Sustainable Management of Filamentous Algae in Freshwater Ecosystems: Insights from Cladophora sp. Life History, Reproductive Tactics, and Growth Ecology
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Morphological Observation
2.2. DNA Extraction, Amplification, and Sequencing
2.3. Preparation of Cladophora sp. for Reproduction and Growth Trials
2.4. Reproduction Trials
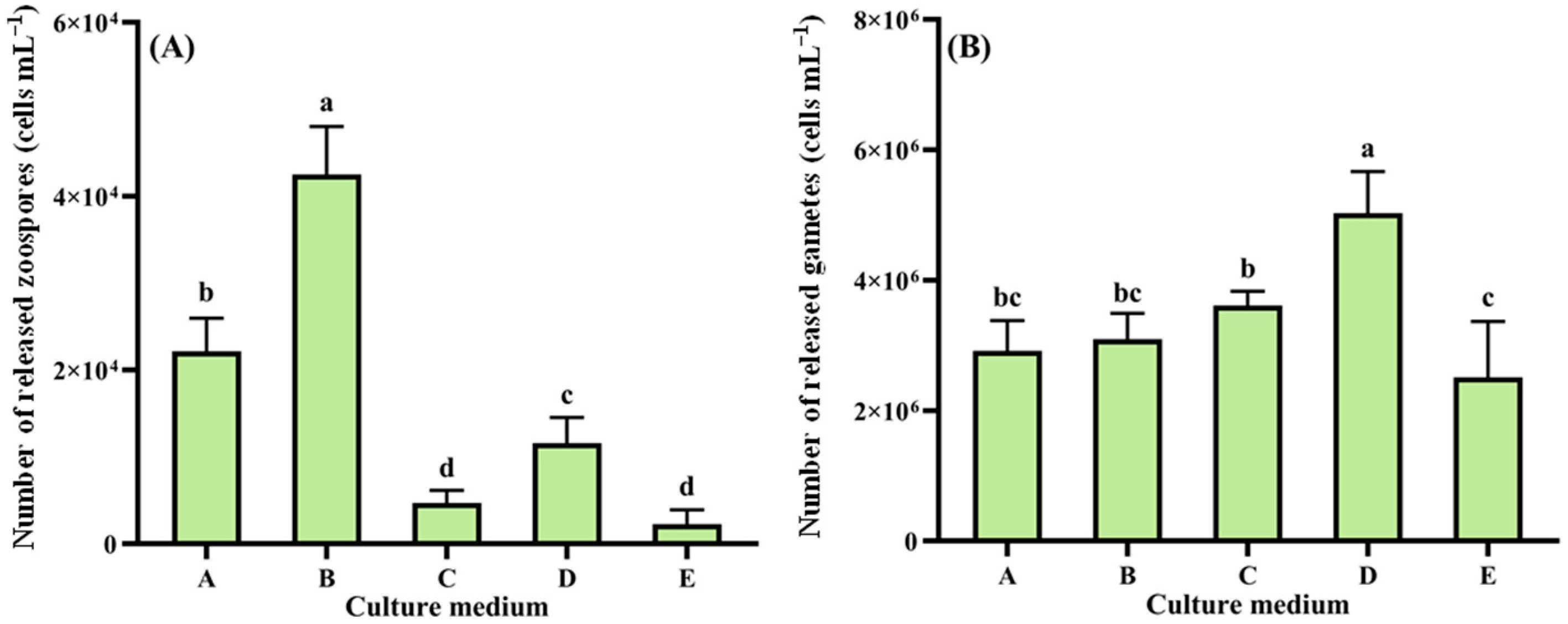
