Efficient Serum-Free Rabies Virus Propagation Using BSR and Vero Cell Lines: A Comparative Evaluation of BioNOC II® Macrocarriers in the BelloStage™-3000 Bioreactor Versus Conventional Microcarriers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rabies Virus Strain, Cell Line, and Cell Culture Medium
2.2. Preparation of Microcarriers
2.3. Cultivation of BSR and Vero Cells on a Techne Magnetic Stirrer
2.4. The Process of Inoculation and Proliferation of the Rabies Virus on Microcarriers
2.5. Cultivation of BSR and Vero Cells in the BelloStage™-3000 Bioreactor System
2.6. Inoculation and Propagation of Rabies Virus on BioNOC II® Macrocarriers
2.7. Assessment of Cell Density on BioNOC II® Macrocarriers
2.8. Glucose Level Monitoring
2.9. Monitoring of pH
2.10. Monitoring of Cellular Infection
2.11. Monitoring of Virus Replication
2.12. Statistical Analysis of Data
3. Results
3.1. Growth of BSR and Vero Cell Lines on Cytodex 1 and Cytodex 3 Microcarriers
3.2. Reproduction of Rabies Virus on Microcarriers Cytodex 1 and Cytodex 3
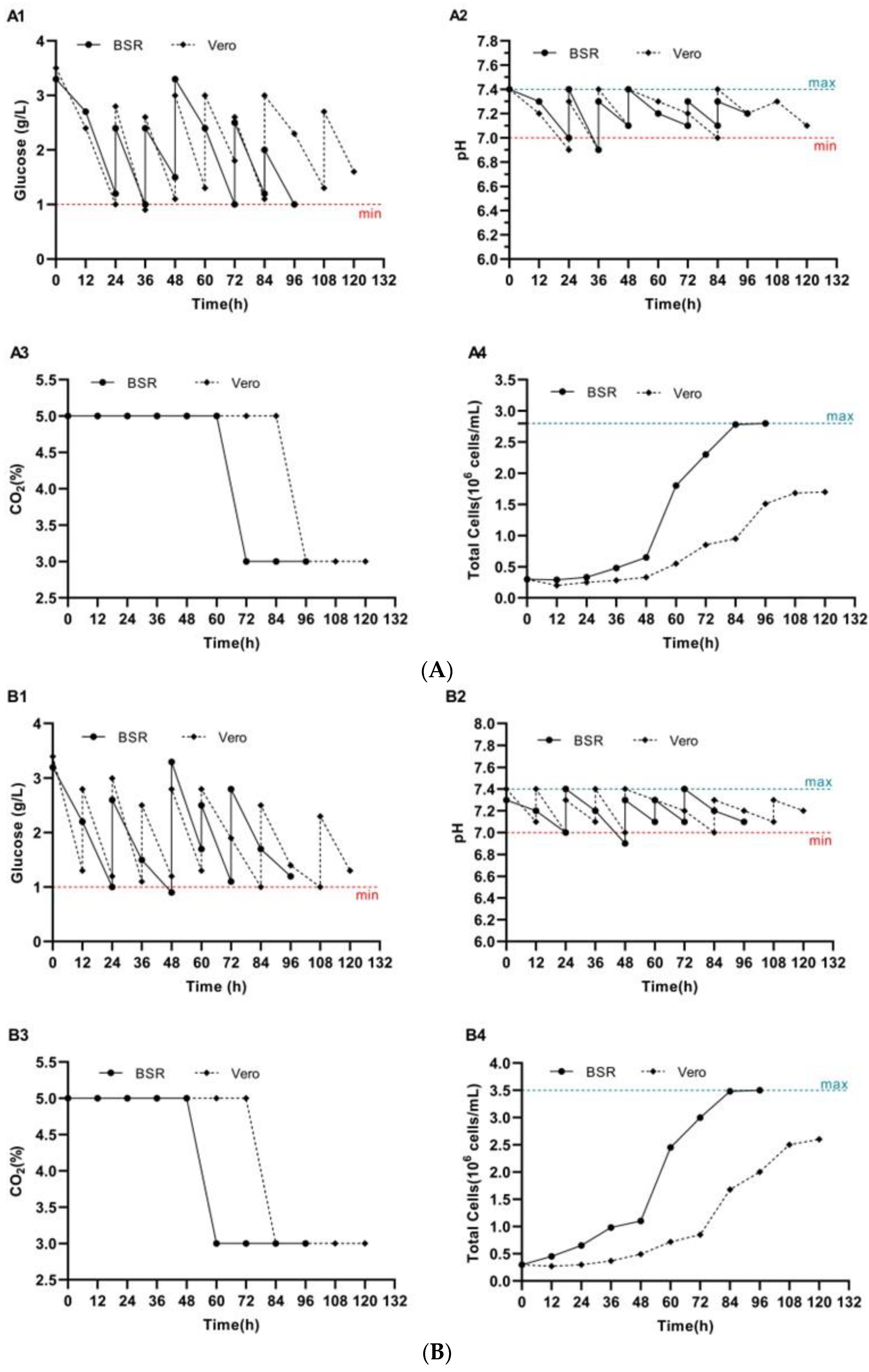
3.3. Growth of BSR and Vero Cell Lines on BioNOC II™ Macrocarriers
3.4. Reproduction of Rabies Virus on BioNOC II® Macrocarriers
3.5. Impact of Micro—And Macrocarriers on Rabies Virus Titers in BSR and Vero Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogino, T. Rhabdoviridae, Rabies Virus. In Encyclopedia of Infection and Immunity; Rezaei, N., Ed.; Elsevier: Oxford, UK, 2022; pp. 219–240. [Google Scholar] [CrossRef]
