Simple Summary
Gray mold, caused by the fungus, Botrytis cinerea, damages over 1400 plant species and leads to serious crop losses worldwide. People often use chemical fungicides, but these will harm the environment and may lose effectiveness after using for long time. As a safer alternative, we developed a tiny triangular RNA particle, called “Bc-triangle,” that silence fungal genes needed for growth and infection. When sprayed on plants, Bc-triangle was more stable and effective than regular RNA, protecting crops for up to 10 days. Tests showed it slowed fungal growth, suppress disease lesion area expansion on leaves, and strongly suppressed the fungus’s target genes. This study highlights RNA nanotechnology as a sustainable and chemical-free strategy for crop protection, with potential benefits for food security and environmental health.
Abstract
Botrytis cinerea, a necrotrophic fungal pathogen responsible for gray mold, poses a severe threat to over 1400 plant species, causing significant pre- and postharvest losses worldwide. RNA interference (RNAi)-based strategies, particularly spray-induced gene silencing (SIGS), have emerged as environmentally friendly alternatives to chemical fungicides. However, the application of naked double-stranded RNA (dsRNA) suffers from poor stability and low cellular uptake. In this study, we engineered a self-assembling triangular RNA nanoparticle, termed Bc-triangle, targeting four virulence genes of B. cinerea—BcDCL1, BcPPI10, BcNMT1 and BcBAC. The nanostructure was designed using RNA origami principles and produced in Escherichia coli. Functional assays demonstrated that Bc-triangle significantly inhibited conidial germination and mycelial growth in vitro, and markedly reduced disease severity in plants. Compared with linear dsRNA, Bc-triangle showed superior persistence and efficacy, with lesion area reduction sustained up to 10 days post-spraying. qRT-PCR analysis revealed substantial downregulation of the target genes, especially BcNMT1, indicating enhanced RNAi activation. These findings establish RNA nanotechnology as a powerful platform for transgene-free, programmable, and sustainable control of fungal pathogens in crop production.
1. Introduction
Botrytis cinerea, the causative agent of gray mold, is a globally prevalent necrotrophic fungal pathogen with an exceptionally broad host range, encompassing vegetables, fruits, ornamental plants, and trees, affecting up to 1400 plant species [1,2]. Annual preharvest losses attributed to B. cinerea are estimated at 10–30%, with postharvest rot rates reaching up to 50%, resulting in over $10 billion in global economic losses [3,4]. The control of B. cinerea remains a major challenge due to its broad host range, rapid reproduction, and strong adaptability [5].
Conventional management strategies predominantly depend on chemical disinfectants. However, their extensive utilization has resulted in the emergence of resistance, heightened environmental concerns, and the intensification of food safety concerns. Consequently, the priority is to cultivate sustainable, precise, and environmentally friendly alternatives.
RNA interference (RNAi) has emerged as a novel approach for plant disease control through gene-specific silencing in pathogens. Spray-induced gene silencing (SIGS) [6,7], which involves the exogenous application of double-stranded RNA (dsRNA) onto plant surfaces, enables transient silencing of essential fungal genes without the need for transgenic plants [8,9]. Despite its promise, SIGS is often limited by the instability of naked dsRNA under field conditions, inefficient uptake by fungal cells, and short protection windows, all of which restrict its practical application [10].
Recent advances in nanotechnology offer new avenues to address these challenges. Nanocarriers such as layered double hydroxides (LDHs), chitosan complexes, and carbon-based materials have been explored to enhance the stability and cellular uptake of dsRNA [11,12,13]. While effective, most of these carriers rely on synthetic or exogenous materials, posing scalability, biocompatibility, and environmental risks [8,13].
Structured RNA nanoparticles—constructed using RNA origami techniques—offer precise spatial arrangement, increased nuclease resistance, and enhanced cellular uptake [8]. In this study, we designed and constructed a triangular RNA nanoparticle (termed Bc-triangle) incorporating siRNAs targeting four key virulence genes of B. cinerea—BcDCL1, BcPPI10, BcNMT1 and BcBAC. We hypothesized that this multivalent nanostructure could enhance RNAi efficiency and provide superior antifungal effects compared to conventional dsRNA [14]. The efficacy of the Bc-triangle was evaluated in vitro and in planta, and its gene-silencing performance was confirmed via quantitative PCR analysis. Our results demonstrate that RNA nanostructures targeting virulence genes can significantly enhance the effectiveness of SIGS, offering a promising new strategy for fungal disease management in crops.
