1. Introduction
Ilex verticillata is a perennial deciduous shrub of the genus
Ilex (family Aquifoliaceae), indigenous to Europe and North America [
1]. Characterized by winter deciduousness, bright red fruits, and an extended fruit-retention period, this species exhibits high ornamental and commercial value, thereby occupying a prominent position in landscape greening projects and the cut-branch ornamental market [
2,
3]. The introduction, popularization and application of
I. verticillata in China have promoted the development of its basic theoretical and applied research. Significant advancements have been made in various areas including tissue culture [
4,
5], stress resistance [
6,
7], transcriptome analysis [
8], and in understanding fruit quality, growth, and development [
9,
10,
11], with transcriptome analysis playing a pivotal role in revealing gene expression patterns and aiding in the improvement of crop varieties.
As one of the primary limiting factors constraining plant growth and geographical distribution [
12], drought stress induces a series of physiological perturbations: it initially causes stomatal closure and a subsequent decline in photosynthetic efficiency, which further leads to osmotic stress and membrane lipid peroxidation, and ultimately results in the degradation of nucleic acids and proteins [
13]. Plants adapt to drought environments through the coordinated regulation of multiple strategies, such as morphological structural modifications, physiological metabolic adjustments, and differential gene expression [
14,
15,
16]. While extensive investigations into these adaptive mechanisms have been conducted in crops and forest tree species, research addressing the drought tolerance and its underlying mechanisms in
I. verticillata remains relatively scarce.
Metabolomics, a discipline utilizing high-throughput detection technologies, conducts qualitative and quantitative analyses of all low-molecular-weight metabolites in biological samples from various growth stages or environmental conditions. Its primary objective is to identify key metabolites that play a role in specific biological processes, such as plant responses to adverse environmental stresses [
17]. This approach has been widely applied to dissect how plants react to different environmental pressures, including drought, high temperatures, salinity, and pests [
18,
19,
20]. For instance, in a metabolomic analysis of garlic (
Allium sativum) seedlings under drought stress, differential metabolites were primarily enriched in the biosynthetic pathways of flavonoids and flavanones, among which caffeic acid and flavone O-malonylhexoside exhibited the most significant upregulation [
21]. Through metabolomic profiling, Long et al. elucidated the dynamic variation patterns of metabolites during kernel development in oil-tea camellia (
Camellia oleifera), revealing marked differences in metabolite composition across different developmental stages [
22]. In a related study, Guo et al. [
23] utilized comparative transcriptomics, metabolomics, and hormone analysis to elucidate the molecular mechanisms underlying maize (
Zea mays B73) adaptation to combined drought and cold stresses, focusing on the stress treatment and recovery phases. They found that the combined stress induced transcriptome-related metabolomic changes, among which raffinose, trehalose-6-phosphate, and proline accumulated, and the abundance of monosaccharides increased.
Significant progress has been made in elucidating the physiological and molecular mechanisms that enable plants to respond to drought stress, yet there remains considerable variation in drought resistance strategies across different species, a reflection of their genetic diversity. As a species that prefers moisture, I. verticillata has rarely been studied in terms of its drought tolerance traits and associated response mechanisms. Therefore, the present study investigates the variation trends of drought-responsive differential metabolites and their related metabolic pathways in I. verticillata. By doing so, we aim to identify key targets and metabolic pathways involved in drought resistance metabolism, which may provide a theoretical basis for future breeding programs focused on developing drought-tolerant I. verticillata cultivars.
3. Results and Analysis
3.1. Effects of Drought Stress on Chlorophyll Content in Leaves of Ilex verticillata
Photosynthesis is the process by which plants convert CO
2 and H
2O into carbon-containing organic compounds, which supplies energy for plants and maintains their normal growth and development. Chlorophyll is a key pigment closely related to photosynthesis and plays a core role in the process of light absorption. As shown in
Figure 1, the chlorophyll content in the leaves of
I. verticillata showed a decreasing trend with the increase in drought duration, and there was a significant difference compared with the control group at the same period when drought stress lasted for 30 days. After rewatering treatment, the chlorophyll content increased to a certain extent. These results indicate that drought stress reduces the chlorophyll content in the leaves of
I. verticillata, inhibits its photosynthesis, and affects the ability of
I. verticillata to cope with drought stress.
3.2. Effects of Drought Stress on Relative Water Content in Leaves of Ilex verticillata
The relative water content (RWC) of plant leaves reflects the water status and water retention capacity of plants, and serves as a crucial indicator for evaluating the strength of plants’ drought resistance. As shown in
Figure 2, there was no significant difference in the leaf RWC of
I. verticillata between the treatment group and the control group on day 0 of treatment. With the extension of drought duration, the leaf RWC decreased continuously, and all values showed significant differences compared with the control group at the same period. After rewatering treatment, the leaf RWC of
I. verticillata increased to a certain extent. These results indicate that drought stress reduces the water retention capacity of
I. verticillata leaves, while rewatering can restore the plant’s water status to some degree. This suggests that
I. verticillata possesses a certain adaptive mechanism when facing drought stress, enabling it to cope with drought environments by regulating leaf water content.
3.3. Effects of Drought Stress on Malondialdehyde (MDA) Content in Ilex verticillata
Under stress conditions, the content of superoxide free radicals in plants increases sharply, triggering lipid peroxidation and generating malondialdehyde (MDA). Excessive accumulation of this substance can promote the cross-linking and polymerization of biological macromolecules such as proteins and nucleic acids, thereby altering the structure and function of cell membranes.
