HSBDF-Derived Bioactive Components Broadly Inhibit Enteroviruses by Targeting 3C Protease and Attenuating Inflammatory Responses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Viruses
2.2. HSBDF and Compounds
2.3. EC50 and CC50 Evaluations
2.4. Plaque Assay
2.5. Time-of-Addition Assay
2.6. Virus Attachment and Internalization Assay
2.7. Determination of the Post-Entry Inhibitory Effect
2.8. RNA-Sequencing (RNA-Seq) Processing and Analysis
2.9. Primary Screening for Antiviral Compounds of HSBDF
2.10. Fluorescence Resonance Energy Transfer (FRET)-Based Protease Assays
2.11. IRES Activity Assay
2.12. In Vitro RNA Primer Extension Assay Using 3Dpol
2.13. Molecular Docking
2.14. Cytokine Expression Analysis
2.15. Antiviral Evaluation In Vivo
2.16. Statistical Analysis
3. Results
3.1. HSBDF Broadly Inhibits Enterovirus Infection
3.2. Transcriptome Analysis of HSBDF for the Treatment of CV-A9 Infection
3.3. Screening of Effective Antiviral Components from HSBDF
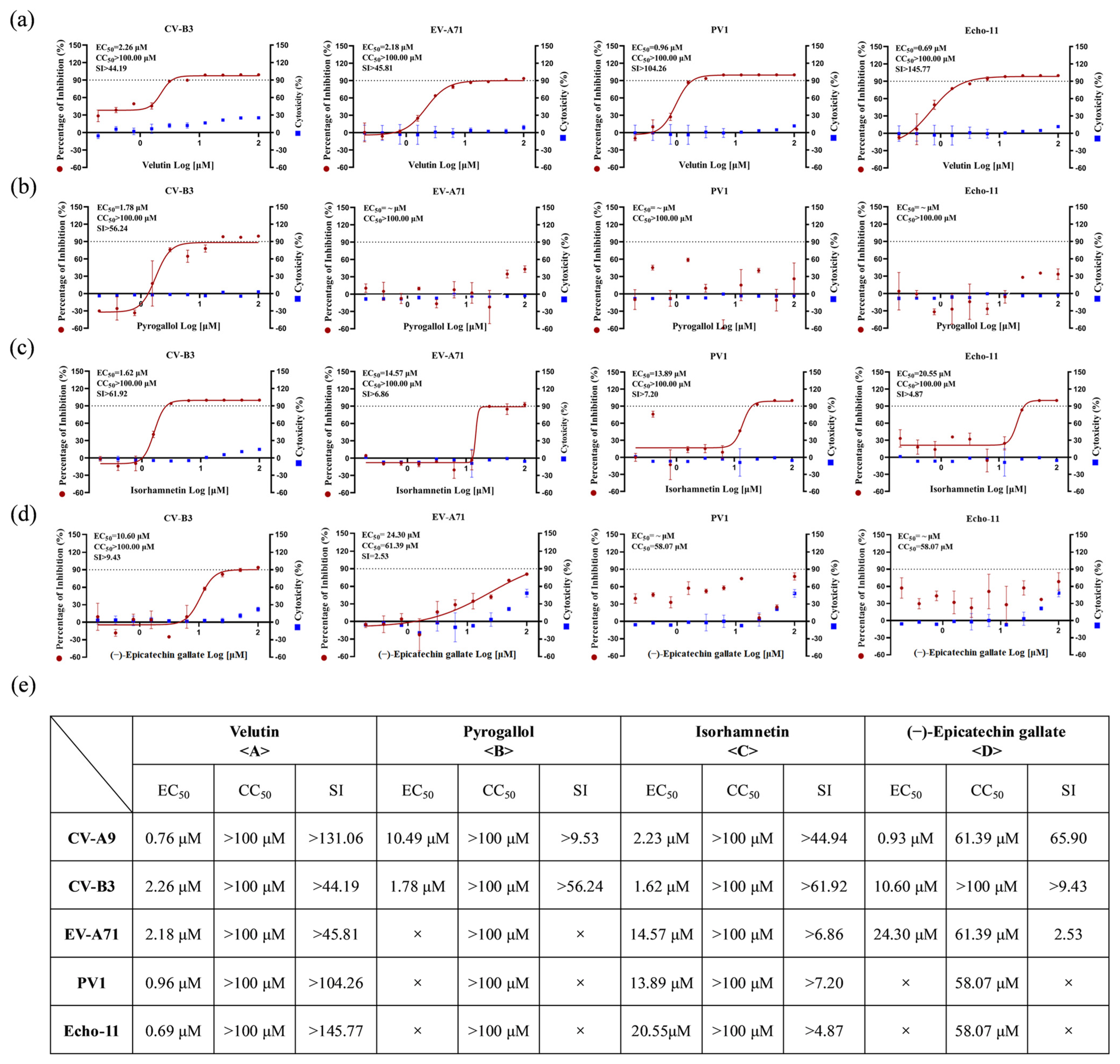
3.4. Bioactive Compounds Exhibit Broad-Spectrum Pan-Cycle Inhibition of Enteroviral Infection
3.5. Bioactive Compounds Targets the Conserved 3C Protease to Suppress Enteroviral Replication
3.6. Bioactive Compounds Demonstrate Anti-Inflammatory Efficacy
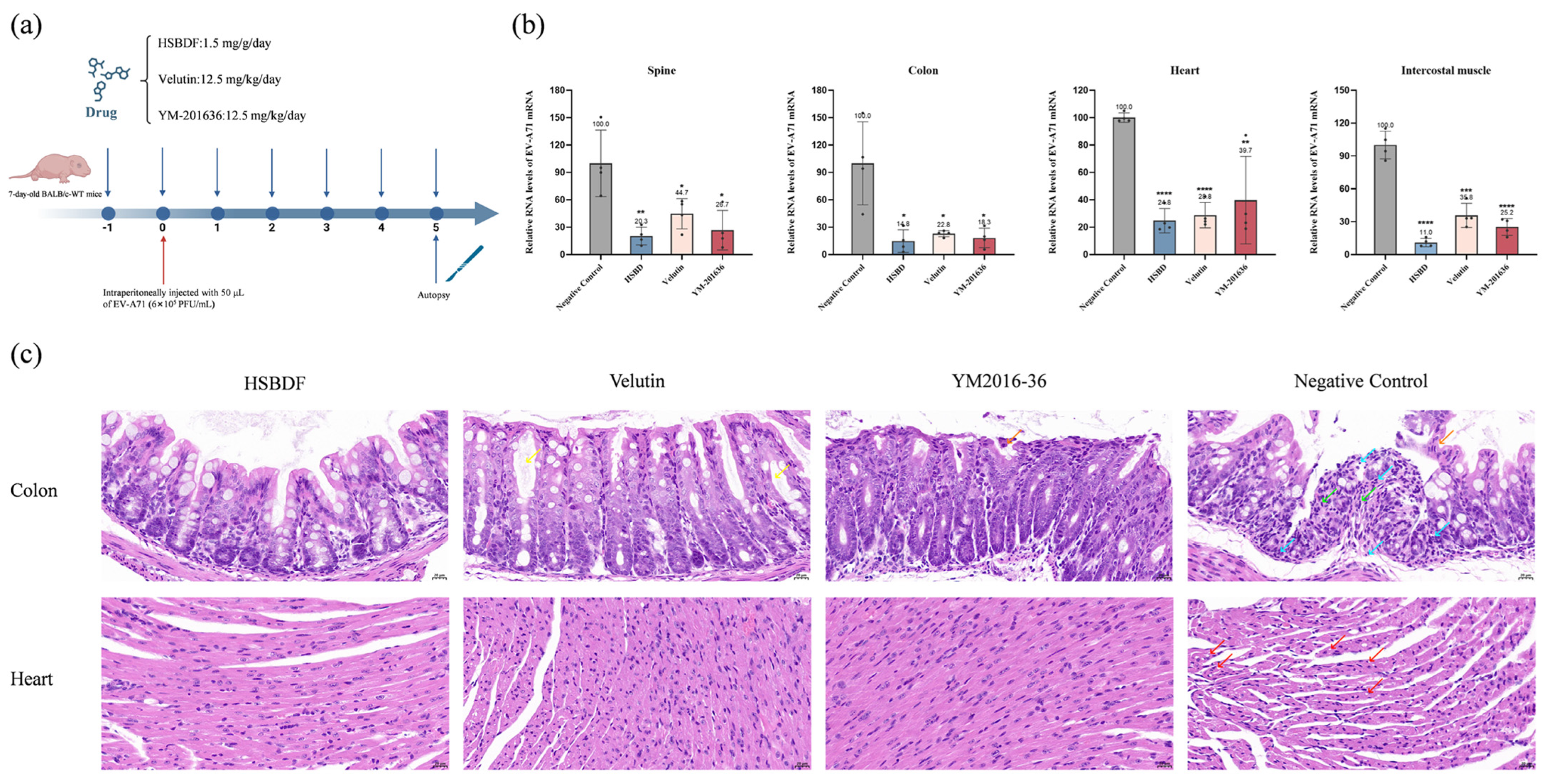
3.7. Active Compounds Inhibit Viral Infection in Suckling Mouse Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baggen, J.; Thibaut, H.J.; Strating, J.; van Kuppeveld, F.J.M. The life cycle of non-polio enteroviruses and how to target it. Nat. Rev. Microbiol. 2018, 16, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Gopalkrishna, V.; Patil, P.R.; Patil, G.P.; Chitambar, S.D. Circulation of multiple enterovirus serotypes causing hand, foot and mouth disease in India. J. Med. Microbiol. 2012, 61 Pt 3, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Xu, D.; Cong, S.; Du, Z.; Li, L.; Zhang, M.; Feng, C.; Bao, G.; Sun, H.; Yang, Z.; et al. Co-infection and enterovirus B: Post EV-A71 mass vaccination scenario in China. BMC Infect. Dis. 2022, 22, 671. [Google Scholar] [CrossRef]
- Palacios, G.; Oberste, M.S. Enteroviruses as agents of emerging infectious diseases. J. Neurovirol. 2005, 11, 424–433. [Google Scholar] [CrossRef]
