.V.; Sedova, N.A. Ecological monitoring of urban groups of stray dogs: An example of the city of petrozavodsk. Russian J. Ecol. 2008, 39, 105–110. [Google Scholar] [CrossRef]
- Dias, R.A.; Guilloux, A.G.A.; Borba, M.R.; de Lourdes Guarnieri, M.C.; Prist, R.; Ferreira, F.; Amaku, M.; Neto, J.S.F.; Stevenson, M. Size and spatial distribution of stray dog population in the University of São Paulo campus, Brazil. Prev. Vet. Med. 2013, 110, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Cunha Silva, L.; Friker, B.; Warembourg, C.; Kanankege, K.; Wera, E.; Berger-González, M.; Alvarez, D.; Dürr, S. Habitat selection by free-roaming domestic dogs in rabies endemic countries in rural and urban settings. Sci. Rep. 2022, 12, 20928. [Google Scholar] [CrossRef]
- Butler, J.R.A.; du Toit, J.T. Diet of free-ranging domestic dogs (Canis familiaris) in rural Zimbabwe: Implications for wild scavengers on the periphery of wildlife reserves. Anim. Conserv. 2002, 5, 29–37. [Google Scholar] [CrossRef]
- Sepúlveda, M.; Pelican, K.; Cross, P.; Eguren, A.; Singer, R. Fine-scale movements of rural free-ranging dogs in conservation areas in the temperate rainforest of the coastal range of southern Chile. Mamm. Biol. 2015, 80, 290–297. [Google Scholar] [CrossRef]
- Tenzin, T.; McKenzie, J.S.; Vanderstichel, R.; Rai, B.D.; Rinzin, K.; Tshering, Y.; Pem, R.; Tshering, C.; Dahal, N.; Dukpa, K. Comparison of mark-resight methods to estimate abundance and rabies vaccination coverage of free-roaming dogs in two urban areas of south Bhutan. Prev. Vet. Med. 2015, 118, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Gibson, A.; Fielding, H.; Ohal, P.; Pandey, P.; Kumar, A.; Singh, S.K.; Airikkala-Otter, I.; Abela-Ridder, B.; Gamble, L.; et al. Free-roaming dog population dynamics in Ranchi, India. Res. Vet. Sci. 2022, 143, 115–123. [Google Scholar] [CrossRef]
- Meunier, N.V.; Gibson, A.D.; Corfmat, J.; Mazeri, S.; Handel, I.G.; Bronsvoort, B.M.D.C.; Gamble, L.; Mellanby, R.J. Reproducibility of the mark-resight method to assess vaccination coverage in free-roaming dogs. Res. Vet. Sci. 2019, 123, 305–310. [Google Scholar] [CrossRef]
- Hu, C.-H.; Yu, P.-H.; Kang, C.-L.; Chen, H.L.; Yen, S.-C. Demography and welfare status of free-roaming dogs in Yangmingshan National Park, Taiwan. Prev. Vet. Med. 2019, 166, 49–55. [Google Scholar] [CrossRef]
- Tiwari, H.K.; Robertson, I.D.; O’Dea, M.; Gogoi-Tiwari, J.; Panvalkar, P.; Bajwa, R.S.; Vanak, A.T. Validation of Application SuperDuplicates (AS) Enumeration Tool for Free-Roaming Dogs (FRD) in Urban Settings of Panchkula Municipal Corporation in North India. Front. Vet. Sci. 2019, 6, 173. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, H.K.; Vanak, A.T.; O’Dea, M.; Gogoi-Tiwari, J.; Robertson, I.D. A Comparative Study of Enumeration Techniques for Free-Roaming Dogs in Rural Baramati, District Pune, India. Front. Vet. Sci. 2018, 5, 104. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, H.K.; Robertson, I.D.; O’Dea, M.; Vanak, A.T. Demographic characteristics of free-roaming dogs (FRD) in rural and urban India following a photographic sight-resight survey. Sci. Rep. 2019, 9, 16562. [Google Scholar] [CrossRef]
- Lyons, M.A.; Malhotra, R.; Thompson, C.W. Investigating the free-roaming dog population and gastrointestinal parasite diversity in Tulúm, México. PLoS ONE 2022, 17, e0276880. [Google Scholar] [CrossRef]
- Smith, L.M.; Goold, C.; Quinnell, R.J.; Munteanu, A.M.; Hartmann, S.; Dalla Villa, P.; Collins, L.M. Population dynamics of free-roaming dogs in two European regions and implications for population control. PLoS ONE 2022, 17, e0266636. [Google Scholar] [CrossRef]