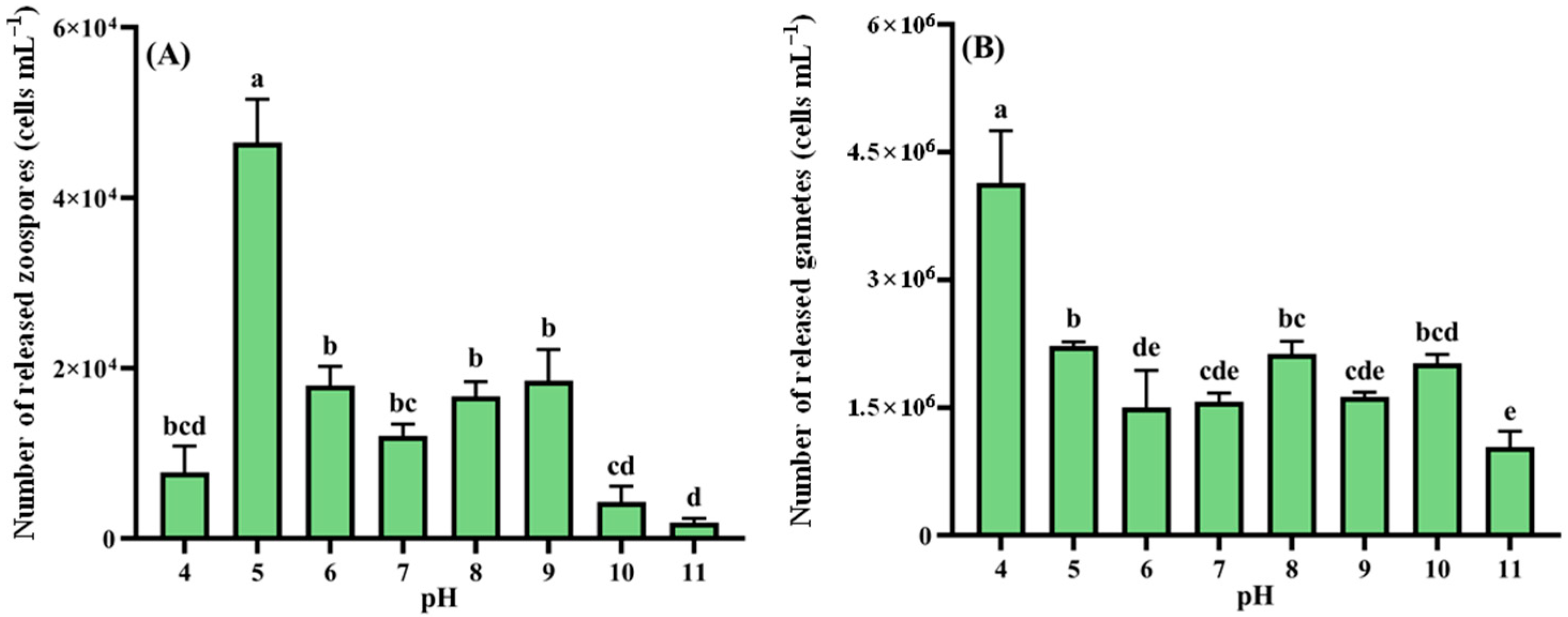
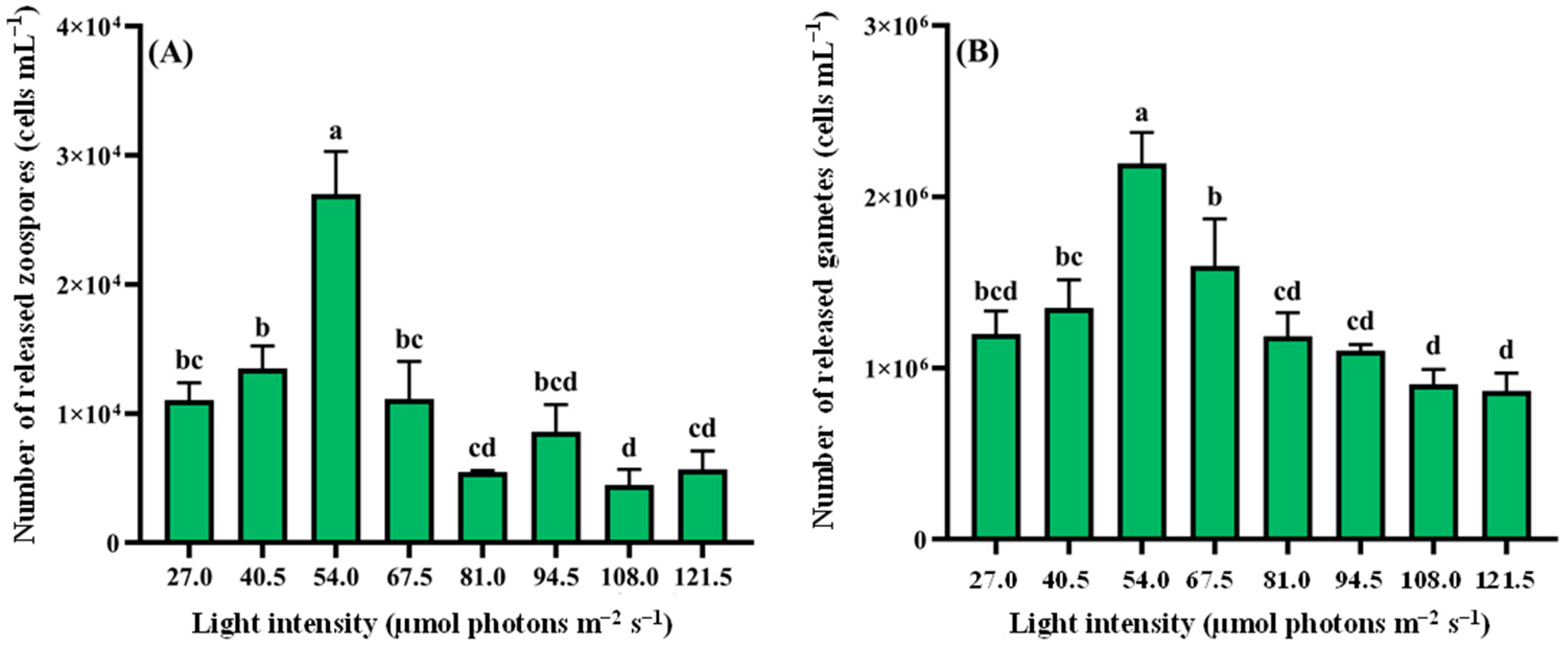
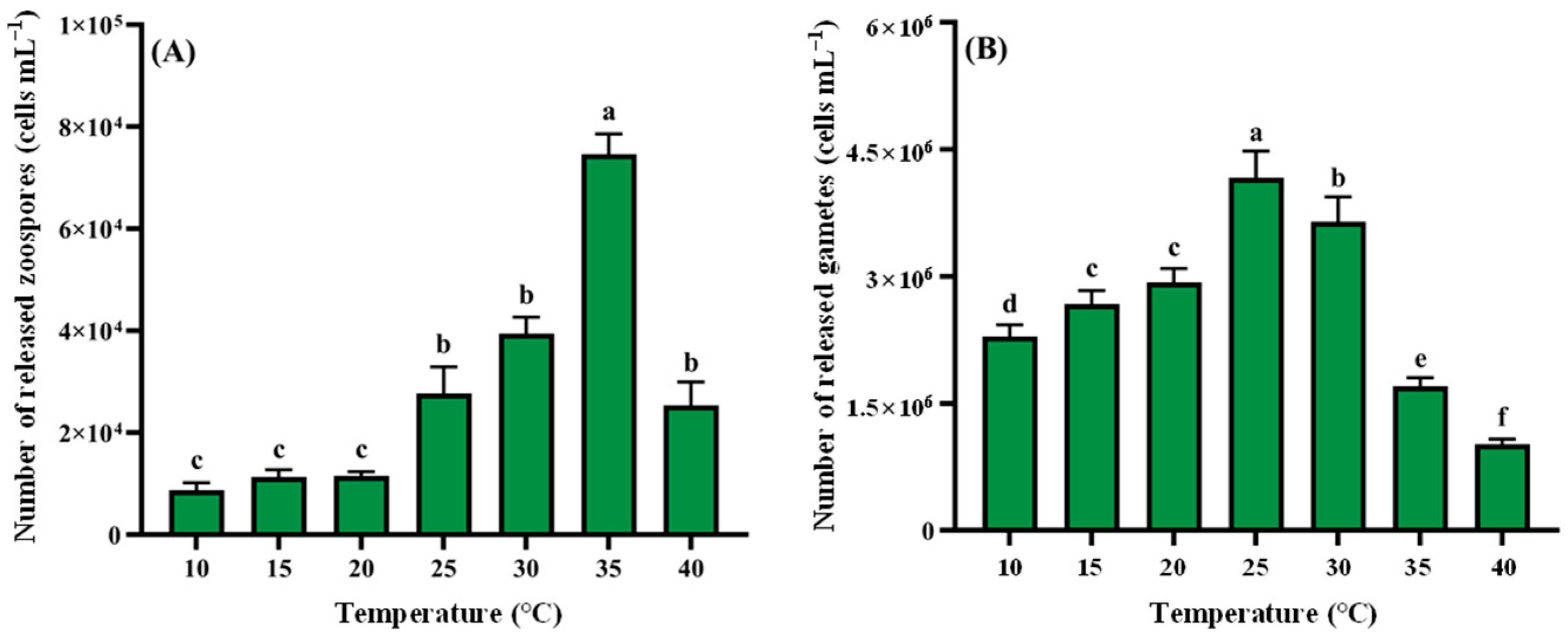
2.4.1. Response of Reproduction to Different Nutrient Conditions and Ecological Factors
- pH: 4, 5, 6, 7, 8, 9, 10, and 11;
- Light intensity: 27.0, 40.5, 54.0, 67.5, 81.0, 94.5, 108.0, and 121.5 µmol photons m−2 s−1;
- Temperature: 10, 15, 20, 25, 30, 35, and 40 °C.