- Yao, X.; Bian, L.L.; Lu, W.W.; Li, J.-X.; Mao, Q.-Y.; Wang, Y.-P.; Gao, F.; Wu, X.; Ye, Q.; Li, X.-L.; et al. Epidemiological and etiological characteristics of herpangina and hand foot mouth diseases in Jiangsu, China, 2013–2014. Hum. Vaccin. Immunother. 2017, 13, 823–830. [Google Scholar] [CrossRef]
- Fischer, T.K.; Simmonds, P.; Harvala, H. The importance of enterovirus surveillance in a post-polio world. Lancet Infect. Dis. 2022, 22, e35–e40. [Google Scholar] [CrossRef]
- Puenpa, J.; Wanlapakorn, N.; Vongpunsawad, S.; Poovorawan, Y. The History of Enterovirus A71 Outbreaks and Molecular Epidemiology in the Asia-Pacific Region. J. Biomed. Sci. 2019, 26, 75. [Google Scholar] [CrossRef]
- Cao, R.G.; Mejias, A.; Leber, A.L.; Wang, H. Clinical and molecular characteristics of the 2022 Enterovirus-D68 outbreak among hospitalized children, Ohio, USA. J. Clin. Virol. 2023, 169, 105618. [Google Scholar] [CrossRef]
- Jartti, M.; Flodström-Tullberg, M.; Hankaniemi, M.M. Enteroviruses: Epidemic potential, challenges and opportunities with vaccines. J. Biomed. Sci. 2024, 31, 73. [Google Scholar] [CrossRef]
- Wang, M.; Tao, L.; Xu, H. Chinese herbal medicines as a source of molecules with anti-enterovirus 71 activity. Chin. Med. 2016, 11, 2. [Google Scholar] [CrossRef]
- Benschop, K.S.; van der Avoort, H.G.; Duizer, E.; Koopmans, M.P. Antivirals against enteroviruses: A critical review from a public-health perspective. Antivir. Ther. 2015, 20, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pang, Z.; Fan, H.; Tong, Y. Advances in anti-EV-A71 drug development research. J. Adv. Res. 2024, 56, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zeng, M.; Chen, Y.Z. The pharmacological mechanism of Huashi Baidu Formula for the treatment of COVID-19 by combined network pharmacology and molecular docking. Ann. Palliat. Med. 2021, 10, 3864–3895. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Tong, X.; Peng, W.; Wei, S.; Sun, T.; Wang, Y.; Zhang, B.; Li, W. Network Pharmacology Prediction and Molecular Docking-Based Strategy to Discover the Potential Pharmacological Mechanism of Huai Hua San Against Ulcerative Colitis. Drug Des. Devel. Ther. 2021, 15, 3255–3276. [Google Scholar] [CrossRef]
- Wei, W.L.; Wu, S.F.; Li, H.J.; Li, Z.-W.; Qu, H.; Yao, C.-L.; Zhang, J.-Q.; Li, J.-Y.; Wu, W.-Y.; Guo, D.-A. Chemical profiling of Huashi Baidu prescription, an effective anti-COVID-19 TCM formula, by UPLC-Q-TOF/MS. Chin. J. Nat. Med. 2021, 19, 473–480. [Google Scholar] [CrossRef]