In this study, we selected a square RNA nanostructure, as reported by Li et al. [15], to design an antifungal RNA fungicide. This was achieved by linking multiple siRNAs targeting four virulence genes Bcin12g06230.1 (Dicer-Like 1, DCL1), Bcin13p01840.1 (Peptidyl prolyl cis-trans isomerase 10, PPI10), Bcin04p04920.1 (N-myristoyltransferase 1, NMT1) and Bcin15p02590.2 (putative adenylate cyclase, BAC)) which was validated as an effective target gene for the RNAi-based management of Botrytis cinerea [16,17].
2. Materials and Methods
2.1. Preparation of Plant Materials and Botrytis cinerea
Tobacco plants (Nicotiana benthamiana) were cultivated in a greenhouse, with an approximate temperature of 26 °C and a photoperiod of 16 h of light and 8 h of darkness. The fungus Botrytis cinerea was cultivated on potato dextrose agar (PDA) medium at 26 °C with 80% humidity. Following a two-week cultivation period, conidia were harvested from the PDA cultures and suspended in sterile deionized water to achieve a final concentration of 5 × 106 conidia/mL for subsequent bioassays.
2.2. Design of RNA Nanoparticles
The gene-specific small interfering RNAs (siRNAs) were designed using the Designer of Small Interfering RNA (DSIR, CEA, Paris, France) algorithm, with selection criteria including a prediction score above 90 and the absence of more than three consecutive identical nucleotides [18]. Potential off-target effects were assessed by performing NCBI BLASTn (version 2.12.0) searches of the siRNA sequences against the tomato genome, using default parameters with the exception of a word size set to 7 [19]. After eliminating off-target siRNAs, five siRNAs were randomly selected to design. A triangular RNA nanoparticle (Bc-triangle) was designed by integrating the five selected siRNAs using tetra-U helix linking motifs [15], hairpin loops (5′-UCCG-3′), and kissing loops (5′-AAGGAGGCA-3′, 5′-AAGCCTCCA-3′), resulting in the formation of a triangle RNA nanostructure. The secondary structure of the RNA square was confirmed using the RNAfold program [20]. In addition, all of these six siRNAs targeting DCL1, PPI10, Nmt1 and BAC were concatenated to form a long double-strand RNA (Bc-dsRNA) as the positive control.
2.3. Plasmid Construction and RNA Nanoparticle Expression in E. coli
The coding sequence of Bc-triangle was subjected to a chemical synthesis process and subsequently cloned into the pET-28a (+) expression vector, a maneuver that utilized the restriction sites Xba I and Bpu1102 I (GenScript, Nanjing, China). The recombinant plasmid was then transformed into the Escherichia coli HT115 (DE3) strain, and RNA expression was induced at 37 °C with 1 mM isopropyl β-D-1-thiogalactoside (IPTG) for a period of 4 h. The kit utilized for dsRNA extraction (RNA-Direct Silencing Technology Co., Ltd., AK011, Shaoxing, China) was employed in accordance with the protocol delineated in the kit manual, and then subjected to agarose gel electrophoresis to verify the production of RNA nanoparticle.
In addition, the reverse complementary sequence of T7 promoter was first appended to the 3′-end of the coding sequence of Bc-dsRNA. This sequence was then chemically synthesized and also inserted into pET28a using Xba I and Bpu1102 I restriction endonucleases. Finally, Bc-dsRNA were performed following the same procedure as Bc-triangle.
2.4. Spore Germination Assay
Conidial suspensions of B. cinerea (1 × 106 spores/mL) were incubated with either No-RNA (water was used as the negative control), Bc-dsRNA, or Bc-triangle (final concentration 100 ng/μL). After 24 h incubation at 26 °C on a glass slide, spore germination was observed under a microscope.
2.5. Mycelial Growth Inhibition Assay
Following the sterilization of PDA (121 °C, 15 min), the medium should be cooled to 50–60 °C, and the Bc-triangle and Bc-dsRNA should be incorporated into the PDA medium. In order to achieve the desired concentrations, 7.5 mL of RNA solution was added to each 150 mL of PDA medium, resulting in final working concentrations of 25, 50, 100, and 200 ng/μL. The control group, which did not receive any treatment, received an equal volume of ddH2O. Mycelial plugs (5 mm) from actively growing B. cinerea cultures were placed at the center of each plate and incubated at 26 °C for 5 days. Colony diameters were measured, and inhibition rates were calculated relative to the No-RNA controls.