As shown in
Figure 3, there was no significant difference between the treatment group and the control group on day 0 of treatment. On day 20 of drought stress, a significant difference was observed between the treatment group and the control group of the same period. The significant difference further increased on day 30 of drought stress, and was alleviated after rewatering. The differences in MDA content indicate that drought stress induces membrane lipid peroxidation in
I. verticillata, which in turn affects its ability to resist drought.
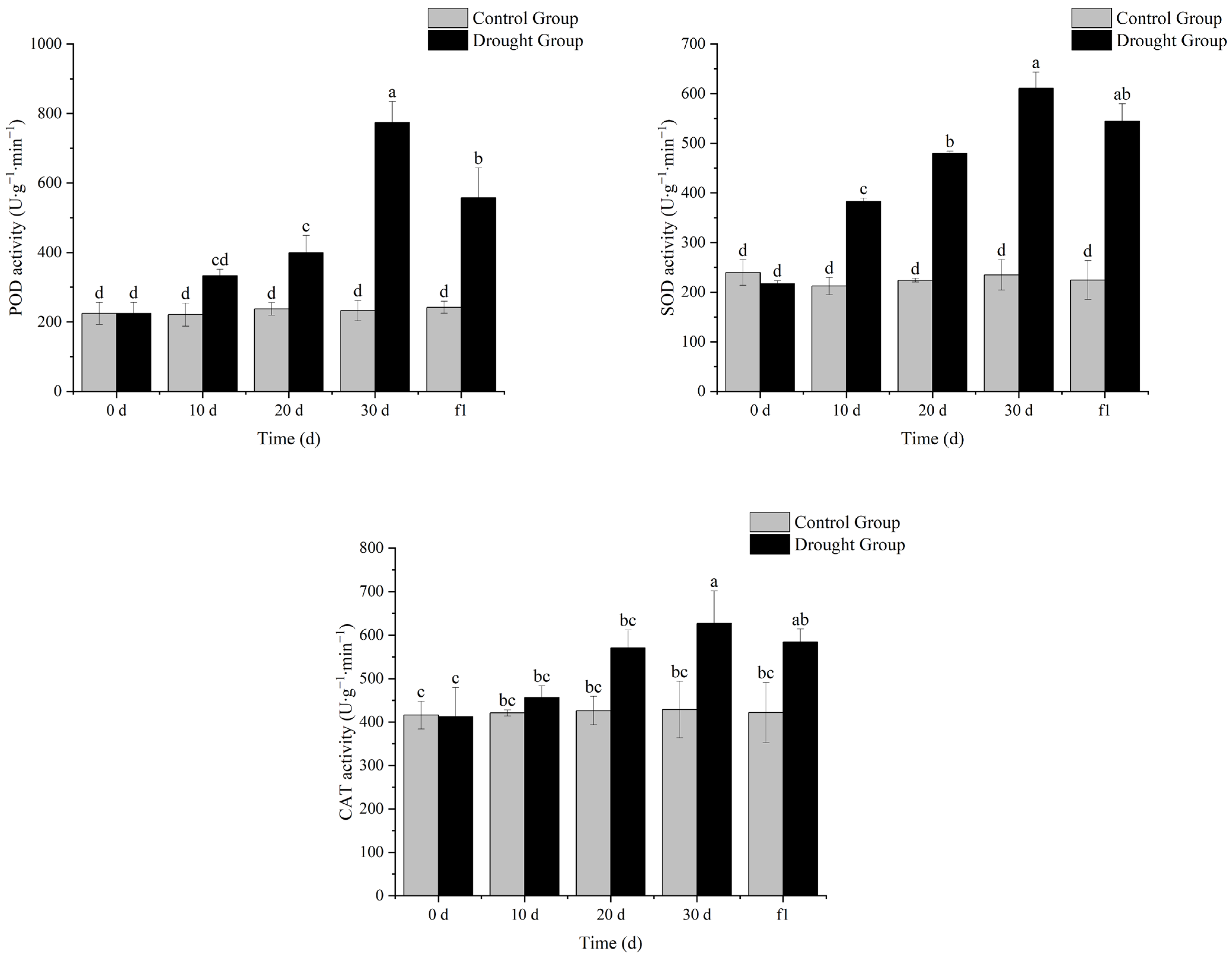
3.4. Effects of Drought Stress on Antioxidant Enzyme Activities in Ilex verticillata
As shown in
Figure 4, with the extension of drought stress, the antioxidant enzyme activity of
I. verticillata increased continuously, and decreased to some extent after rewatering. In particular, the activity of superoxide dismutase (SOD) showed a significant difference compared with the control group at the same period on the 10th day of drought, while the activities of peroxidase (POD) and catalase (CAT) showed significant differences compared with the control group at the same period on the 20th and 30th days of drought, respectively. These results indicate that under drought stress, membrane lipid peroxidation occurs in
I. verticillata, and the plant resists drought by increasing antioxidant enzyme activity. The longer the drought stress lasts, the higher the antioxidant enzyme activity of
I. verticillata becomes, which suggests that
I. verticillata has a certain adaptive mechanism and can gradually enhance its antioxidant capacity to cope with more severe drought stress.