- Yin, C. Progress in the Development of Animal Rabies Vaccines in China. China CDC Wkly. 2021, 3, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, K.; Ben Zakour, M.; Kallel, H. Purification of rabies virus produced in Vero cells grown in serum free medium. Vaccine 2019, 37, 7052–7060. [Google Scholar] [CrossRef]
- Kaye, A.D.; Perilloux, D.M.; Field, E.; Orvin, C.A.; Zaheri, S.C.; Upshaw, W.C.; Behara, R.; Parker-Actlis, T.Q.; Kaye, A.M.; Ahmadzadeh, S.; et al. Rabies Vaccine for Prophylaxis and Treatment of Rabies: A Narrative Review. Cureus 2024, 16, e62429. [Google Scholar] [CrossRef] [PubMed]
- Natesan, K.; Isloor, S.; Vinayagamurthy, B.; Ramakrishnaiah, S.; Doddamane, R.; Fooks, A.R. Developments in Rabies Vaccines: The Path Traversed from Pasteur to the Modern Era of Immunization. Vaccines 2023, 11, 756. [Google Scholar] [CrossRef]
- Wunner, W.H.; Briggs, D.J. Rabies in the 21 century. PLoS Negl. Trop. Dis. 2010, 4, e591. [Google Scholar] [CrossRef]
- Bourhy, H.; Dautry-Varsat, A.; Hotez, P.J.; Salomon, J. Rabies, still neglected after 125 years of vaccination. PLoS Negl. Trop. Dis. 2010, 4, e839. [Google Scholar] [CrossRef]
- Kabeto, J.M.; Tewabe, Y.T.; Haile, A.W.; Gemechu, W. Rabies: A Neglected Zoonotic Disease and its Public Health Concern in Ethiopia. J. Zoonotic Dis. 2021, 5, 1–11. [Google Scholar] [CrossRef]
- World Health Organization. Rabies: Key Facts; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/news-room/fact-sheets/detail/rabies (accessed on 10 July 2025).