For mycelial growth assays, PDA plates supplemented with RNA formulations (25–200 ng µL−1) were inoculated with 5 mm mycelial plugs from the growing edge of B. cinerea cultures. Colony diameter was measured after 5 days of incubation at 25 °C, and inhibition rate was calculated as follows:
where C is the colony diameter of the control, and T is the colony diameter of the treatment.
2.6. In Planta Assay on Tobacco
Four-week-old Nicotiana benthamiana plants were sprayed with 5 mL of either sterile water, Bc-dsRNA, or Bc-triangle (200 ng/μL). At 3, 7, and 10 days post spraying (dps), individual leaves were inoculated with B. cinerea mycelial plugs (1 per leaf). Disease progression was assessed after 3 days by measuring necrotic lesion areas by ImageJ 1.53k (NIH, Bethesda, MD, USA). Data were analyzed from three biological replicates.
2.7. Quantitative RT-PCR Analysis
Mycelia from treated PDA plates were collected for RNA extraction using TRIzol reagent (Solarbio, Beijing, China). cDNA synthesis was performed using a reverse transcription kit (Takara, Dalian, China). Quantitative real-time PCR (qRT-PCR) was conducted using gene-specific primers for BcDCL1, BcNMT1 and BcBAC with a standard SYBR Green system (Vazyme, Nanjing, China). Gene expression level was normalized to an internal reference gene, and relative expression was calculated using the 2−ΔΔCt method [21].
2.8. Statistics Analysis
All experiments were conducted with at least three independent biological replicates. Data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test in GraphPad Prism 9.0 (GraphPad Software, Boston, MA, USA). Results are expressed as mean ± standard deviation (SD). Differences were considered statistically significant at p < 0.05.
2.9. Cryo-EM Imaging
RNA NPs was transcribed in vitro using the expression vectors and the T7 RiboMAX™ Express Large Scale RNA Production System (Gatan, Pleasanton, CA, USA) following the manufacturer’s protocol. The RNA NPs was purified by DNase I digestion for 15 min at 37 °C, followed by lithium chloride precipitation, and then melted into Tris-Mg buffer (10 mM Tris-HCl, 10 mM Mg(OAc)2 at a concentration of 3 μM. The RNA nanostructures were confirmed using cryo-EM following the description provided by Li et al. [15].
3. Results
3.1. Design and Production of B. cinerea-Targeted Triangle RNA Nanoparticles
To construct a B. cinerea-targeted RNA nanostructure, we selected four virulence genes, DCL1, PPI10, Nmt1 and BAC, which have been verified as effective target genes for RNAi-based management of B. cinerea [16]. To design B. cinerea-targeted siRNAs, we utilized DSIR and randomly screened out six siRNAs for these four target genes (Table 1). These six siRNAs served as structural motifs for the assembly of triangle RNA NPs utilizing hairpin loops, kissing loops, and the tetra-U motif. Computational prediction using RNAfold confirmed that the Bc-triangle adopts a thermodynamically stable conformation with a free energy of approximately −340 kcal mol−1, suggesting strong intramolecular interactions and folding efficiency (Figure 1a). This assembly was validated using the RNAfold program and named Bc-triangle (Figure 1b). The coding DNA sequence of the Bc-triangle is provided in Table 1. The nanostructure of the Bc-triangle was characterized using cryo-EM. Results showed that this NP could self-assemble into the expected structures (Figure S1).

Table 1.
siRNA used in RNA NP.
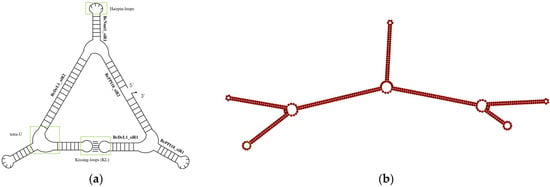
Figure 1.
Design model of a triangle RNA nanoparticle (a) and its secondary structure confirmed by RNAfold (b).