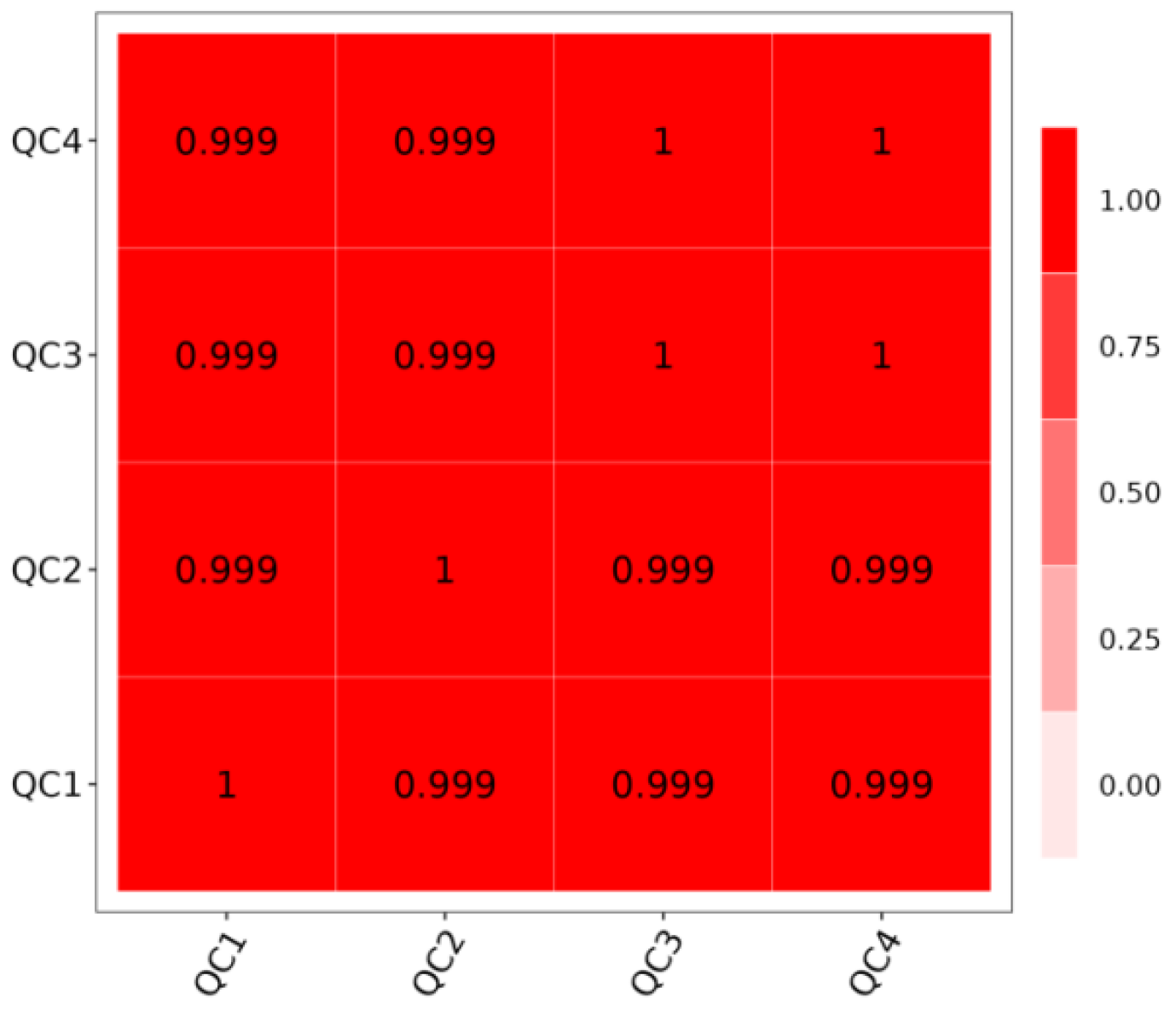
3.5. Sample Correlation Analysis
Quality Control (QC) is a crucial step to ensure the reliability and accuracy of experimental results. As demonstrated in
Figure 5, the sample correlation coefficients are all near 1, signifying strong linear relationships and high repeatability among the samples. This high repeatability is indicative of the samples’ suitability for further analysis.
3.6. PCA of Total Samples
Principal Component Analysis (PCA) was employed to evaluate the overall distribution among all tested samples and the reliability of the analysis process, with the variance explained by the first principal component (PC1) being 14.93%, indicating a clear distinguishability between groups and good aggregation within groups. As shown in
Figure 6, the 10 treatment groups of samples exhibited clear distinguishability between groups, while the samples within each group showed good aggregation and repeatability, indicating that the tested samples were reliable. The PCA results revealed that the direction with the largest total data variation (PC1) explained 14.93% of the total data variability, and the direction with the second-largest total data variation (PC2) explained 9.81% of the total data variability. The above results demonstrated that there were significant differences in the metabolites among the 10 groups of samples.
3.7. Differential Metabolite Analysis
Differential metabolites were screened using the thresholds of VIP > 1.0, FC > 2.0 or FC < 0.5, and
p value < 0.05, with the results shown in
Table 1. Analysis of
Table 1 reveals the following:
After 10 days of drought stress, the study identified 68 metabolites with differential expression, comprising 40 metabolites that were significantly up-regulated and 28 that were significantly down-regulated. After 20 days of drought stress, a comparable number of metabolites were up-regulated and down-regulated, with 113 metabolites showing increased expression and 117 metabolites showing decreased expression. On the 30th day of drought stress, the number of differential metabolites decreased to 165, and the number of up-regulated metabolites was significantly greater than that of down-regulated metabolites; On the 1st day after rewatering, the number of differential metabolites reached the maximum (239), including 130 up-regulated metabolites and 109 down-regulated metabolites.