- Cleaton, J.M.; Blanton, J.D.; Dilius, P.; Ludder, F.; Crowdis, K.; Medley, A.; Chipman, R.; Estime, F.; Maciel, E.; Wallace, R.M. Use of photography to identify free-roaming dogs during sight-resight surveys: Impacts on estimates of population size and vaccination coverage, Haiti 2016. Vaccine X 2019, 2, 100025. [Google Scholar] [CrossRef]
- Font, E. Spacing and social organization: Urban stray dogs revisited. Appl. Anim. Behav. Sci. 1987, 17, 319–328. [Google Scholar] [CrossRef]
- Belsare, A.V.; Gompper, M.E. Assessing demographic and epidemiologic parameters of rural dog populations in India during mass vaccination campaigns. Prev. Vet. Med. 2013, 111, 139–146. [Google Scholar] [CrossRef]
- Majumder, S.; Chatterjee, A.; Bhadra, A. A dog’s day with humans—Time activity budget of free-ranging dogs in India. Curr. Sci. 2014, 106, 874–878. [Google Scholar]
- Belo, V.S.; Struchiner, C.J.; Werneck, G.L.; Teixeira Neto, R.G.; Tonelli, G.B.; de Carvalho Júnior, C.G.; Ribeiro, R.A.N.; da Silva, E.S. Abundance, survival, recruitment and effectiveness of sterilization of free-roaming dogs: A capture and recapture study in Brazil. PLoS ONE 2017, 12, e0187233. [Google Scholar] [CrossRef]
- Kitala, P.; McDermott, J.; Kyule, M.; Gathuma, J.; Perry, B.; Wandeler, A. Dog ecology and demography information to support the planning of rabies control in Machakos District, Kenya. Acta Trop. 2001, 78, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Bingham, J. Demography and dog-human relationships of the dog population in Zimbabwean communal lands. Vet. Rec. 2000, 147, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Jamett, G.; Cleaveland, S.; Cunningham, A.A.; Bronsvoort, B.M.d. Demography of domestic dogs in rural and urban areas of the Coquimbo region of Chile and implications for disease transmission. Prev. Vet. Med. 2010, 94, 272–281. [Google Scholar] [CrossRef]
- Czupryna, A.M.; Brown, J.S.; Bigambo, M.A.; Whelan, C.J.; Mehta, S.D.; Santymire, R.M.; Lankester, F.J.; Faust, L.J. Ecology and Demography of Free-Roaming Domestic Dogs in Rural Villages near Serengeti National Park in Tanzania. PLoS ONE 2016, 11, e0167092. [Google Scholar] [CrossRef]
- Melo, S.N.d.; da Silva, E.S.; Barbosa, D.S.; Teixeira-Neto, R.G.; Lacorte, G.A.; Horta, M.A.P.; Cardoso, D.T.; Werneck, G.L.; Struchiner, C.J.; Belo, V.S. Effects of Gender, Sterilization, and Environment on the Spatial Distribution of Free-Roaming Dogs: An Intervention Study in an Urban Setting. Front. Vet. Sci. 2020, 7, 289. [Google Scholar] [CrossRef]
- Conan, A.; Akerele, O.; Simpson, G.; Reininghaus, B.; van Rooyen, J.; Knobel, D. Population Dynamics of Owned, Free-Roaming Dogs: Implications for Rabies Control. PLoS Negl. Trop. Dis. 2015, 9, e0004177. [Google Scholar] [CrossRef]
- Borchelt, P.L. Aggressive behavior of dogs kept as companion animals: Classification and influence of sex, reproductive status and breed. Appl. Anim. Ethol. 1983, 10, 45–61. [Google Scholar] [CrossRef]
- Wright, J.C.; Nesselrote, M.S. Classification of behavior problems in dogs: Distributions of age, breed, sex and reproductive status. Appl. Anim. Behav. Sci. 1987, 19, 169–178. [Google Scholar] [CrossRef]
- Morters, M.K.; McKinley, T.J.; Restif, O.; Conlan, A.J.; Cleaveland, S.; Hampson, K.; Whay, H.R.; Damriyasa, I.M.; Wood, J.L. The demography of free-roaming dog populations and applications to disease and population control. J. Appl. Ecol. 2014, 51, 1096–1106. [Google Scholar] [CrossRef]