3.2. Production of the Triangle RNA Nanoparticles
The coding cassette was cloned into the pET-28a (+) vector and expressed in Escherichia coli strain HT115 (DE3). Following IPTG induction, total RNA was extracted and analyzed by agarose gel electrophoresis. A distinct RNA band of approximately 250–500 bp was detected in bacterial lysates harboring the Bc-triangle construct, whereas no such band was observed in the empty vector control (Figure 2). These results confirmed the successful expression and accumulation of the designed RNA nanostructure in E. coli.
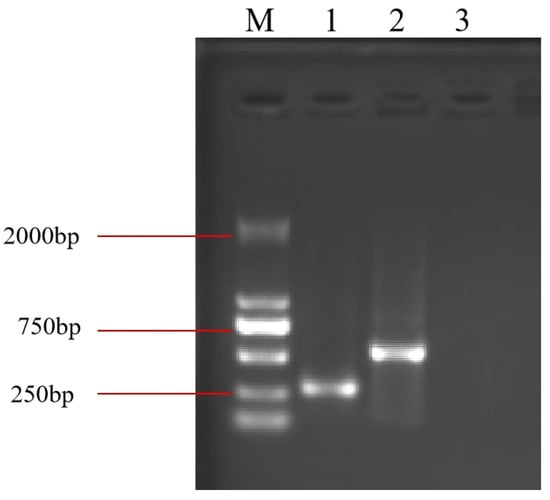
Figure 2.
RNA extracted from induced E. coli harboring the empty plasmid or from the two recombinant plasmids. M: DL 2000 marker; 1: Bc-triangle; 2: Bc-dsRNA; 3: pET-28a.
3.3. Bc-Triangle Strongly Inhibits B. cinerea Spore Germination
To assess the antifungal efficacy of Bc-triangle during the early stages of fungal development, its effect on Botrytis cinerea conidial germination was investigated. After 24 h of incubation, spores in the water-treated control group (No-RNA) germinated normally, while those treated with dsRNA exhibited partial inhibition of germination. Notably, spores treated with Bc-triangle exhibited a near-complete inhibition of germination, with negligible germ tube formation (Figure 3). These results suggest that Bc-triangle exerts potent inhibitory activity at the initial infection stage, outperforming traditional dsRNA.
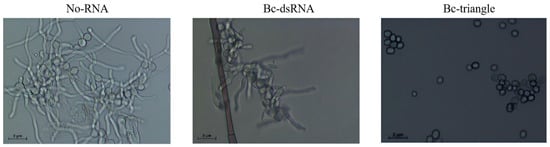
Figure 3.
Effect of Bc-triangle with concentration of 100 ng/uL for 10 h on spore germination of Botrytis cinerea. No-RNA served as negative control.
3.4. Suppression of Mycelial Growth by Bc-Triangle
The antifungal efficacy of Bc-triangle against vegetative hyphal expansion was tested on PDA medium supplemented with the final concentrations of 25, 50, 100 ng/μL and 200 ng/μL. After 5 days of incubation, Bc-triangle exhibited a concentration-dependent inhibition of mycelial growth (Figure 4a).
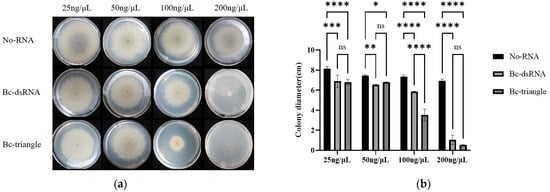
Figure 4.
Effect of Bc-triangle with different concentrations on the growth of Botrytis cinerea mycelium on PDA plate. (a) Colony size of B. cinerea on PDA plates about control, dsRNAs and Bc-triangle groups. (b) The relative colony size of B. cinerea. Results are expressed as the means ± SEM of four biological replicates. Asterisk indicates the statistically significant differences according to one-way analysis of variance with Tukey test (ns: p > 0.05; *: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001).
At 100 ng/μL, Bc-triangle achieved an average inhibition rate of 52.35%, significantly higher than the 20.19% observed for dsRNA. At 200 ng/μL, Bc-triangle suppressed hyphal growth by 95.02%, whereas dsRNA achieved 87.31% inhibition (Figure 4b).
These findings indicate that while both RNA treatments are effective at high concentrations, Bc-triangle exhibits significantly greater antifungal potency at equivalent doses, likely due to its enhanced stability and improved cellular uptake of the nanoparticle structure.