- Liu, J.; Yang, W.; Liu, Y.; Lu, C.; Ruan, L.; Zhao, C.; Huo, R.; Shen, X.; Miao, Q.; Lv, W.; et al. Combination of Hua Shi Bai Du granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): A single-center, open-label, randomized controlled trial. Phytomedicine 2021, 91, 153671. [Google Scholar] [CrossRef]
- Xu, H.; Li, S.; Liu, J.; Cheng, J.; Kang, L.; Li, W.; Zhong, Y.; Wei, C.; Fu, L.; Qi, J.; et al. Bioactive compounds from Huashi Baidu decoction possess both antiviral and anti-inflammatory effects against COVID-19. Proc. Natl. Acad. Sci. USA 2023, 120, e2301775120. [Google Scholar] [CrossRef]
- Yu, Z.; Zheng, Y.; Chen, B.; Lv, J.; Zhu, X.; Shang, B.; Xv, Y.; Tao, R.; Yang, Y.; Cong, J.; et al. Efficacy and safety of Huashi Baidu granule plus Nirmatrelvir-Ritonavir combination therapy in patients with high-risk factors infected with Omicron (B.1.1.529): A multi-arm single-center, open-label, randomized controlled trial. Phytomedicine 2023, 120, 155025. [Google Scholar] [CrossRef]
- Pei, P.; Qin, H.; Chen, J.; Wang, F.; He, C.; He, S.; Hong, B.; Liu, K.; Qiao, R.; Fan, H.; et al. Computational design of ultrashort peptide inhibitors of the receptor-binding domain of the SARS-CoV-2 S protein. Brief Bioinform. 2021, 22, bbab243. [Google Scholar]
- He, S.T.; Qin, H.; Guan, L.; Liu, K.; Hong, B.; Zhang, X.; Lou, F.; Li, M.; Lin, W.; Chen, Y.; et al. Bovine lactoferrin inhibits SARS-CoV-2 and SARS-CoV-1 by targeting the RdRp complex and alleviates viral infection in the hamster model. J. Med. Virol. 2023, 95, e28281. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Lou, F.; Hu, R.; Chen, Y.; Li, M.; An, X.; Song, L.; Tong, Y.; Fan, H. 2’-Fucosyllactose Inhibits Coxsackievirus Class A Type 9 Infection by Blocking Virus Attachment and Internalisation. Int. J. Mol. Sci. 2022, 23, 13727. [Google Scholar] [CrossRef]
- Luo, Y.; Tan, C.W.; Xie, S.Z.; Chen, Y.; Yao, Y.-L.; Zhao, K.; Zhu, Y.; Wang, Q.; Liu, M.-Q.; Yang, X.-L.; et al. Identification of ZDHHC17 as a Potential Drug Target for Swine Acute Diarrhea Syndrome Coronavirus Infection. mBio 2021, 12, e0234221. [Google Scholar] [CrossRef] [PubMed]