2.4.2. Orthogonal Experiment Design
2.4.3. Propagule Observation
2.5. Growth Trials
2.5.1. Response of Growth to Different Nutrient Conditions
- Five formulations: (Culture medium A–E);
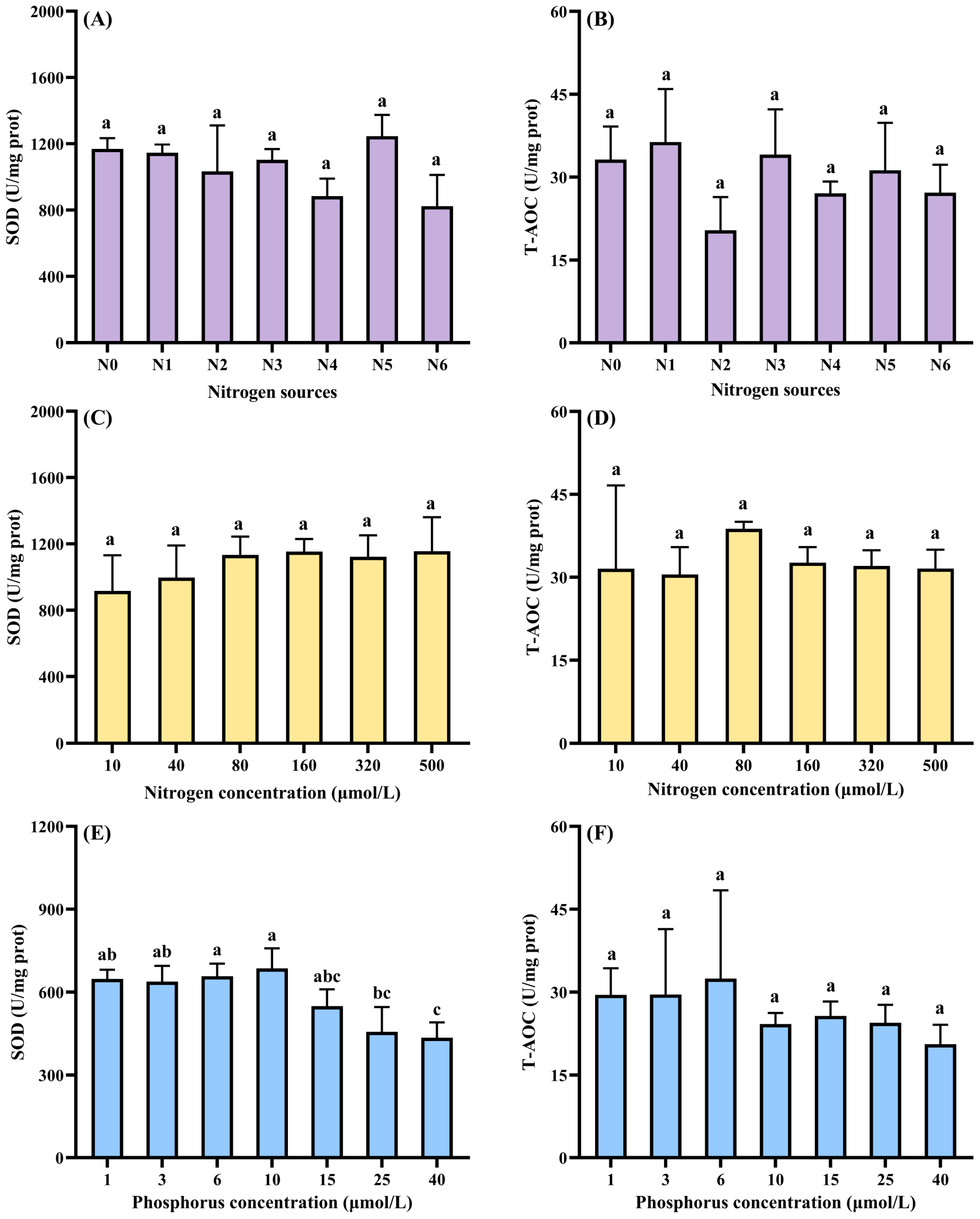
- Six nitrogen sources (160 µmol L−1 total N): NO3−-N (N1), NH4+-N (N2), Urea-N (N3), NO3−-N:NH4+-N = 1:1 (N4), NO3−-N:Urea-N = 1:1 (N5), NH4+-N:Urea-N = 1:1 (N6), and a N-free control (N0);
- Seven nitrogen concentrations (10 µmol L−1 total P): 0, 10, 40, 80, 160, 320, and 500 µmol L−1; NO3−-N was the nitrogen source;
- Eight phosphorus concentrations (160 µmol L−1 total N): 0, 1, 3, 6, 10, 15, 25, and 40 µmol L−1; K2HPO4 was the phosphorus source.