- Totton, S.C.; Wandeler, A.I.; Ribble, C.S.; Rosatte, R.C.; McEwen, S.A. Stray dog population health in Jodhpur, India in the wake of an animal birth control (ABC) program. Prev. Vet. Med. 2011, 98, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Reece, J.F.; Chawla, S.K.; Hiby, E.F.; Hiby, L.R. Fecundity and longevity of roaming dogs in Jaipur, India. BMC Vet. Res. 2008, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Reece, J. Timing of oestrus and reproductive behaviour in Indian street dogs. Vet. Rec. 2002, 150, 450–451. [Google Scholar] [CrossRef]
- Ortega-Pacheco, A.; Segura-Correa, J.; Jimenez-Coello, M.; Forsberg, C.L. Reproductive patterns and reproductive pathologies of stray bitches in the tropics. Theriogenology 2007, 67, 382–390. [Google Scholar] [CrossRef]
- Pal, S.K. Population ecology of free-ranging urban dogs in West Bengal, India. Acta Theriol. 2001, 46, 69–78. [Google Scholar] [CrossRef]
- Pal, S.K. Reproductive behaviour of free-ranging rural dogs in West Bengal, India. Acta Theriol. 2003, 48, 271–281. [Google Scholar] [CrossRef]
- Kalthoum, S.; Ben Salah, C.; Rzeigui, H.; Gharbi, R.; Guesmi, K.; Ben Salem, A.; Ferchichi, S.; Zammel, F.; Fatnassi, N.; Bahloul, C.; et al. Owned and free-roaming dogs in the North West of Tunisia: Estimation, characteristics and application for the control of dog rabies. Heliyon 2021, 7, e08347. [Google Scholar] [CrossRef]
- Brill, G.; Kartal, T.; Yadav, D.P.; Bhyan, M.; Patel, C.; Sheoran, S.K.; Patel, P.; Painuly, B.; Chaudhari, A. Seasonal Patterns of Oestrus and Reproduction in Street Dogs of Indian Cities. Front. Vet. Sci. 2022, 9, 821424. [Google Scholar] [CrossRef] [PubMed]
- Kisiel, L.M.; Jones-Bitton, A.; Sargeant, J.M.; Coe, J.B.; Flockhart, D.T.T.; Canales Vargas, E.J.; Greer, A.L. Modeling the effect of surgical sterilization on owned dog population size in Villa de Tezontepec, Hidalgo, Mexico, using an individual-based computer simulation model. PLoS ONE 2018, 13, e0198209. [Google Scholar] [CrossRef] [PubMed]
- Yoak, A.J.; Reece, J.F.; Gehrt, S.D.; Hamilton, I.M. Optimizing free-roaming dog control programs using agent-based models. Ecosyst. Model 2016, 341, 53–61. [Google Scholar] [CrossRef]
- Davlin, S.L.; VonVille, H.M. Canine rabies vaccination and domestic dog population characteristics in the developing world: A systematic review. Vaccine 2012, 30, 3492–3502. [Google Scholar] [CrossRef]


| Data | Categories | Method of Estimation |
|---|---|---|
| GPS coordinates | Latitude and longitude | GPS recording in Epicollect5 app. |
| Block | The block number | Google My Maps current location |
| Sex | Male/Female/Unknown | Observation of reproductive organs |
| Reproductive status | Intact/Spayed or Neutered | Male: Observation of reproductive organs Female: Observation of circular brands on thighs and/or caretaker interviews |
| Age | Puppy/Adult | Observation of dogs if they depend on their mothers |
| Physical characteristics and other prominent appearances | Coat color: Multicolored/Two-colored/ Mono-colored Prominent appearances: Natural marks and/or scars Ear shape: Erect/Drop/Semi-erect Tail shape: Bobbed/Docked/Curly/Whip/ Saber | Observation |
| Variables | n (%) | Mean | Variance | SD | Median | IQR |
|---|---|---|---|---|---|---|
| Number of FRDs in each block | 156 | 10.49 | 164.03 | 12.81 | 7 | 4–14.5 |
| Presence of food source | 156 | |||||
| No | 132 (84.62) | |||||
| Yes | 24 (15.38) | |||||
| Year of observations 2018 2019 2021 | 156 52 (33.33) 52 (33.33) 52 (33.33) | |||||
| Block area size (km2) | 156 | 0.11 | 0.003 | 0.06 | 0.09 | 0.06–0.13 |