- Diep, J.; Ooi, Y.S.; Wilkinson, A.W.; Peters, C.E.; Foy, E.; Johnson, J.R.; Zengel, J.; Ding, S.; Weng, K.-F.; Laufman, O.; et al. Enterovirus pathogenesis requires the host methyltransferase SETD3. Nat. Microbiol. 2019, 4, 2523–2537. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Liang, Y.; Tian, M.; Ruan, Z.; Su, R.; Shereen, M.A.; Yin, J.; Wu, K.; Guo, J.; Zhang, Q.; et al. Inhibition of PIKFYVE kinase interferes ESCRT pathway to suppress RNA virus replication. J. Med. Virol. 2023, 95, e28527. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Han, Q.; Wang, L.; Wang, B.; Chen, J.; Cai, B.; Wu, C.; Zhu, X.; Liu, F.; Han, D.; et al. Jinhua Qinggan granules attenuates acute lung injury by promotion of neutrophil apoptosis and inhibition of TLR4/MyD88/NF-κB pathway. J. Ethnopharmacol. 2023, 301, 115763. [Google Scholar] [CrossRef]
- Kong, F.X.; Liu, H.; Xu, T.; Li, S.-J.; Li, W.; Lu, H.; Ma, N.-N.; Wang, Y.-L.; Shi, J.-H.; Yang, Y.-R.; et al. RG108 attenuates acute kidney injury by inhibiting P38 MAPK/FOS and JNK/JUN pathways. Int. Immunopharmacol. 2024, 142 Pt A, 113077. [Google Scholar] [CrossRef]
- Livingston, M.J.; Zhang, M.; Kwon, S.H.; Chen, J.-K.; Li, H.; Manicassamy, S.; Dong, Z. Autophagy activates EGR1 via MAPK/ERK to induce FGF2 in renal tubular cells for fibroblast activation and fibrosis during maladaptive kidney repair. Autophagy 2024, 20, 1032–1053. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Liu, Z.; Zhou, L.; Huang, J.; Luo, X.; Luo, Y.; Li, J.; Lin, Y.; Lai, J.; et al. TXNIP knockdown protects rats against bupivacaine-induced spinal neurotoxicity via the inhibition of oxidative stress and apoptosis. Free Radic. Biol. Med. 2024, 219, 1–16. [Google Scholar] [CrossRef]
- Ma, Q.; Li, R.; Pan, W.; Huang, W.; Liu, B.; Xie, Y.; Wang, Z.; Li, C.; Jiang, H.; Huang, J.; et al. Phillyrin (KD-1) exerts anti-viral and anti-inflammatory activities against novel coronavirus (SARS-CoV-2) and human coronavirus 229E (HCoV-229E) by suppressing the nuclear factor kappa B (NF-κB) signaling pathway. Phytomedicine 2020, 78, 153296. [Google Scholar] [CrossRef]
- Herring, S.; Oda, J.M.; Wagoner, J.; Kirchmeier, D.; O’cOnnor, A.; Nelson, E.A.; Huang, Q.; Liang, Y.; DeWald, L.E.; Johansen, L.M.; et al. Inhibition of Arenaviruses by Combinations of Orally Available Approved Drugs. Antimicrob. Agents Chemother. 2021, 65, 10-1128. [Google Scholar] [CrossRef]