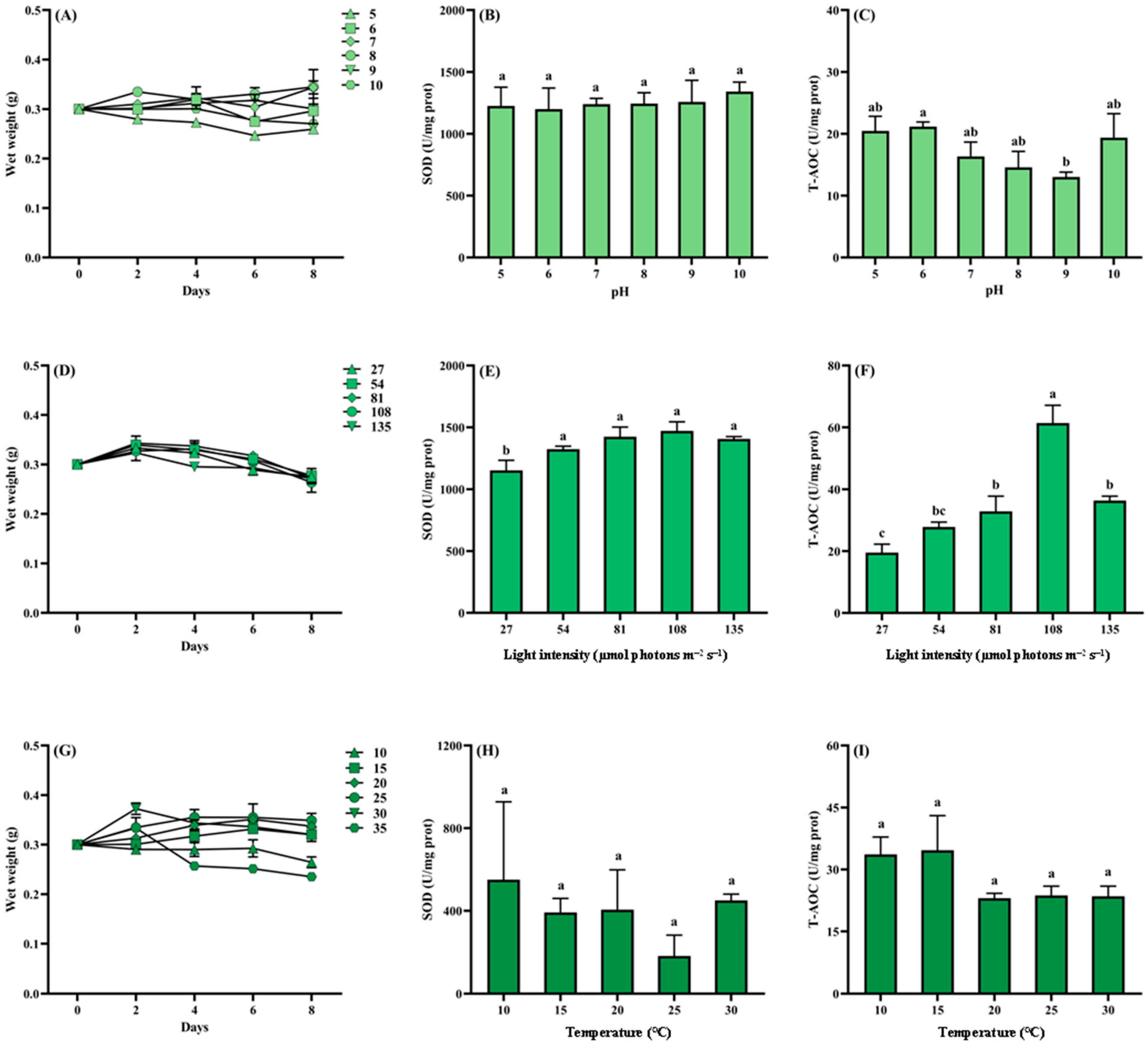
2.5.2. Response of Growth to Different Ecological Factors
2.5.3. Determination of Growth and Antioxidant Parameters
2.6. Statistics Analysis
3. Results
3.1. Life History Observation
3.2. Reproductive Response
3.2.1. Nutrient Conditions
3.2.2. pH Levels
3.2.3. Light Intensity
3.2.4. Temperature
3.2.5. Orthogonal Experiment Analysis
3.3. Growth Response
3.3.1. Growth Response to Nutrient Conditions
3.3.2. Growth Response to Ecological Factors
4. Discussion
4.1. Life History
4.2. Reproductive and Growth Response to Nutrient Conditions
4.3. Reproductive and Growth Response to Ecological Factors
4.4. The Synergy of Driving Factors and Implications for Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| N | Nitrogen |
| P | Phosphorus |
| SOD | Superoxide dismutase |
| T-AOC | Total antioxidant capacity |
| HCO3− | Bicarbonate |
| ITS | Internal transcribed spacer |
References
- Tang, Y.; Wang, C.; Cheng, Y.; Sun, Y.; Zhao, L.; Qian, C.; Yang, Y. Investigation on the key factors of filamentous algae occurrence in aquaculture ponds. J. Fish. China 2024, 48, 049119. [Google Scholar]
- Gámez, T.E.; Manning, S.R. Blooming in the Anthropocene: Perspectives on the development of freshwater blooms, changing phytoplankton communities, and mitigation strategies. Harmful Algae 2025, 147, 102883. [Google Scholar] [CrossRef]
- Díaz-Martínez, S.; Boedeker, C.; Zuccarello, G.C. Contrasting patterns of population structure and reproduction strategies of three sympatric species of Cladophoraceae endemic to Lake Baikal. Phycologia 2021, 60, 120–130. [Google Scholar] [CrossRef]
- Usandizaga, S.; Valenzuela, P.; Gaitán-Espitia, J.D.; Destombe, C.; Guillemin, M.L. Reproductive effort in the domesticated red alga Agarophyton chilense: Differences between farms and natural populations. J. Appl. Phycol. 2021, 33, 1149–1156. [Google Scholar] [CrossRef]
- Agrawal, S.C. Factors controlling induction of reproduction in algae—Review: The text. Folia Microbiol. 2012, 57, 387–407. [Google Scholar] [CrossRef]
- Sato, Y.; Nagoe, H.; Nishihara, G.N.; Terada, R. The photosynthetic response of cultivated juvenile and mature Undaria pinnatifida (Laminariales) sporophytes to light and temperature. J. Appl. Phycol. 2021, 33, 3437–3448. [Google Scholar] [CrossRef]
- Vliet, N.H.; Small, S.L.; Weigel, B.L. Effects of sori incubation temperature on Nereocystis luetkeana gametophyte and sporophyte development. Integr. Comp. Biol. 2023, 63, S480. [Google Scholar]
- Li, H.; Kim, H.; Shin, K.; Hyun, B.; Kim, Y.S.; Kim, J.-H. Effects of temperature and light on the growth and reproduction of an endophytic pest alga Ectocarpus siliculosus (Ectocarpales) found in wild Gracilaria textorii (Gracilariales). Aquaculture 2022, 560, 738526. [Google Scholar] [CrossRef]
- Kalita, T.L.; Titlyanov, E.A. The effect of temperature on infradian rhythms of reproduction in Ulva fenestrata postels et ruprecht, 1840 (Chlorophyta: Ulvales). Russ. J. Mar. Biol. 2011, 37, 52–61. [Google Scholar] [CrossRef]
- Mason, C.P. Ecology of Cladophora in farm ponds. Ecology 1965, 46, 421–429. [Google Scholar] [CrossRef]
- Guo, H.; Yao, J.; Sun, Z.; Duan, D. Effect of temperature, irradiance on the growth of the green alga Caulerpa lentillifera (Bryopsidophyceae, Chlorophyta). J. Appl. Phycol. 2015, 27, 879–885. [Google Scholar] [CrossRef]
- Qi, F.; Li, X.-D.; Zhao, Y.-H.; Lei, Y.-Z.; Li, Y.-H. Effects of salinity, light intensity and temperature on photosynthesis in alga Cladophera expansal. J. Dalian Fish. Univ. 2008, 23, 382–386. [Google Scholar]
- Luo, W.; Gong, X.; Gao, W.; Xia, Y.; Zhang, B. Effects of light intensity on spore germination, early development of the sporelings of Scytosiphon lomentaria and the dynamic change of epiphytic algae. J. Fish. China 2014, 38, 11. [Google Scholar]