- Selleck, M.; Koppes, P.; Jareb, C.; Shwiff, S.; Liu, L.; Shwiff, S.A. The Economic Landscape of Global Rabies: A Scoping Review and Future Directions. Trop. Med. Infect. Dis. 2025, 10, 222. [Google Scholar] [CrossRef]
- Khan, B.; Shrivastava, N.; Sheikh, N.; Singh, P.; Jha, H.; Parmar, H. Rabies Vaccines: Journey from Classical to Modern Era. Vet. Vaccine 2025, 4, 100105. [Google Scholar] [CrossRef]
- Abela-Ridder, B.; Knopf, L.; Martin, S.; Taylor, L.; Torres, G.; De Balogh, K. 2016: The beginning of the end of rabies? Lancet Glob. Health 2016, 4, e780–e781. [Google Scholar] [CrossRef] [PubMed]
- Kabzhanova, A.M.; Mukhanbetkaliyev, E.E.; Yesembekova, G.N.; Berdikulov, M.A.; Abdrakhmanov, S.K. Spatio-Temporal Analysis of the Epizootic Situation of Animal Rabies in Kazakhstan; S Seifullin Kazakh Agro Technical University: Astana, Kazakhstan, 2022; Volume 144, pp. 51–58. [Google Scholar]
- Minghui, R.; Stone, M.; Semedo, M.H.; Nel, L. New global strategic plan to eliminate dog-mediated rabies by 2030. Lancet Glob. Health 2018, 6, e828–e829. [Google Scholar] [CrossRef]
- Subbiahanadar Chelladurai, K.; Selvan Christyraj, J.D.; Rajagopalan, K.; Yesudhason, B.V.; Venkatachalam, S.; Mohan, M.; Chellathurai Vasantha, N.; Selvan Christyraj, J.R.S. Alternative to FBS in animal cell culture—An overview and future perspective. Heliyon 2021, 7, e07686. [Google Scholar] [CrossRef]
- van der Valk, J.; Bieback, K.; Buta, C.; Cochrane, B.; Dirks, W.G.; Fu, J.; Hickman, J.; Hohensee, C.; Kolar, R.; Liebsch, M.; et al. Fetal bovine serum (FBS): Past–present–future. ALTEX—Altern. Anim. Exp. 2018, 35, 99–118. [Google Scholar] [CrossRef]
- Wu, S.; Rish, A.J.; Skomo, A.; Zhao, Y.; Drennen, J.K.; Anderson, C.A. Rapid serum-free/suspension adaptation: Medium development using a definitive screening design for Chinese hamster ovary cells. Biotechnol. Prog. 2021, 37, e3154. [Google Scholar] [CrossRef]
- Berrie, D.M.; Waters, R.C.; Montoya, C.; Chatel, A.; Vela, E.M. Development of a high-yield live-virus vaccine production platform using a novel fixed-bed bioreactor. Vaccine 2020, 38, 3639–3645. [Google Scholar] [CrossRef]
- Lennaertz, A.; Knowles, S.; Drugmand, J.C.; Castillo, J. Viral vector production in the integrity® iCELLis® single-use fixed-bed bioreactor, from bench-scale to industrial scale. BMC Proc. 2013, 7 (Suppl. 6), 59. [Google Scholar] [CrossRef]
- Goral, V.N.; Hong, Y.; Scibek, J.J.; Sun, Y.; Romeo, L.E.; Rao, A.; Manning, D.; Zhou, Y.; Schultes, J.A.; Tjong, V.; et al. Innovative fixed bed bioreactor platform: Enabling linearlyscalable adherent cell biomanufacturing with real-timebiomass prediction from nutrient consumption. Biotechnol. J. 2024, 19, e2300635. [Google Scholar] [CrossRef]
- Lesch, H.P.; Valonen, P.; Karhinen, M. Evaluation of the Single-Use Fixed-Bed Bioreactors in Scalable Virus Production. Biotechnol. J. 2021, 16, e2000020. [Google Scholar] [CrossRef]
- Li, J.; Pan, R.; Yue, F.; Gao, T.; Wu, X.; Shi, L.; Wang, Y.; Zhao, D.; Lan, Z.; Chen, H.; et al. Evaluation of the Efficacy of the Vaccine Production Process in Removing Residual Host Cell DNA from the Vero Cell Rabies Vaccine. Vaccines 2024, 12, 1379. [Google Scholar] [CrossRef]