- Wagoner, J.; Herring, S.; Hsiang, T.Y.; Ianevski, A.; Biering, S.B.; Xu, S.; Hoffmann, M.; Pöhlmann, S.; Gale, M.; Aittokallio, T.; et al. Combinations of Host- and Virus-Targeting Antiviral Drugs Confer Synergistic Suppression of SARS-CoV-2. Microbiol. Spectr. 2022, 10, e0333122. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Chen, S.; Cheng, A.; Wang, M. Roles of the Picornaviral 3C Proteinase in the Viral Life Cycle and Host Cells. Viruses 2016, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xi, X.; Lei, X.; Zhang, X.; Cui, S.; Wang, J.; Jin, Q.; Zhao, Z. Correction: Enterovirus 71 Protease 2Apro Targets MAVS to Inhibit Anti-Viral Type I Interferon Responses. PLoS Pathog. 2024, 20, e1012209. [Google Scholar] [CrossRef]
- Weng, K.F.; Li, M.L.; Hung, C.T.; Shih, S.-R. Enterovirus 71 3C protease cleaves a novel target CstF-64 and inhibits cellular polyadenylation. PLoS Pathog. 2009, 5, e1000593. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Lei, P.; Li, Z.; Chen, F.; Chen, Q.; Wang, Y.; Gong, J.; Tang, Q.; Liu, X.; et al. ANXA2 Facilitates Enterovirus 71 Infection by Interacting with 3D Polymerase and PI4KB to Assist the Assembly of Replication Organelles. Virol. Sin. 2021, 36, 1387–1399. [Google Scholar] [CrossRef]
- Lacerda, R.; Menezes, J.; Romão, L. More than just scanning: The importance of cap-independent mRNA translation initiation for cellular stress response and cancer. Cell. Mol. Life Sci. 2017, 74, 1659–1680. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, B.; Chen, X.; Song, N.; Wu, J.; Li, G.; Yu, P.; Han, Y.; Liu, J.; Qin, C. GS-9620 inhibits enterovirus 71 replication mainly through the NF-κB and PI3K-AKT signaling pathways. Antivir. Res. 2018, 153, 39–48. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Z.; Huang, M.; Zeng, J. Predicting Severe Enterovirus 71-Infected Hand, Foot, and Mouth Disease: Cytokines and Chemokines. Mediat. Inflamm. 2020, 2020, 9273241. [Google Scholar] [CrossRef]
- Cao, Z.; Ding, Y.; Ke, Z.; Cao, L.; Li, N.; Ding, G.; Wang, Z.; Xiao, W. Luteoloside Acts as 3C Protease Inhibitor of Enterovirus 71 In Vitro. PLoS ONE 2016, 11, e0148693. [Google Scholar] [CrossRef]
- Yao, C.; Xi, C.; Hu, K.; Gao, W.; Cai, X.; Qin, J.; Lv, S.; Du, C.; Wei, Y. Inhibition of enterovirus 71 replication and viral 3C protease by quercetin. Virol. J. 2018, 15, 116. [Google Scholar] [CrossRef]