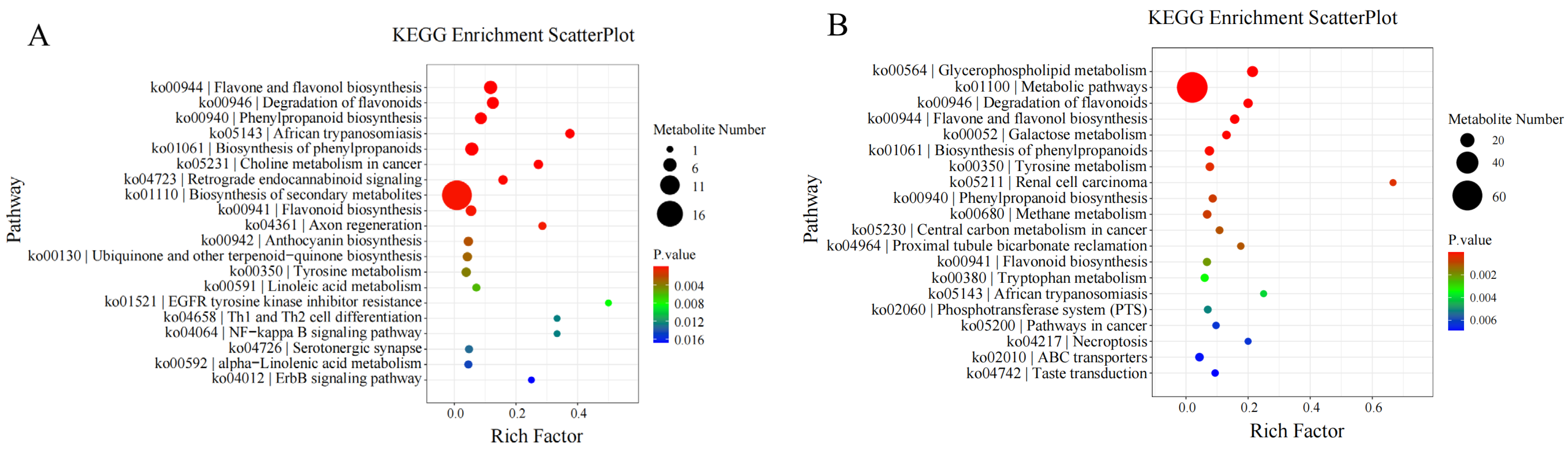
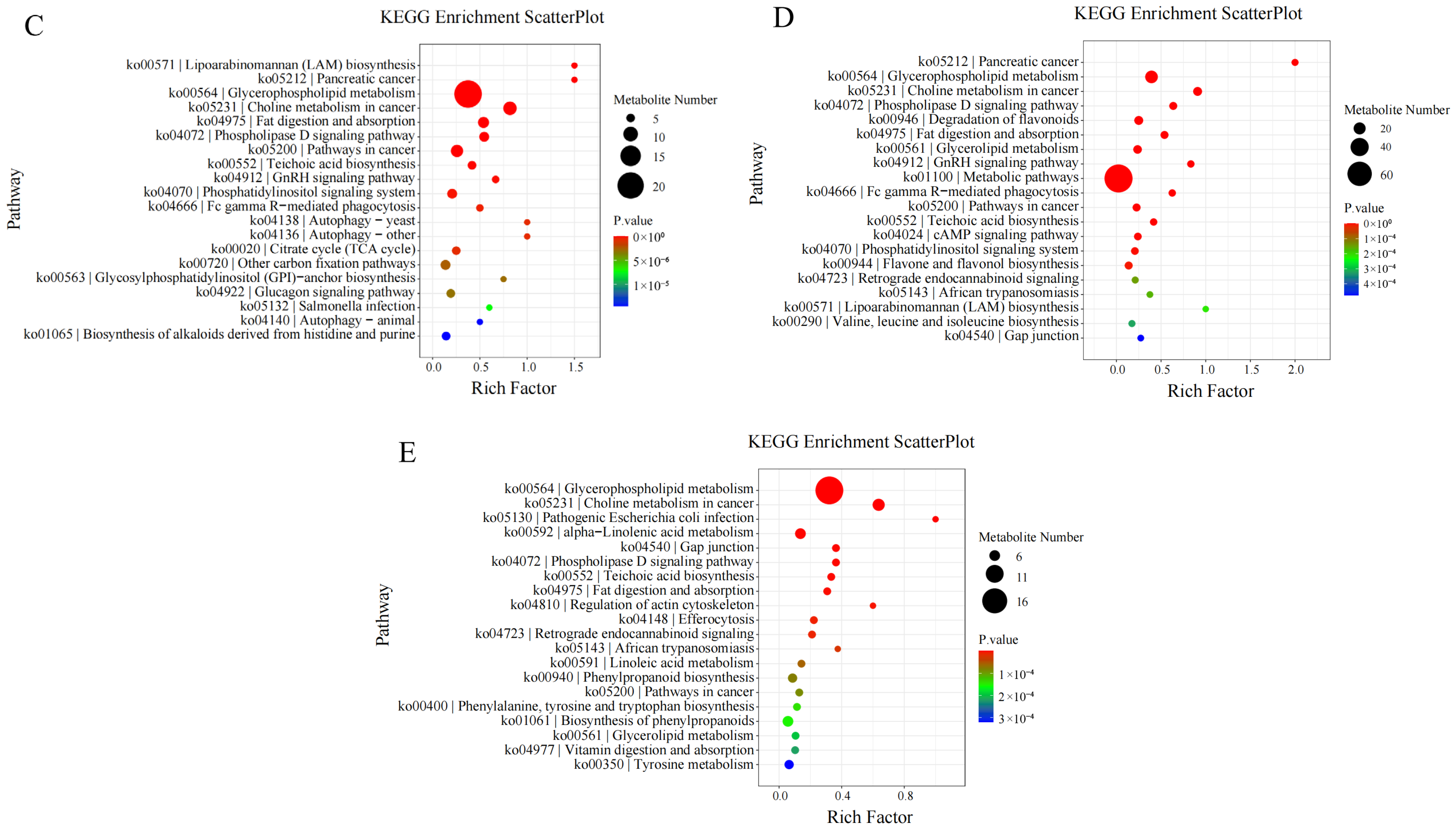
3.8. KEGG Enrichment Analysis of Differential Metabolites
KEGG enrichment analysis of differential metabolites showed that:
On the 10th day of drought stress, differential metabolites were mostly enriched in the “biosynthesis of secondary metabolites” pathway. On the 20th day of drought stress, they were mostly enriched in “metabolic pathways”. On the 30th day of drought stress, they were enriched in “glycerophospholipid metabolism”. On the 1st day after rewatering, they were again mostly enriched in “metabolic pathways”.
Furthermore, longitudinal KEGG enrichment analysis of differential metabolites involved in
I. verticillata’s response to drought stress revealed that throughout the entire drought and rewatering process, differential metabolites were mainly enriched in the “glycerophospholipid metabolism” pathway (
Figure 7).
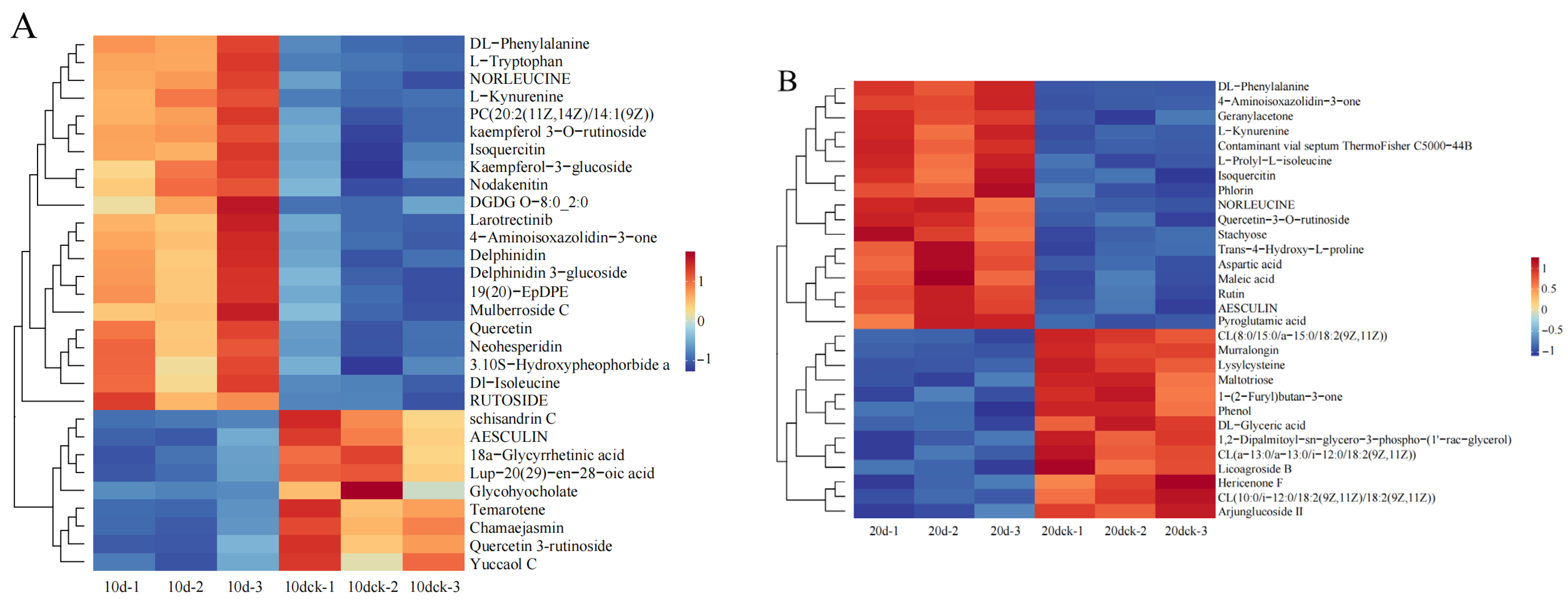