3.5. Bc-Triangle Confers Enhanced Protection Against B. cinerea Infection in Planta
To evaluate the efficacy of Bc-triangle under more realistic conditions, we conducted infection assays on whole tobacco (Nicotiana benthamiana) plants. Plants were foliar-sprayed with Bc-triangle, dsRNA, or water (negative control) at 200 ng/μL followed by challenged with B. cinerea mycelial plugs at 3, 7, and 10 days post-spraying (dps). Lesion area measurements revealed that Bc-triangle conferred more robust and sustained protection against fungal infection compared to dsRNA (Figure 5a,b).
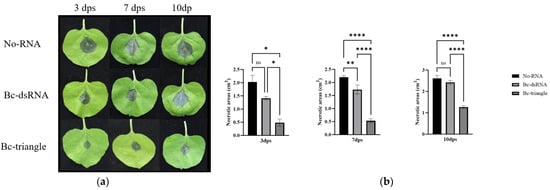
Figure 5.
Protective efficacy of the Bc-triangle against B. cinerea infection on tobacco plants. (a) Necrosis of B. cinerea on tobacco leaves at 3, 7 and 10 dps. (b) The necrotic areas on tobacco leaves at 3, 7 and 10 dps. The results are expressed as the means ± SEMs of three leaves. Asterisk indicates the statistically significant differences according to one-way analysis of variance with Tukey test (ns: p > 0.05; *: p < 0.05; **: p < 0.01; ****: p < 0.0001).
At 3 dpt, lesion areas on Bc-triangle–treated leaves averaged 0.4–0.6 cm2, significantly smaller than those in dsRNA-treated (1.3–1.5 cm2) and control plants (2.0–2.3 cm2) (Figure 4a). Even at 10 dpt, Bc-triangle maintained strong protection, with lesion areas remaining below 1.3 cm2, while dsRNA and control treatments showed extensive tissue maceration (2.3–2.7 cm2) (Figure 4b).
These results indicate that Bc-triangle provides extended protection compared with conventional dsRNA, prolonging the effective disease-suppression window to at least 10 days after spraying.
3.6. Bc-Triangle Enhances Gene Silencing Efficiency in B. cinerea
To confirm whether the antifungal effect of Bc-triangle was associated through RNAi-mediated gene silencing, three genes (DCL1, NMT1 and BcBAC) of the four target genes were selected to detect their expression levels using qRT-PCR. Results showed that both genes were significantly downregulated in Bc-triangle treatment groups compared to the No-RNA group. However, with regard to the BcPPI10 gene, it cannot be discounted that fluctuations in gene expression levels may occur during the course of the gray mold growth cycle. Notably, Bc-triangle induced stronger gene silencing than dsRNA (Figure 6). These findings confirm that Bc-triangle more effectively delivers siRNA payloads into fungal cells and triggers efficient gene silencing.
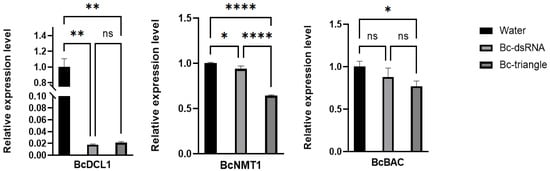
Figure 6.
Expression levels of two target genes on B. cinerea treated by Bc-dsRNA and Bc-triangle. The Actin gene of B. cinerea was used as the internal control. The results are expressed as the means ± SEM of three biological replicates. Asterisk indicates the statistically significant differences according to one-way analysis of variance with Tukey test (ns: p > 0.05; *: p < 0.05; **: p < 0.01; ****: p < 0.0001.
4. Discussion
In this study, we successfully developed a triangular RNA nanoparticle, termed Bc-triangle, that targets Botrytis cinerea virulence genes—BcDCL1, BcPPI10, BcNMT1 and BcBAC—identified in our previous work [17]. The Bc-triangle nanostructure demonstrated superior antifungal efficacy compared to conventional naked dsRNA across all tested parameters, including inhibition of spore germination, suppression of hyphal expansion, reduction in plant lesion size, and enhancement of gene silencing efficiency [8,17]. However, it was not possible to detect PPI10 at any expression level. The underlying reason for the observed absence of PPI10 expression could be attributed to the specific expression of this gene during the course of pathogen-plant interactions. Consequently, in the course of conducting experiments on mycelium in the presence of RNA nanoparticles, the expression of the mycelium could not be detected.