- Lin, Y.J.; Chang, Y.C.; Hsiao, N.W.; Hsieh, J.-L.; Wang, C.-Y.; Kung, S.-H.; Tsai, F.-J.; Lan, Y.-C.; Lin, C.-W. Fisetin and rutin as 3C protease inhibitors of enterovirus A71. J. Virol. Methods 2012, 182, 93–98. [Google Scholar] [CrossRef]
- Lei, X.; Xiao, X.; Wang, J. Innate Immunity Evasion by Enteroviruses: Insights into Virus-Host Interaction. Viruses 2016, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Li, Q. Immune Evasion of Enteroviruses Under Innate Immune Monitoring. Front. Microbiol. 2018, 9, 1866. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wang, J.; Fan, T.; Qin, B.; Guo, L.; Lei, X.; Wang, J.; Wang, M.; Jin, Q. Crystal structure of human enterovirus 71 3C protease. J. Mol. Biol. 2011, 408, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Xie, C.; Kang, J.; Li, Z.; Schauss, A.G.; Badger, T.M.; Nagarajan, S.; Wu, T.; Wu, X. The açaí flavonoid velutin is a potent anti-inflammatory agent: Blockade of LPS-mediated TNF-α and IL-6 production through inhibiting NF-κB activation and MAPK pathway. J. Nutr. Biochem. 2012, 23, 1184–1191. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, R.; Guan, L.; Li, S.; Liu, C.; Huang, G.; Lou, F.; Jiang, H.; Wang, S.; Pang, Z.; Wang, Y.; et al. HSBDF-Derived Bioactive Components Broadly Inhibit Enteroviruses by Targeting 3C Protease and Attenuating Inflammatory Responses. Biology 2025, 14, 1615. https://doi.org/10.3390/biology14111615
Hu R, Guan L, Li S, Liu C, Huang G, Lou F, Jiang H, Wang S, Pang Z, Wang Y, et al. HSBDF-Derived Bioactive Components Broadly Inhibit Enteroviruses by Targeting 3C Protease and Attenuating Inflammatory Responses. Biology. 2025; 14(11):1615. https://doi.org/10.3390/biology14111615
Chicago/Turabian StyleHu, Ruolan, Lin Guan, Siyue Li, Chunlin Liu, Gang Huang, Fuxing Lou, Hongzheng Jiang, Shuqi Wang, Zehan Pang, Yaxin Wang, and et al. 2025. "HSBDF-Derived Bioactive Components Broadly Inhibit Enteroviruses by Targeting 3C Protease and Attenuating Inflammatory Responses" Biology 14, no. 11: 1615. https://doi.org/10.3390/biology14111615
APA StyleHu, R., Guan, L., Li, S., Liu, C., Huang, G., Lou, F., Jiang, H., Wang, S., Pang, Z., Wang, Y., Li, Z., Zhang, H., Tong, Y., Fan, H., & Hong, B. (2025). HSBDF-Derived Bioactive Components Broadly Inhibit Enteroviruses by Targeting 3C Protease and Attenuating Inflammatory Responses. Biology, 14(11), 1615. https://doi.org/10.3390/biology14111615