- Tatsumi, M.; Layton, C.; Cameron, M.J.; Shelamoff, V.; Johnson, C.R.; Wright, J.T. Interactive effects of canopy-driven changes in light, scour and water flow on microscopic recruits in kelp. Mar. Environ. Res. 2021, 171, 105450. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Luo, Q.; Chen, H. Physiological and biochemical responses of different species of chlorophyta to simulated acid rain stress. J. Trop. Oceanogr. 2023, 42, 115–123. [Google Scholar]
- Zhao, Z.F.; Liu, Z.Y.; Qin, S.; Wang, X.H.; Song, W.L.; Liu, K.; Zhuang, L.C.; Xiao, S.Z.; Zhong, Z.H. Impacts of low pH and low salinity induced by acid rain on the photosynthetic activity of green tidal alga Ulva prolifera. Photosynthetica 2021, 59, 468–477. [Google Scholar] [CrossRef]
- Han, W.; Xu, Y.; Jiang, Y.; Yao, Y.; Cao, X.; Jiang, A.; Chen, L.; Xing, R. Effects of N/P ratio, salinity and pH on growth and photosynthesis of sea weed Chaetomorpha valida. J. Dalian Ocean Univ. 2019, 34, 776–784. [Google Scholar]
- Çelekli, A.; Arslanargun, H.; Soysal, Ç.; Gültekin, E.; Bozkurt, H. Biochemical responses of filamentous algae in different aquatic ecosystems in South East Turkey and associated water quality parameters. Ecotoxicol. Environ. Saf. 2016, 133, 403–412. [Google Scholar] [CrossRef]
- Parker, J.E.; Maberly, S.C. Biological response to lake remediation by phosphate stripping: Control of Cladophora. Freshw. Biol. 2000, 44, 303–309. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; McCarthy, M.J.; Zhu, G.; Qin, B.; Li, Y.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, K.; Lv, J.; Peng, X.; Tang, Y.; Zhao, L.; Cheng, Y.; Liu, Q. Effects of Nitrogen and Phosphorus in Sediment on the Occurrence of Cladophora sp. (Cladophoraceae) in Aquaculture Ponds. Biology 2024, 13, 739. [Google Scholar] [CrossRef] [PubMed]
- de Paula Silva, P.H.; Paul, N.A.; de Nys, R.; Mata, L. Enhanced production of green tide algal biomass through additional carbon supply. PLoS ONE 2013, 8, e81164. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Shuai, L.; Lu, B.; Wang, S.; Chen, J.; Wang, G. Effects of temperature and irradiance on filament development of Grateloupia turuturu (Halymeniaceae, Rhodophyta). J. Appl. Phycol. 2013, 25, 1881–1886. [Google Scholar] [CrossRef]
- Waite, I.R.; Van Metre, P.C.; Moran, P.W.; Konrad, C.P.; Nowell, L.H.; Meador, M.R.; Munn, M.D.; Schmidt, T.S.; Gellis, A.C.; Carlisle, D.M.; et al. Multiple in-stream stressors degrade biological assemblages in five U.S. regions. Sci. Total Environ. 2021, 800, 149350. [Google Scholar] [CrossRef]
- Xu, G.; Terada, R.; Watanabe, Y.; Nishihara, G.N. The occurrence of Phycocalidia tanegashimensis (Bangiaceae) in the splash zone may be related to the tolerance of photochemical efficiency to temperature, irradiance, desiccation, and salinity. J. Appl. Phycol. 2021, 33, 3427–3435. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Bing, X.; Tan, Y.; Zhou, Q.; Jiang, J.; Zhu, Y. Influencing factors for the growth of Cladophora and its cell damage and destruction mechanism: Implication for prevention and treatment. Water 2024, 16, 1890. [Google Scholar] [CrossRef]
- Leliaert, F.; De Clerck, O.; Verbruggen, H.; Boedeker, C.; Coppejans, E. Molecular phylogeny of the Siphonocladales (Chlorophyta: Cladophorophyceae). Mol. Phylogenet. Evol. 2007, 44, 1237–1256. [Google Scholar] [CrossRef] [PubMed]
- Sogin, M.L.; Gunderson, J.H. Structural diversity of eukaryotic small subunit ribosomal RNAs. Evolutionary implications. Ann. N. Y. Acad. Sci. 1987, 503, 125–139. [Google Scholar] [CrossRef]
- Bakker, F.T.; Olsen, J.L.; Stam, W.T.; van den Hoke, C. Nuclear ribosomal DNA internal transcribed spacer regions (ITS1 and ITS2) define discrete biogeographic groups in Cladophora albida (Chlorophyta). J. Phycol. 1992, 28, 839–845. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, L.; Zhao, L.; Qian, C.; Lun, F.; Wang, C.; Zheng, H.; Tang, B.; Cheng, Y.; Guo, X. Inhibitory Effects and Oxidative Damages in Cladophora sp. (Cladophoraceae) Exposed to Berberine. Aquac. Rep. 2022, 27, 101357. [Google Scholar] [CrossRef]