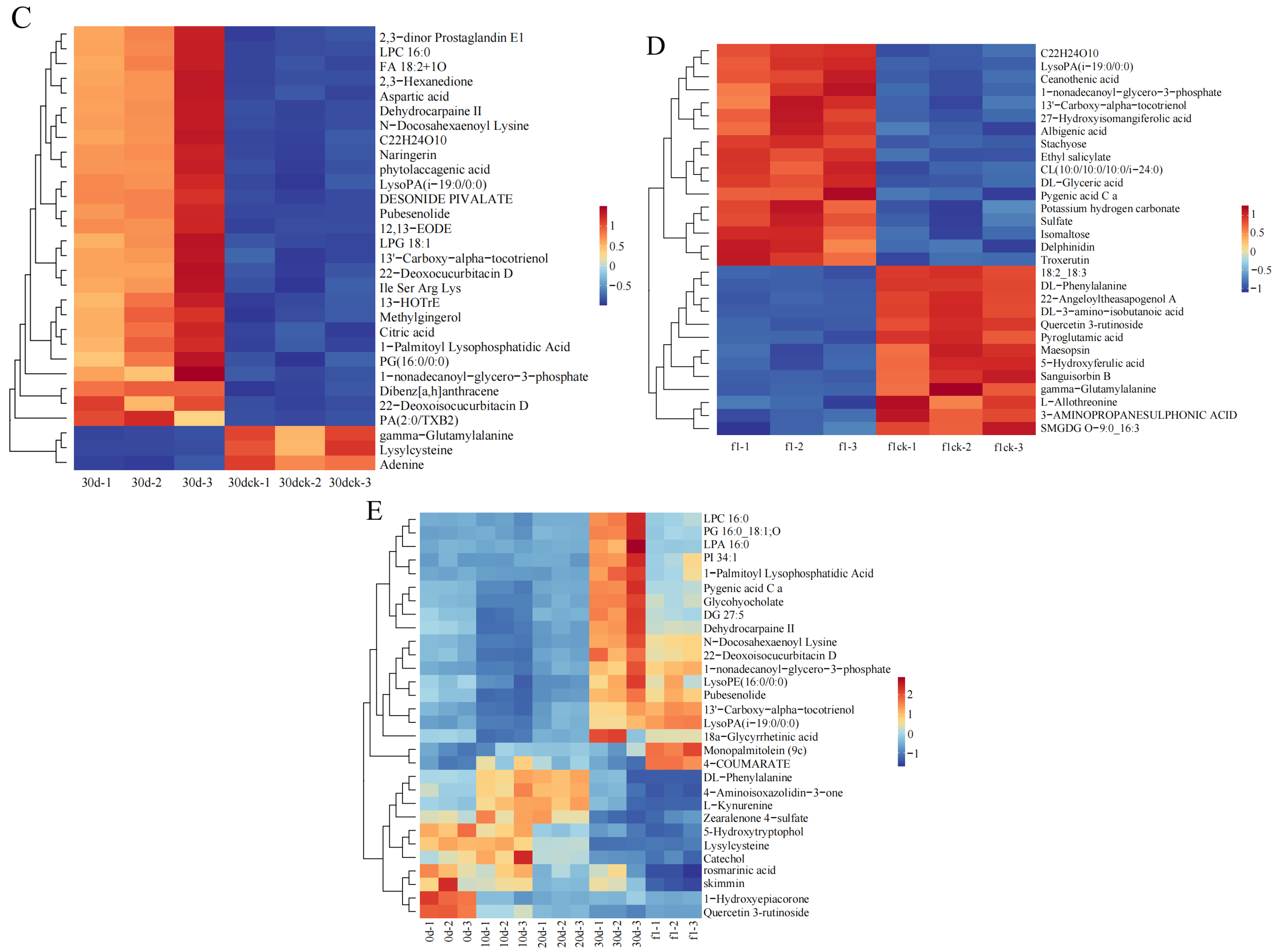
3.9. Cluster Analysis of Differential Metabolites
The heatmap of cluster analysis on the top 30 differential metabolites in each group indicates that
I. verticillata exhibits varying metabolite profiles across different drought periods, as observed in
Figure 8.
On the 10th day of drought stress, most of the differentially expressed metabolites were phenylpropanoids and polyketides enriched in the biosynthetic pathway of secondary metabolites. Among them, all were up-regulated except for aesculin, schisandrin C, and yuccaol C, whose expression levels were down-regulated. On the 20th day of drought stress, the differentially expressed metabolites were mostly organic acids and their derivatives, including maleic acid, pyroglutamic acid, and aspartic acid. On the 30th day of drought stress, the differentially expressed metabolites were mostly lipids and lipid-like molecules enriched in the glycerophospholipid metabolism pathway, such as 1-acyl-sn-glycero-3-phosphoglycerol (LPG 18:1), phosphatidylglycerol, and phosphatidic acid. On the 1st day after rewatering, the differentially expressed metabolites were mostly enriched in the glycerophospholipid metabolism pathway and metabolic pathways. Among them, lipid and lipid-like molecules accounted for the largest proportion of metabolites, and the expression levels of most of these metabolites enriched in the glycerophospholipid metabolism pathway (e.g., lysophosphatidic acid, cardiolipin were up-regulated.
In addition, the screening of longitudinally differentially expressed metabolites during the drought and rewatering process showed that these metabolites were mainly enriched in the glycerophospholipid metabolism pathway and metabolic pathways. Most of these metabolites (e.g., 1-acyl-sn-glycerol-3-phosphate, Phosphatidylglycerol, 1-phosphatidyl-D-inositol) were up-regulated with the extension of drought stress duration and down-regulated after rewatering.