- Mohammed, J.; Mengesha, A.; Hurisa, B.; Mulugeta, D.; Waldetensai, A.; Tesera, Y.; Gemechu, W.; Bizuneh, A.; Getachaw, D.; Desalegn, A. Sequential Adaptation of Vero Cell Lines in Serum Free Medium for Fixed Rabies Virus Propagation. Int. J. Appl. Sci. Res. Rev. 2021, 8, 20. [Google Scholar]
- Trabelsi, K.; Rourou, S.; Loukil, H.; Majoul, S.; Kallel, H. Comparison of Various Culture Modes for the Production of Rabies Virus by Vero Cells Grown on Microcarriers in a 2-l Bioreactor. Enzym. Microb. Biotechnol. 2005, 36, 514–519. [Google Scholar] [CrossRef]
- Trabelsi, K.; Zakour, M.B.; Jordan, I.; Sandig, V.; Rourou, S.; Kallel, H. Development of an efficient veterinary rabies vaccine production process in the avian suspension cell line AGE1.CR.pIX. BMC Biotechnol. 2022, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Onen, E.; Bezawada, S. Pilot-Scale Production of Lyophilized Inactivated Rabies Vaccine Candidate in Vero Cells under Fully Animal Component-Free Conditions Using Microcarrier Technology and Laboratory Animal Trials. J. Biomed. Sci. Eng. 2022, 15, 157–178. [Google Scholar] [CrossRef]
- Rourou, S.; Ben Zakkour, M.; Kallel, H. Adaptation of Vero cells to suspension growth for rabies virus production in different serum free media. Vaccine 2019, 37, 6987–6995. [Google Scholar] [CrossRef]
- Hassanzadeh, S.M.; Zavareh, A.; Shokrgozar, M.A.; Ramezani, A.; Fayaz, A. High vero cell density and rabies virus proliferation on fibracel disks versus cytodex-1 in spinner flask. Pak. J. Biol. Sci. PJBS 2011, 14, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, A.Y.; Antoniazzi, M.M.; Galdino, P.L.; Azambuja, N.; Jorge, S.A., Jr.; Pereira, C.A. Rabies virus production in high vero cell density cultures on macroporous microcarriers. Biotechnol. Bioeng. 2004, 85, 506–515. [Google Scholar] [CrossRef]
- Guo, H.; Ding, X.; Hua, D.; Liu, M.; Yang, M.; Gong, Y.; Ye, N.; Chen, X.; He, J.; Zhang, Y.; et al. Enhancing Dengue Virus Production and Immunogenicity with Celcradle™ Bioreactor: A Comparative Study with Traditional Cell Culture Methods. Vaccines 2024, 12, 563. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Gemmiti, C.V.; Guldberg, R.E. Shear stress magnitude and duration modulates matrix composition and tensile mechanical properties in engineered cartilaginous tissue. Biotechnol. Bioeng. 2009, 104, 809–820. [Google Scholar] [CrossRef]
- Rourou, S.; van der Ark, A.; van der Velden, T.; Kallel, H. A microcarrier cell culture process for propagating rabies virus in Vero cells grown in a stirred bioreactor under fully animal component free conditions. Vaccine 2007, 25, 3879–3889. [Google Scholar] [CrossRef]
- Kallel, H.; Rourou, S.; Majoul, S.; Loukil, H. A novel process for the production of a veterinary rabies vaccine in BHK-21 cells grown on microcarriers in a 20-l bioreactor. Appl. Microbiol. Biotechnol. 2003, 61, 441–446. [Google Scholar] [CrossRef]
- Rourou, S.; van Der Ark, A.; Majoul, S.; Trabelsi, K.; van Der Velden, T.; Kallel, H. A novel animal-component-free medium for rabies virus production in Vero cells grown on Cytodex 1 microcarriers in a stirred bioreactor. Appl. Microbiol. Biotechnol. 2009, 85, 53–63. [Google Scholar] [CrossRef]