- Zhong, J.; Zhao, Q.; He, P.; Shao, L. Selection and optimization of inhibitors derived from plants for Cladophora sp. J. Shanghai Ocean Univ. 2023, 32, 234–243. [Google Scholar]
- Liu, X.; Chen, Y. A review on the ecology of Cladophora. J. Lake Sci. 2018, 30, 881–896. [Google Scholar] [CrossRef]
- Ley, S.-H.; Chu, W.; Hsia, I.-T.; Yu, M.-K.; Lin, K.-E.; Liu, K.-S.; Lo, C.-Y.; Chen, Y.-H. The mass culture of unicellular green algae. Acta Hydrobiol. Sin. 1959, 4, 462–472. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Sutkowy, M.; Lenarczyk, J.; Kłosowski, G. Effect of culture medium on the growth of microscopic algae (Chlorophyceae) biomass showing biosorption potential: A case study Pseudopediastrum boryanum. Phycol. Res. 2019, 67, 112–119. [Google Scholar] [CrossRef]
- Vildanova, G.I.; Allaguvatova, R.Z.; Kunsbaeva, D.F.; Sukhanova, N.V.; Gaysina, L.A. Application of Chlorella vulgaris Beijerinck as a Biostimulant for Growing Cucumber Seedlings in Hydroponics. BioTech 2023, 12, 42. [Google Scholar] [CrossRef]
- Hariz, H.B.; Lawton, R.J.; Craggs, R.J. Novel assay for attached filamentous algae productivity and nutrient removal. J. Appl. Phycol. 2023, 35, 251–264. [Google Scholar] [CrossRef]
- Lester, W.W.; Adams, M.S.; Farmer, A.M. Effects of light and temperature on photosynthesis of the nuisance alga Cladophora glomerata (L.) Kutz from Green Bay, Lake Michigan. New Phytol. 1988, 109, 53–58. [Google Scholar] [CrossRef]
- Monaco, M.E. An Ecological Investigation of Cladophora glomerata and Its Minimal Light Requirements for Colonization. Master’s Thesis, The Ohio State University, Columbus, OH, USA, 1984. [Google Scholar]
- Mantai, K.E.; Haase, C. Some factors affecting photosynthesis and respiration measurements in Cladophora glomerata. J. Great Lakes Res. 1977, 3, 191–195. [Google Scholar] [CrossRef]
- Marks, J.C.; Cummings, M.P. DNA Sequence Variation in the Ribosomal Internal Transcribed Spacer Region of Freshwater Cladophora Species (Chlorophyta). J. Phycol. 1996, 32, 1035–1042. [Google Scholar] [CrossRef]
- Ensminger, I.; Hagen, C.; Braune, W. Strategies providing success in a variable habitat: I. Relationships of environmental factors and dominance of Cladophora glomerata. Plant Cell Environ. 2000, 23, 1119–1128. [Google Scholar] [CrossRef]
- Dodds, W.K.; Gudder, D.A. The Ecology of Cladophora. J. Phycol. 1992, 28, 415–427. [Google Scholar] [CrossRef]
- Parodi, E.R.; Cáceres, E.J. Life history of Cladophora surera sp. nov. (Cladophorales, Ulvophyceae). Phycol. Res. 1995, 43, 223–231. [Google Scholar] [CrossRef]
- Romanova, E.V.; Kravtsova, L.S.; Izhboldina, L.A.; Khanaev, I.V.; Sherbakov, D.Y. Identification of filamentous green algae from an area of local biogenic pollution of Lake Baikal (Listvennichnyi Bay) using SSU 18S rDNA. Russ. J. Genet. Appl. Res. 2015, 5, 118–125. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, G. Effect of nitrogen to phosphorus ratios on cell proliferation in marine micro algae. Chin. J. Oceanol. Limnol. 2011, 29, 739–745. [Google Scholar] [CrossRef]
- Malkin, S.Y.; Guildford, S.J.; Hecky, R.E. Modeling the growth response of Cladophora in a Laurentian Great Lake to the exotic invader Dreissena and to lake warming. Limnol. Oceanogr. 2008, 53, 1111–1124. [Google Scholar] [CrossRef]
- Higgins, S.N.; Malkin, S.Y.; Howell, E.T.; Guildford, S.J.; Campbell, L.; Hiriart-Baer, V.; Hecky, R.E. An ecological review of Cladophora glomerata (Chlorophyta) in the Laurentian Great Lakes. J. Phycol. 2008, 44, 839–854. [Google Scholar] [CrossRef]
- Tomlinson, L.M.; Auer, M.T.; Bootsma, H.A.; Owens, E.M. The Great Lakes Cladophora Model: Development, testing, and application to Lake Michigan. J. Great Lakes Res. 2010, 36, 287–297. [Google Scholar] [CrossRef]