The dramatic reduction in spore germination upon Bc-triangle treatment suggests efficient early-stage RNAi interference. It is known that germinating conidia are highly sensitive to environmental signals, and rapid siRNA uptake during this stage may interfere with initial infection processes such as appressorium formation or adhesion [22,23]. The enhanced performance of Bc-triangle compared to dsRNA also supports previous findings that RNA nanoparticles can improve intracellular delivery and retention [24,25]. The observed dose-dependent inhibition of mycelial growth further illustrates the functional robustness of the nanostructure. At 200 ng/μL, the Bc-triangle inhibited over 95% of fungal colony expansion—significantly higher than dsRNA at the same concentration—highlighting improved bioavailability and efficiency of gene silencing in fungal tissues.
One of the most notable outcomes is the long-lasting disease protection achieved by a single foliar spray of Bc-triangle. While the protective effect of naked dsRNA significantly diminished after 7 days, Bc-triangle maintained significant antifungal activity up to 10 days post-spraying. This extended protective window is critical for field applications where environmental factors such as rain, UV radiation, and nucleases rapidly degrade dsRNA [26,27,28]. The compact and folded architecture of the RNA nanoparticle likely confers resistance to degradation and facilitates gradual siRNA release, consistent with similar observations in biomedical RNA delivery studies [25,29].
When compared to synthetic/organic carriers (e.g., layered double hydroxide clay sLDH or chitosan nanoparticles), pure RNA nanoparticles have been demonstrated to exhibit superior stability and RNAi persistence in terms of efficacy, with a significant inhibition of spore germination and mycelial growth [8]. In terms of cost, this process can utilize microorganisms (such as E. coli) for scale-up, thereby eliminating preparation steps involving carrier materials and complexation, significantly reducing costs. With regard to environmental compatibility, pure RNA nanoparticles are fully biodegradable and pose no risk of carrier residue, whereas synthetic carriers may lead to non-target accumulation or ecotoxicity concerns [30]. In comparison to alternative control methodologies, chemical pesticides (e.g., carbendazim) have been observed to readily induce resistance and environmental contamination. Furthermore, genetically modified RNAi crops encounter regulatory challenges in obtaining approval for genetic modification [10]. Conversely, SIGS technology does not necessitate genetic modification and permits flexible application. Additionally, biological agents (e.g., antagonistic bacteria) demonstrate diminished efficacy under environmental constraints, whereas RNA nanoparticles exhibit enhanced stability. In this study, the protective window of Bc-triangle was observed to last up to 10 days, while naked dsRNA was found to last 3–7 days, though this was slightly shorter than the carrier-complexed dsRNA (e.g., sLDH-dsRNA was found to reach 20 days) [8,9,30].
5. Conclusions
Our results provide strong evidence that RNA origami-based nanostructures can significantly improve the efficacy and durability of SIGS-mediated fungal disease control. The Bc-triangle RNA nanoparticle not only enables efficient multigene silencing but also enhances RNA stability and in planta retention, overcoming critical limitations of conventional dsRNA. This study advances the development of self-delivering, environmentally friendly RNA biopesticides and opens new avenues for applying RNA nanotechnology in sustainable agriculture. Further work is needed to elucidate the uptake and translocation mechanisms of RNA nanostructures in both host plants and pathogens and to assess their long-term performance under field conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology14111616/s1, Figure S1: Bc-triangle cryo-EM Results.