3.10. Analysis of Enriched Pathways for Differential Metabolites
Analysis of the top 20 KEGG metabolic pathways revealed that drought stress impacted the glycerophospholipid metabolism and other metabolic pathways in I. verticillata. Based on these findings, we compared the differential metabolites related to the glycerophospholipid metabolic pathway between two pairs of groups: 30 d vs. 30 dck, and f1 vs. f1 ck. The results showed that: on the 30th day of drought stress, the content of phosphatidylcholine in the leaves of I. verticillata was lower than that in the control group (CK), while the contents of phosphatidic acid, cardiolipin, and lysophosphates were higher. After rewatering, the contents of phosphatidic acid and cardiolipin in the leaves of I. verticillata were lower than those in the control group, while the contents of phosphatidylglycerol, lecithin, and CDP-choline were higher than those in the control group.
These results suggest that under drought stress, I. verticillata may regulate the composition of membrane phospholipids to cope with water deficit and maintain the integrity of cell membranes. After rewatering, it may restore the normal function of membranes and promote hydration by increasing specific phospholipid components.
In addition, by comparing the differentially expressed metabolites related to metabolic pathways between the two stages (20 d vs. 20 dck and f1 vs. f1 ck), it was found that on the 20th day of drought stress, the contents of DL-phenylalanine and pyroglutamic acid were higher than those in the control group, while the contents of cardiolipin and DL-glyceric acid were lower than those in the control group.
Following rewatering, the regulation trends of the aforementioned metabolites reversed compared to those observed on the 20th day of drought stress. These findings suggest that, under drought stress, I. verticillata may mitigate water deficiency by up-regulating specific stress-resistant metabolites (e.g., phenylalanine and pyroglutamic acid), and simultaneously regulating membrane lipids and energy metabolism to minimize consumption. After rewatering, it restores normal growth and metabolic status by adjusting the levels of these metabolites.
4. Discussion
Relative water content, chlorophyll content, MDA content, and antioxidant enzyme activity are important physiological indicators when plants are subjected to drought stress. Determining these indicators helps quantify the intensity of drought stress and analyze the physiological mechanisms of plant drought resistance.
In this study, Ilex verticillata showed a clear gradient of physiological responses during drought stress: the relative water content of leaves continued to decrease with the extension of drought duration, with a significant difference from the control group at 30 days, and gradually increased after rehydration, indicating that the plants responded to stress by dynamically regulating the balance between water absorption and transpiration. The decreasing trend of chlorophyll content is closely related to the protection of the photosynthetic system—water deficit inhibits the activity of chlorophyll synthase and accelerates chlorophyll degradation, thereby reducing light energy capture to decrease transpiration water consumption, which is a typical “drought avoidance” strategy. The continuous increase in antioxidant enzyme activity reflects the “active defense” mechanism, which reduces membrane lipid peroxidation by scavenging reactive oxygen species. However, the MDA content increased significantly after 20 days of drought, reflecting the progressive process of cell membrane structure damage with the aggravation of stress. The reverse adjustment of each indicator after rehydration confirms the reversibility and repair potential of the physiological responses of I. verticillata.
Untargeted metabolomics technology has the advantages of high throughput, integrity, and objectivity. Leveraging multivariate statistical analysis techniques, the study conducts both qualitative and quantitative assessments of the chemical compositions of plants across various treatments. This facilitates a comprehensive exploration of the characteristics and variation patterns of different chemical components during plant growth and development, leading to its widespread application in plant research. KEGG enrichment analysis, a method that involves data preprocessing, functional annotation, and pathway enrichment analysis, reveals the effects of different treatment groups on plants by comparing metabolome data with the KEGG database.
In this study, untargeted metabolomic analysis was performed on each treatment group under drought and rewatering treatments, with a focus on the comparison groups of 20 d vs. 20 dck, 30 d vs. 30 dck, and f1 vs. f1 ck. The analysis results showed that in the 30 d vs. 30 dck comparison group, the number of significantly up-regulated differential metabolites was greater than that of significantly down-regulated ones; while in the 20 d vs. 20 dck and f1 vs. f1 ck comparison groups, the total number of significantly up-regulated and down-regulated differential metabolites was basically the same. Based on the above results, the top 30 metabolites in the three comparison groups were selected for analysis. It was found that the three comparison groups contained relatively high contents of three types of metabolites, namely lipids and lipid-like molecules, phenylpropanoids and polyketides, and organic acids and their derivatives.