- Offersgaard, A.; Duarte Hernandez, C.R.; Pihl, A.F.; Costa, R.; Venkatesan, N.P.; Lin, X.; Van Pham, L.; Feng, S.; Fahnøe, U.; Scheel, T.K.H.; et al. SARS-CoV-2 Production in a Scalable High Cell Density Bioreactor. Vaccines 2021, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Toriniwa, H.; Komiya, T. Japanese encephalitis virus production in Vero cells with serum-free medium using a novel oscillating bioreactor. Biol. J. Int. Assoc. Biol. Stand. 2007, 35, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wu, J.C.; Wang, K.C.; Chiang, Y.W.; Lai, C.W.; Chung, Y.C.; Hu, Y.C. Baculovirus-mediated production of HDV-like particles in BHK cells using a novel oscillating bioreactor. J. Biotechnol. 2005, 118, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Weng, T.C.; Tseng, Y.F.; Chiang, J.R.; Lee, M.S.; Hu, A.Y. Evaluation of novel disposable bioreactors on pandemic influenza virus production. PLoS ONE 2019, 14, e0220803. [Google Scholar] [CrossRef]
- Rhazi, H.; Safini, N.; Mikou, K.; Alhyane, M.; Tadlaoui, K.O.; Lin, X.; Venkatesan, N.P.; Elharrak, M. Production of small ruminant morbillivirus, rift valley fever virus and lumpy skin disease virus in CelCradle™-500A bioreactors. BMC Vet. Res. 2021, 17, 93. [Google Scholar] [CrossRef]
- Esco VacciXcell. TideXcell: The Gentle Giant of Adherent Bioprocessing. Available online: https://escovaccixcell.com/assets/central_download/document/9010289_Vaccixcell_Product%20Guide_A4_Brochure_vE_LR.pdf (accessed on 10 July 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amanova, Z.; Sametova, Z.; Turyskeldy, S.; Kurmasheva, A.; Abitayev, R.; Ussembay, A.; Kondibaeva, Z.; Toktyrova, D.; Mazbayeva, D.; Nurabayev, S.; et al. Efficient Serum-Free Rabies Virus Propagation Using BSR and Vero Cell Lines: A Comparative Evaluation of BioNOC II® Macrocarriers in the BelloStage™-3000 Bioreactor Versus Conventional Microcarriers. Biology 2025, 14, 1455. https://doi.org/10.3390/biology14101455
Amanova Z, Sametova Z, Turyskeldy S, Kurmasheva A, Abitayev R, Ussembay A, Kondibaeva Z, Toktyrova D, Mazbayeva D, Nurabayev S, et al. Efficient Serum-Free Rabies Virus Propagation Using BSR and Vero Cell Lines: A Comparative Evaluation of BioNOC II® Macrocarriers in the BelloStage™-3000 Bioreactor Versus Conventional Microcarriers. Biology. 2025; 14(10):1455. https://doi.org/10.3390/biology14101455
Chicago/Turabian StyleAmanova, Zhanat, Zhanna Sametova, Sholpan Turyskeldy, Alina Kurmasheva, Ruslan Abitayev, Abdurakhman Ussembay, Zhanat Kondibaeva, Dariya Toktyrova, Dana Mazbayeva, Sergazy Nurabayev, and et al. 2025. "Efficient Serum-Free Rabies Virus Propagation Using BSR and Vero Cell Lines: A Comparative Evaluation of BioNOC II® Macrocarriers in the BelloStage™-3000 Bioreactor Versus Conventional Microcarriers" Biology 14, no. 10: 1455. https://doi.org/10.3390/biology14101455
APA StyleAmanova, Z., Sametova, Z., Turyskeldy, S., Kurmasheva, A., Abitayev, R., Ussembay, A., Kondibaeva, Z., Toktyrova, D., Mazbayeva, D., Nurabayev, S., Kerimbayev, A., & Bulatov, Y. (2025). Efficient Serum-Free Rabies Virus Propagation Using BSR and Vero Cell Lines: A Comparative Evaluation of BioNOC II® Macrocarriers in the BelloStage™-3000 Bioreactor Versus Conventional Microcarriers. Biology, 14(10), 1455. https://doi.org/10.3390/biology14101455