Author Contributions
Y.C.: Data curation, Methodology, Writing—Original draft preparation, Validation. Y.L.: Writing—Review and Editing, Validation. Y.H.: Methodology, Data Curation, Validation. F.W.: Conceptualization, Methodology, Supervision, Project Administration, Funding Acquisition. W.J. (Corresponding author): Conceptualization, Methodology, Writing—Review and Editing, Supervision, Project Administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of China, grant number 32172496.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials. The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Li, H.; Chen, Y.; Zhang, Z.; Li, B.; Qin, G.; Tian, S. Pathogenic Mechanisms and Control Strategies of Botrytis cinerea Causing Post-Harvest Decay in Fruits and Vegetables. Food Qual. Saf. 2018, 2, 111–119. [Google Scholar] [CrossRef]
- Shaw, M.W.; Emmanuel, C.J.; Emilda, D.; Terhem, R.B.; Shafia, A.; Tsamaidi, D.; Emblow, M.; Van Kan, J.A.L. Analysis of Cryptic, Systemic Botrytis Infections in Symptomless Hosts. Front. Plant Sci. 2016, 7, 625. [Google Scholar] [CrossRef]
- Spada, M.; Pugliesi, C.; Fambrini, M.; Pecchia, S. Silencing of the Slt2-Type MAP Kinase Bmp3 in Botrytis cinerea by Application of Exogenous dsRNA Affects Fungal Growth and Virulence on Lactuca sativa. Int. J. Mol. Sci. 2021, 22, 5362. [Google Scholar] [CrossRef]
- Ullah, I.; Yuan, W.; Khalil, H.B.; Khan, M.R.; Lak, F.; Uzair, M.; Abbas, A.; Mirzadi Gohari, A.; Wu, H. Understanding Botrytis cinerea Infection and Gray Mold Management: A Review Paper on Deciphering the Rose’s Thorn. Phytopathol. Res. 2024, 6, 42. [Google Scholar] [CrossRef]
- Spada, M.; Pugliesi, C.; Fambrini, M.; Pecchia, S. Challenges and Opportunities Arising from Host–Botrytis cinerea Interactions to Outline Novel and Sustainable Control Strategies: The Key Role of RNA Interference. Int. J. Mol. Sci. 2024, 25, 6798. [Google Scholar] [CrossRef]
- McLaughlin, M.S.; Roy, M.; Abbasi, P.A.; Carisse, O.; Yurgel, S.N.; Ali, S. Why Do We Need Alternative Methods for Fungal Disease Management in Plants? Plants 2023, 12, 3822. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Sherif, S.M. RNAi-Based Biofungicides as a Promising Next-Generation Strategy for Controlling Devastating Gray Mold Diseases. Int. J. Mol. Sci. 2020, 21, 2072. [Google Scholar] [CrossRef]
- Wu, F.; Yan, L.; Zhao, X.; Lv, C.; Jin, W. Development of an RNA Nanostructure for Effective Botrytis cinerea Control through Spray-Induced Gene Silencing without an Extra Nanocarrier. J. Fungi 2024, 10, 483. [Google Scholar] [CrossRef]
- Nerva, L.; Sandrini, M.; Gambino, G.; Chitarra, W. Double-Stranded RNAs (dsRNAs) as a Sustainable Tool against Gray Mold (Botrytis cinerea) in Grapevine: Effectiveness of Different Application Methods in an Open-Air Environment. Biomolecules 2020, 10, 200. [Google Scholar] [CrossRef]
- Qiao, L.; Lan, C.; Capriotti, L.; Ah-Fong, A.; Nino Sanchez, J.; Hamby, R.; Heller, J.; Zhao, H.; Glass, N.L.; Judelson, H.S.; et al. Spray-induced Gene Silencing for Disease Control Is Dependent on the Efficiency of Pathogen RNA Uptake. Plant Biotechnol. J. 2021, 19, 1756–1768. [Google Scholar] [CrossRef]
- Qiao, L.; Niño-Sánchez, J.; Hamby, R.; Capriotti, L.; Chen, A.; Mezzetti, B.; Jin, H. Artificial Nanovesicles for dsRNA Delivery in Spray-Induced Gene Silencing for Crop Protection. Plant Biotechnol. J. 2023, 21, 854–865. [Google Scholar] [CrossRef]
- Spada, M.; Pugliesi, C.; Fambrini, M.; Palpacelli, D.; Caneo, A.; Pecchia, S. Spray-Induced Gene Silencing (SIGS): Nanocarrier-Mediated dsRNA Delivery Improves RNAi Efficiency in the Management of Lettuce Gray Mold Caused by Botrytis cinerea. Agronomy 2025, 15, 194. [Google Scholar] [CrossRef]
- Verma, K.; Modgil, M. RNA Interference (RNAi) Mediated Technique for Combating Plant Diseases: Harnessing Nanoparticles for Effective Delivery and Enhanced Efficacy. Plant Cell Tissue Organ Cult. PCTOC 2024, 157, 53. [Google Scholar] [CrossRef]
- Capriotti, L.; Molesini, B.; Pandolfini, T.; Jin, H.; Baraldi, E.; Cecchin, M.; Mezzetti, B.; Sabbadini, S. RNA Interference-Based Strategies to Control Botrytis cinerea Infection in Cultivated Strawberry. Plant Cell Rep. 2024, 43, 201. [Google Scholar] [CrossRef]
- Li, M.; Zheng, M.; Wu, S.; Tian, C.; Liu, D.; Weizmann, Y.; Jiang, W.; Wang, G.; Mao, C. In Vivo Production of RNA Nanostructures via Programmed Folding of Single-Stranded RNAs. Nat. Commun. 2018, 9, 2196. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Weiberg, A.; Lin, F.-M.; Thomma, B.P.H.J.; Huang, H.-D.; Jin, H. Bidirectional Cross-Kingdom RNAi and Fungal Uptake of External RNAs Confer Plant Protection. Nat. Plants 2016, 2, 16151. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Huang, Y.; Jiang, W.; Jin, W. Genome-Wide Identification and Validation of Tomato-Encoded sRNA as the Cross-Species Antifungal Factors Targeting the Virulence Genes of Botrytis cinerea. Front. Plant Sci. 2023, 14, 1072181. [Google Scholar] [CrossRef] [PubMed]
- Vert, J.-P.; Foveau, N.; Lajaunie, C.; Vandenbrouck, Y. An Accurate and Interpretable Model for siRNA Efficacy Prediction. BMC Bioinform. 2006, 7, 520. [Google Scholar] [CrossRef]
- BLAST: Basic Local Alignment Search Tool. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 2 July 2025).