| Number of intact adult female dogs | 156 | 5.24 | 25.90 | 5.09 | 4 | 2–7 |
| Number of intact adult male dogs | 156 | 2.88 | 17.22 | 4.15 | 2 | 1–4 |
| Proportion of building area within each block | 156 | 0.09 | 0.007 | 0.08 | 0.078 | 0.03–0.17 |
| Number of Dogs: n (%) | Year | ||
|---|---|---|---|
| 2018 | 2019 | 2021 | |
| Total dogs | 529 | 577 | 531 |
| Age | |||
| Adults | 480 (90.7%) | 526 (91.2%) | 507 (95.5%) |
| Puppies | 49 (9.3%) | 51 (8.8%) | 24 (4.5%) |
| Sex | |||
| Male | 184 (34.8%) | 149 (25.8%) | 176 (33.1%) |
| Female | 302 (57.1%) | 308 (53.4%) | 303 (57.1%) |
| Unknown | 43 (8.1%) | 120 (20.8%) | 52 (9.8%) |
| Reproductive status | |||
| Intact | 470 (88.8%) | 563 (97.6%) | 449 (84.6%) |
| Neutered or spayed | 59 (11.2%) | 14 (2.4%) | 82 (15.4%) |
| Variables | Coefficient (SE) | p-Value |
|---|---|---|
| Block area size (km2) | 35.56 (18.26) | 0.053 |
| Number of intact adult female dogs | 2.27 (0.07) | <0.001 |
| Number of intact adult male dogs | 2.83 (0.10) | <0.001 |
| Variables | Negative Binomial Regression a | Negative Binomial Mixed Regression b | ||
|---|---|---|---|---|
| Coefficient (SE) | p-Value | Coefficient (SE) | p-Value | |
| Intercept | 1.25 (0.08) | <0.001 | 1.25 (0.08) | <0.001 |
| Number of intact adult female dogs | 0.15 (0.01) | <0.001 | 0.15 (0.01) | <0.001 |
| Block variance σ | - | - | 7.52 × 10−31 (3.72 × 10−16) | - |
| Number of Sighting Occasions | Number of Dogs Sighted: n (%) | ||
|---|---|---|---|
| Six Occasions | Three Morning Occasions | Three Evening Occasions | |
| 0 | - | 135 (25.42%) | 92 (17.33%) |
| 1 | 173 (32.58%) | 168 (31.64%) | 184 (34.65%) |
| 2 | 86 (16.2%) | 104 (19.59%) | 131 (24.67%) |
| 3 | 67 (12.62%) | 124 (23.35%) | 124 (23.35%) |
| 4 | 67 (12.62%) | - | - |
| 5 | 76 (14.31%) | - | - |
| 6 | 62 (11.68%) | - | - |
| Variables | Mean ± SEM | 95% CI | p-Value |
|---|---|---|---|
| Survey period | <0.01 | ||
| Morning | 51.7 ± 4.0 | 43.8–59.7 | |
| Evening | 64.4 ± 4.0 | 56.4–72.3 | |
| Building area level | 0.74 | ||
| Low | 56.6 ± 5.0 | 46.5–66.7 | |
| High | 59.5 ± 4.1 | 51.2–67.8 | |
| Presence of food source | 0.53 | ||
| No | 56.8 ± 2.7 | 51.3–62.2 | |
| Yes | 59.3 ± 5.9 | 47.4–71.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phiriyaphokhai, T.; Patanasatienkul, T.; Kittisiam, T.; Kasemsuwan, S.; Leelahapongsathon, K. Population Estimation and Demographic Characteristics of Free-Roaming Dogs on Kasetsart University Kamphaeng Saen Campus, Thailand: Implications for Rabies Control. Biology 2025, 14, 808. https://doi.org/10.3390/biology14070808
Phiriyaphokhai T, Patanasatienkul T, Kittisiam T, Kasemsuwan S, Leelahapongsathon K. Population Estimation and Demographic Characteristics of Free-Roaming Dogs on Kasetsart University Kamphaeng Saen Campus, Thailand: Implications for Rabies Control. Biology. 2025; 14(7):808. https://doi.org/10.3390/biology14070808
Chicago/Turabian StylePhiriyaphokhai, Tita, Thitiwan Patanasatienkul, Tipsarp Kittisiam, Suwicha Kasemsuwan, and Kansuda Leelahapongsathon. 2025. "Population Estimation and Demographic Characteristics of Free-Roaming Dogs on Kasetsart University Kamphaeng Saen Campus, Thailand: Implications for Rabies Control" Biology 14, no. 7: 808. https://doi.org/10.3390/biology14070808
APA StylePhiriyaphokhai, T., Patanasatienkul, T., Kittisiam, T., Kasemsuwan, S., & Leelahapongsathon, K. (2025). Population Estimation and Demographic Characteristics of Free-Roaming Dogs on Kasetsart University Kamphaeng Saen Campus, Thailand: Implications for Rabies Control. Biology, 14(7), 808. https://doi.org/10.3390/biology14070808