Lipids, including phospholipids and triglycerides, alongside lipid-like substances like carotenoids, are essential constituents of plant cell membranes. They play a pivotal role in preserving the structural integrity and ensuring the fluidity of these membranes, which is critical for cellular functions. In this study, among the top 30 metabolites, on the 20th day of drought stress, there were 5 lipid and lipid-like metabolites; on the 30th day, the number increased to 17; and on the 1st day after rewatering, there were 11 lipid and lipid-like metabolites. Among them, most lipid and lipid-like metabolite contents were down-regulated on the 20th day of drought stress. This may occur because drought increased reactive oxygen species (ROS) levels in
I. verticillata, causing lipid peroxidation and cell membrane damage. Therefore, plants may reduce lipid synthesis to lower peroxide production, thereby alleviating cell membrane damage. On the 30th day of drought stress, the contents of all 17 lipid and lipid-li. The metabolites were up-regulated, indicating that drought stress can damage the cell membrane of
I. verticillata. However, the accumulation of lipids and lipid-like substances can mitigate this issue by enhancing the stability and protective capacity of the cell membrane. Following rewatering treatment, it was observed that most lipid and lipid-like metabolites remained up-regulated, whereas a minority were down-regulated. This may be because rewatering
I. verticillata may stimulate lipid synthesis and membrane reconstruction, thus increasing the levels of related lipid metabolites. It can be seen that the underlying mechanisms of the aforementioned physiological changes are closely related to the dynamic remodeling of lipid metabolism in metabolomics, and that lipid metabolism under drought stress in
I. verticillata exhibits a typical ‘suppression followed by promotion’ pattern. Shen et al. [
24] found that lipid metabolites in tea leaves exhibited an obvious temporal pattern of “first inhibition then promotion” under drought stress. Michael Ackah made the same observation in
Morus alba L. [
25].
In addition, Ling et al. [
26] discovered that under drought stress, phosphatidic acid (PA) is the most critical responsive lipid in somatic embryos of
Picea asperata. Its content increases continuously, functioning not only as a membrane structural component but also as a signaling molecule to activate downstream protein kinases, regulating embryo development and the expression of stress-resistant genes. No significant inhibition of lipid synthesis was observed throughout the process. Qu et al. [
27] found that the drought-tolerant cultivar “Huajin” showed continuously enhanced lipid synthesis capacity under drought stress. The proportion of unsaturated fatty acids in membrane lipid components increased continuously, accompanied by the persistent accumulation of signaling lipids such as PA and PIP, which activated the expression of downstream drought-resistant genes. This showed a significant difference from the “first inhibition then promotion” pattern. This indicates that the response of lipid metabolism in woody plants to drought stress does not follow a single fixed pattern, but rather exhibits significant species specificity and environmental adaptive differentiation. The essence of such differences is closely related to the plants’ own drought tolerance potential and metabolic regulatory flexibility. The “first inhibition then promotion” pattern of lipid metabolism formed by
I. verticillata in response to drought stress also provides an important theoretical basis for targeted breeding of drought-tolerant varieties.
5. Conclusions
Ilex verticillata exhibits distinct physiological response gradients and dynamically coupled characteristics with lipid metabolism under drought stress. In the early stage of drought, the relative water content of leaves decreases continuously, and chlorophyll synthesis is inhibited to reduce transpiration water consumption, while the activity of antioxidant enzymes increases to scavenge reactive oxygen species. Metabolomic analysis shows that 5 lipid and lipid-like metabolites among the top 30 differential metabolites are significantly down-regulated after 20 days of drought. It is speculated that the plant reduces the production of peroxidation substrates by inhibiting lipid synthesis to lower the risk of membrane lipid peroxidation damage, reflecting the synergy between the “drought avoidance” strategy and defensive metabolic inhibition. In the late stage of drought, the relative water content of leaves is significantly different from that of the control group, the activity of antioxidant enzymes continues to increase, and the content of MDA further intensifies, reflecting the progressive aggravation of membrane damage. At this time, lipid metabolism undergoes a transitional change, with all 17 lipid and lipid-like metabolites being up-regulated, including membrane lipid components such as phosphatidylcholine (PC) and phosphatidylglycerol (PG). By increasing membrane lipid saturation and accumulating membrane structural components, the stability and protective properties of cell membranes are enhanced, alleviating drought-induced membrane damage, thus forming an integrated response of “active defense” and compensatory metabolic activation.
Glycerophospholipid metabolism is a key pathway commonly enriched under both drought and rehydration conditions, and its dynamic changes run through the entire process of I. verticillata’s response to drought. In the early stage of drought, the contents of glycerophospholipids such as PC and phosphatidylethanolamine (PE) are down-regulated, reducing peroxidation-sensitive substrates to mitigate oxidative damage. In the late stage of drought, a large amount of membrane lipids such as PC and PG accumulate, remodeling membrane lipid composition to maintain membrane fluidity and integrity. After rehydration, 11 lipid metabolites remain up-regulated, and a small number are down-regulated, indicating that the glycerophospholipid metabolic pathway remains active. It accelerates the repair of the membrane system by stimulating lipid synthesis and membrane structure reconstruction, confirming the core regulatory role of this pathway in drought adaptation and rehydration recovery.
This study is the first to reveal the molecular basis of the “first inhibition then promotion” pattern of lipid metabolism in I. verticillata: that is, through metabolic inhibition in the early stage of drought to reduce energy consumption and oxidation risk, metabolic activation in the later stage to achieve membrane structure repair and function maintenance, and rapid reconstruction of membrane lipid homeostasis after rehydration, which reflects the phased adaptation strategy of I. verticillata to drought stress. As a core hub, the glycerophospholipid metabolic pathway, whose key metabolites (such as 1-acyl-sn-glycerol-3-phosphate glycerol and phosphatidylcholine) accumulate in a time-dependent manner and are closely related to the regulation of membrane stability, provides important targets for the breeding of drought-tolerant varieties. The research results enrich the theory of plant stress metabolic regulation and provide a typical example for analyzing the drought resistance mechanism of woody plants.