- RNAfold Web Server. Available online: http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi (accessed on 2 July 2025).
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Leroch, M.; Kleber, A.; Silva, E.; Coenen, T.; Koppenhöfer, D.; Shmaryahu, A.; Valenzuela, P.D.T.; Hahn, M. Transcriptome Profiling of Botrytis cinerea Conidial Germination Reveals Upregulation of Infection-Related Genes during the Prepenetration Stage. Eukaryot. Cell 2013, 12, 614–626. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Zhou, J.; Dong, H.; Bai, X.; Liu, W.; Gu, Z. Pathogenicity, Infection Process, Physiological and Biochemical Effects of Metarhizium rileyi against Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) Larvae. Egypt. J. Biol. Pest Control 2024, 34, 19. [Google Scholar] [CrossRef]
- Li, M.; Sun, X.; Yin, M.; Shen, J.; Yan, S. Recent Advances in Nanoparticle-Mediated Co-Delivery System: A Promising Strategy in Medical and Agricultural Field. Int. J. Mol. Sci. 2023, 24, 5121. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, Q.; Lan, C.; Tang, T.; Wang, K.; Shen, J.; Niu, D. Nanoparticle Carriers Enhance RNA Stability and Uptake Efficiency and Prolong the Protection against Rhizoctonia solani. Phytopathol. Res. 2023, 5, 2. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Tang, X. RNAi-Based Pest Control: Production, Application and the Fate of dsRNA. Front. Bioeng. Biotechnol. 2022, 10, 1080576. [Google Scholar] [CrossRef] [PubMed]
- Hoang, B.T.L.; Fletcher, S.J.; Brosnan, C.A.; Ghodke, A.B.; Manzie, N.; Mitter, N. RNAi as a Foliar Spray: Efficiency and Challenges to Field Applications. Int. J. Mol. Sci. 2022, 23, 6639. [Google Scholar] [CrossRef]
- McLoughlin, A.G.; Wytinck, N.; Walker, P.L.; Girard, I.J.; Rashid, K.Y.; de Kievit, T.; Fernando, W.G.D.; Whyard, S.; Belmonte, M.F. Identification and Application of Exogenous dsRNA Confers Plant Protection against Sclerotinia sclerotiorum and Botrytis cinerea. Sci. Rep. 2018, 8, 7320. [Google Scholar] [CrossRef]
- Pérez-Pizá, M.C.; Sautua, F.J.; Szparaga, A.; Bohata, A.; Kocira, S.; Carmona, M.A. New Tools for the Management of Fungal Pathogens in Extensive Cropping Systems for Friendly Environments. Crit. Rev. Plant Sci. 2024, 43, 63–93. [Google Scholar] [CrossRef]
- Niño-Sánchez, J.; Sambasivam, P.T.; Sawyer, A.; Hamby, R.; Chen, A.; Czislowski, E.; Li, P.; Manzie, N.; Gardiner, D.M.; Ford, R.; et al. BioClayTM Prolongs RNA Interference-Mediated Crop Protection against Botrytis cinerea. J. Integr. Plant Biol. 2022, 64, 2187–2198. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).