- Ross, M.E.; Davis, K.; McColl, R.; Stanley, M.S.; Day, J.G.; Semião, A.J.C. Nitrogen uptake by the macro-algae Cladophora coelothrix and Cladophora parriaudii: Influence on growth, nitrogen preference and biochemical composition. Algal Res. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Middleton, C.M.; Frost, P.C. Stoichiometric and growth responses of a freshwater filamentous green alga to varying nutrient supplies: Slow and steady wins the race. Freshw. Biol. 2014, 59, 2225–2234. [Google Scholar] [CrossRef]
- Zeng, L.Z. The Study of the Effects of Different Environment Factors on Growth, Physiological Biochemistry Compositions and Preliminary Culture Observation of Cladophora expansa. Master’s Thesis, Shantou University, Shantou, China, 2016. [Google Scholar]
- Choo, K.; Snoeijs, P.; Pedersén, M. Uptake of inorganic carbon by Cladophora glomerata (Chlorophyta) from the Baltic Sea. J. Phycol. 2002, 38, 493–502. [Google Scholar] [CrossRef]
- Sheath, R.G.; Cole, K.M. Biogeography of stream macroalgae in North America. J. Phycol. 1992, 28, 448–460. [Google Scholar] [CrossRef]
- Whitton, B.A. Biology of Cladophora in freshwaters. Water Res. 1970, 4, 457–476. [Google Scholar] [CrossRef]
- Mine, I.; Sekida, S.; Okuda, K. Cell wall and cell growth characteristics of giant-celled algae. Phycol. Res. 2015, 63, 77–84. [Google Scholar] [CrossRef]
- Mine, I.; Suzuki, S.; Li, K.F.; Sekida, S. pH-dependent maintenance of cell wall integrity in the giant-celled green alga Valonia utricularis. Cytologia 2018, 83, 99–102. [Google Scholar] [CrossRef]
- Luo, D.; Huang, W.; Ding, M.; Li, L. Effects of increased nutrient loading and water level caused by extreme flooding on Hydrilla verticillata, periphyton and water properties. J. Lake Sci. 2025, 37, 184–193. [Google Scholar] [CrossRef]
- Ishii, Y.; Ishii, H.; Kuroha, T.; Yokoyama, R.; Deguchi, R.; Nishitani, K.; Minagawa, J.; Kawata, M.; Takahashi, S.; Maruyama, S. Environmental pH signals the release of monosaccharides from cell wall in coral symbiotic alga. eLife 2023, 12, e80628. [Google Scholar] [CrossRef]
- Watson, B.S.; Sumner, L.W. Isolation of Cell Wall Proteins from Medicago sativa Stems. In Methods in Molecular Biology: Plant Proteomics Methods and Protocols; Thiellement, H., Zivy, M., Damerval, C., Méchin, V., Eds.; Humana Press: Totowa, NJ, USA, 2007; Volume 355, pp. 79–92. [Google Scholar]
- Zemke-White, W.L.; Clements, K.D.; Harris, P.J. Acid lysis of macroalgae by marine herbivorous fishes: Effects of acid pH on cell wall porosity. J. Exp. Mar. Biol. Ecol. 2000, 245, 57–68. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, N.; Yuan, J.; Zhu, W.; Li, Y. The sporogenesis is partly regulated by oxidative signal in Ulva prolifera: A physiological and transcriptomic perspective. Algal Res. 2023, 70, 102991. [Google Scholar] [CrossRef]
- Hollarsmith, J.A.; Buschmann, A.H.; Camus, C.; Grosholz, E.D. Varying reproductive success under ocean warming and acidification across giant kelp (Macrocystis pyrifera) populations. J. Exp. Mar. Biol. Ecol. 2020, 522, 151247. [Google Scholar] [CrossRef]
- Kavale, M.G.; Largo, D.B.; De la Torre, E.O.; Baritugo, A.T.; Azcuna-Montaño, M. Plantlets directly developed from secondary phyllodes of Sargassum siliquosum J. Agardh: Implication for seedling production during the off-reproductive season. Aquaculture 2023, 563, 738977. [Google Scholar] [CrossRef]
- Juijuljerm, R.; Vanijajiva, O.; Chittapun, S. The potential of using akinetes as seed starters for Cladophora glomerata cultivation: Germination and growth of akinetes under different light intensities and humic concentrations. Algal Res. 2021, 60, 102478. [Google Scholar] [CrossRef]
- Zou, X.-X.; Xing, S.-S.; Su, X.; Zhu, J.; Huang, H.-Q.; Bao, S.-X. The effects of temperature, salinity and irradiance upon the growth of Sargassum polycystum C. Agardh (Phaeophyceae). J. Appl. Phycol. 2017, 30, 1207–1215. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Dong, S.; Cao, S. Sexual fusion and life history of Scytosiphon lomentaria (Scytosiphonaceae, Phaeophyceae) in Dalian, china. J. Ocean Univ. China 2011, 10, 170–176. [Google Scholar] [CrossRef]
- Robinson, P.K.; Hawkes, H.A. Studies on the growth of Cladophora glomerata in laboratory continuous-flow culture. Br. Phycol. J. 2007, 21, 437–444. [Google Scholar] [CrossRef]
- Hall, D.J.; Walmsley, R.D. Effect of temperature on germination of Rhizoclonium riparium (Siphonocladales, Chlorophyta) akinetes and zoospores. J. Phycol. 1991, 27, 537–539. [Google Scholar] [CrossRef]
- Dethier, M.N. Pattern and process in tidepool algae: Factors influencing seasonality and distribution. Bot. Mar. 1982, 25, 55–66. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, Y.; Zhang, M.; Lv, X.; Li, Y.; Zhang, J.; Wang, X.; Gao, X.; Zhao, X.; Wang, X. The Distribution and Succession of Filamentous Algae in the Southern Taihang Catchment under Different Nutrient Regimes. Water 2024, 16, 2453. [Google Scholar] [CrossRef]
- Ensminger, I.; Xyländer, M.; Hagen, C.; Braune, W. Strategies providing success in a variable habitat: III. Dynamic control of photosynthesis in Cladophora glomerata. Plant Cell Environ. 2001, 24, 769–779. [Google Scholar] [CrossRef]
- Green, L.; Fong, P. The good, the bad and the Ulva: The density dependent role of macroalgal subsidies in influencing diversity and trophic structure of an estuarine community. Oikos 2016, 125, 988–1000. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, Z.; Luo, Y.; Bai, Y.; Fan, J. Bioremediation of phenolic pollutants by algae-current status and challenges. Bioresour. Technol. 2022, 350, 126930. [Google Scholar] [CrossRef] [PubMed]
- Zulkifly, S.B.; Graham, J.M.; Young, E.B.; Mayer, R.J.; Piotrowski, M.J.; Smith, I.; Graham, L.E. The Genus Cladophora Kutzing (Ulvophyceae) as a Globally Distributed Ecological Engineer. J. Phycol. 2013, 49, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Bhandari, G.; Turco, R.F.; Aminikhoei, Z.; Bhatt, K.; Simsek, H. Algae in wastewater treatment, mechanism, and application of biomass for production of value-added product. Environ. Pollut. 2022, 309, 119688. [Google Scholar] [CrossRef] [PubMed]

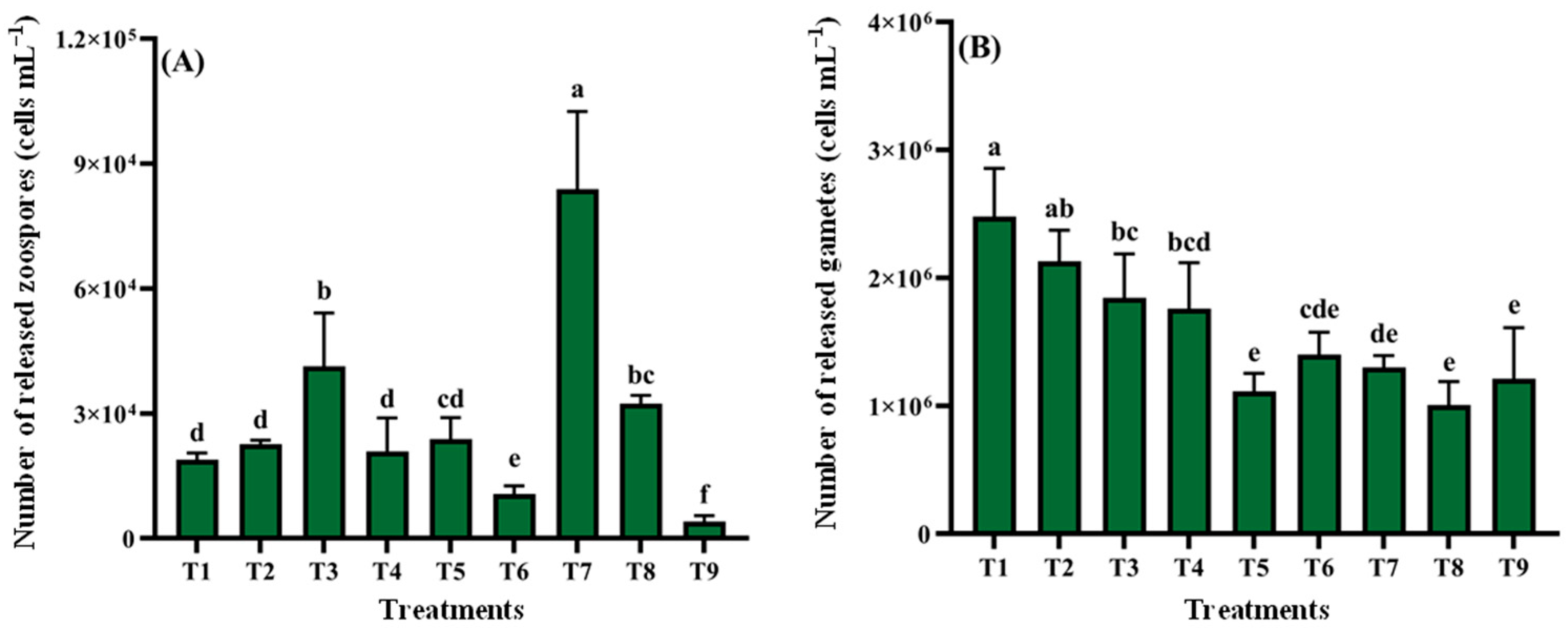

| Treatments | Zoospore | Gamete | ||||||
|---|---|---|---|---|---|---|---|---|
| Culture Medium | pH | Temperature (°C) | Light Intensity (μmol·m−2·s−1) | Culture Medium | pH | Temperature (°C) | Light Intensity (μmol·m−2·s−1) | |
| T1 | A | 5 | 25 | 40.5 | C | 4 | 20 | 67.5 |
| T2 | A | 6 | 30 | 54.0 | C | 8 | 25 | 54.0 |
| T3 | A | 9 | 35 | 67.5 | C | 10 | 30 | 40.5 |
| T4 | B | 5 | 30 | 67.5 | D | 4 | 25 | 40.5 |
| T5 | B | 6 | 35 | 40.5 | D | 8 | 30 | 67.5 |
| T6 | B | 9 | 25 | 54.0 | D | 10 | 20 | 54.0 |
| T7 | D | 5 | 35 | 54.0 | B | 4 | 30 | 54.0 |
| T8 | D | 6 | 25 | 67.5 | B | 8 | 20 | 40.5 |
| T9 | D | 9 | 30 | 40.5 | B | 10 | 25 | 67.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Guo, L.; Tan, C.; Tang, Y.; Ma, Y.; Zhang, Z.; Cheng, Y.; Qian, C. Sustainable Management of Filamentous Algae in Freshwater Ecosystems: Insights from Cladophora sp. Life History, Reproductive Tactics, and Growth Ecology. Biology 2025, 14, 1671. https://doi.org/10.3390/biology14121671
Zhao L, Guo L, Tan C, Tang Y, Ma Y, Zhang Z, Cheng Y, Qian C. Sustainable Management of Filamentous Algae in Freshwater Ecosystems: Insights from Cladophora sp. Life History, Reproductive Tactics, and Growth Ecology. Biology. 2025; 14(12):1671. https://doi.org/10.3390/biology14121671
Chicago/Turabian StyleZhao, Liangjie, Liangxin Guo, Chenxi Tan, Yongtao Tang, Yuanye Ma, Zhen Zhang, Yongxu Cheng, and Chen Qian. 2025. "Sustainable Management of Filamentous Algae in Freshwater Ecosystems: Insights from Cladophora sp. Life History, Reproductive Tactics, and Growth Ecology" Biology 14, no. 12: 1671. https://doi.org/10.3390/biology14121671
APA StyleZhao, L., Guo, L., Tan, C., Tang, Y., Ma, Y., Zhang, Z., Cheng, Y., & Qian, C. (2025). Sustainable Management of Filamentous Algae in Freshwater Ecosystems: Insights from Cladophora sp. Life History, Reproductive Tactics, and Growth Ecology. Biology, 14(12), 1671. https://doi.org/10.3390/biology14121671







