Possible Involvement of Leptin in Pathogenesis of Periodontal Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Human Periodontal Ligament Cells for Cell Cultures
2.2. Performing of Periodontal Ligament Cell Cultures and Their Stimulation
2.3. RNA Isolation and RT-qPCR Analysis
2.4. Analysis of Cytokine Concentrations in Periodontal Ligament Cell Culture Supernatants
2.5. Statistical Analysis
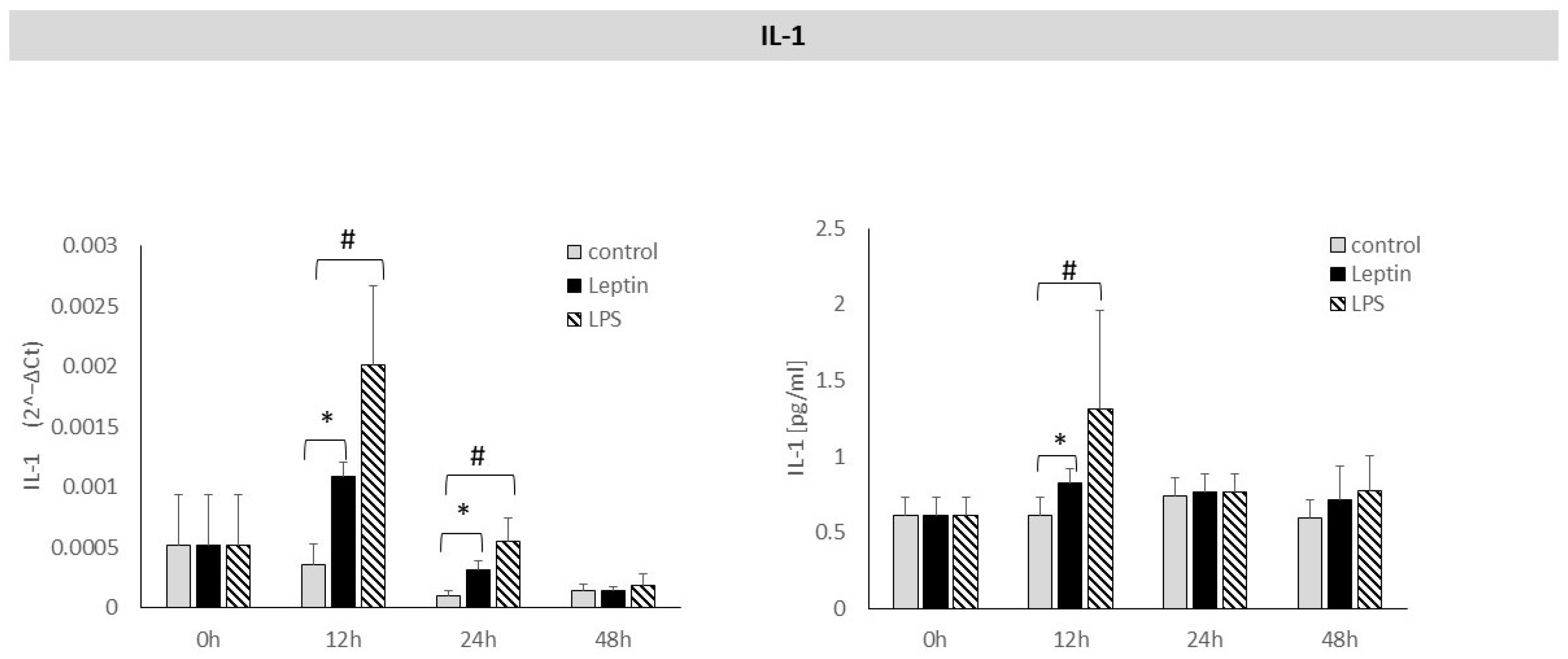
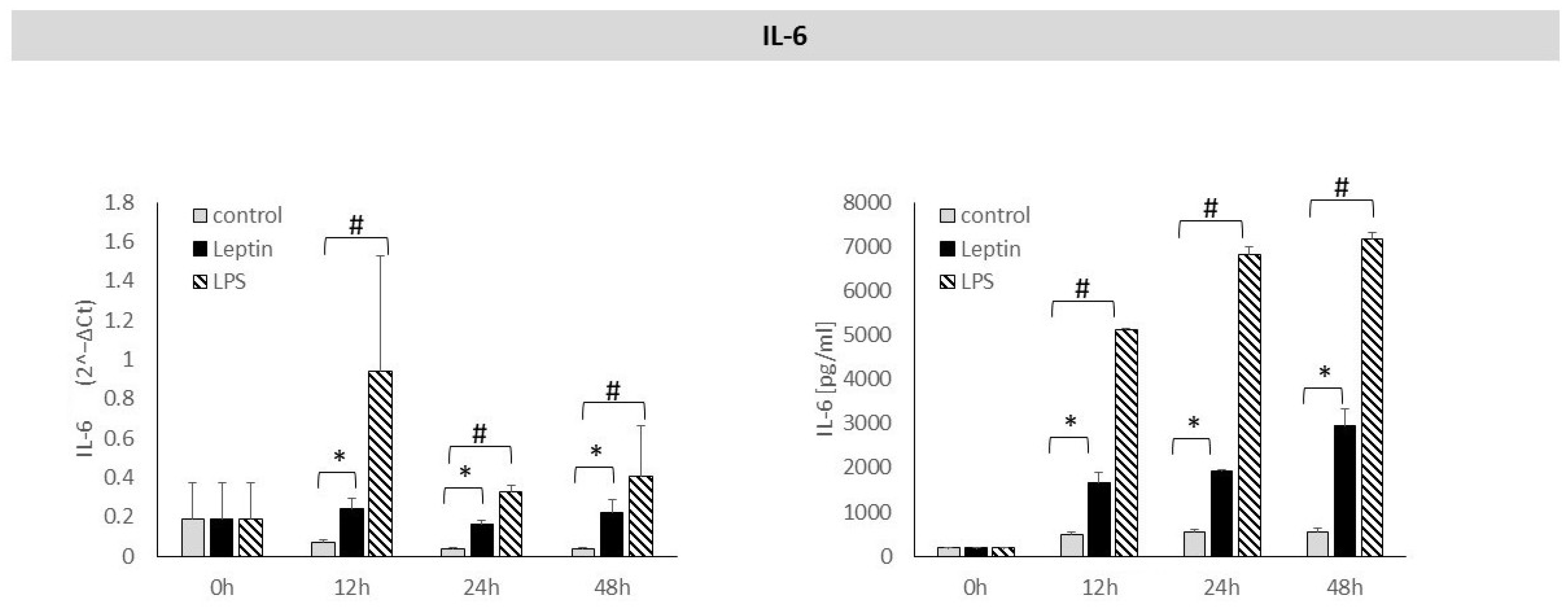
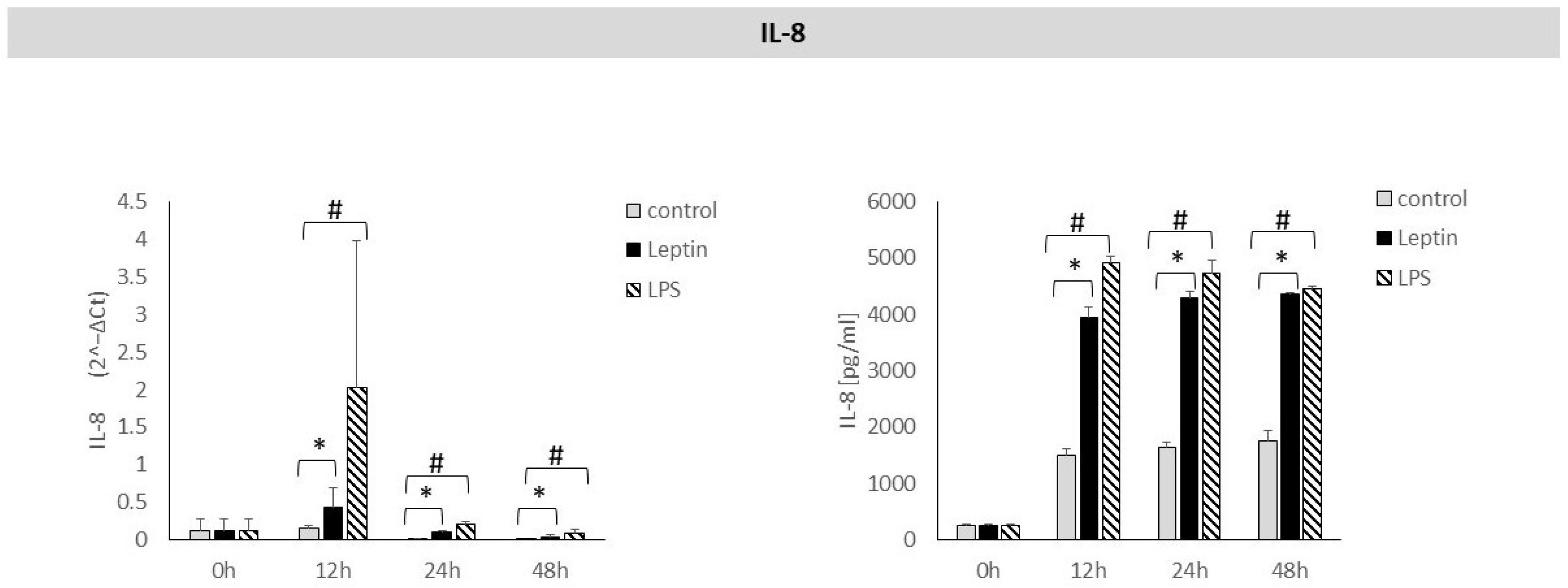
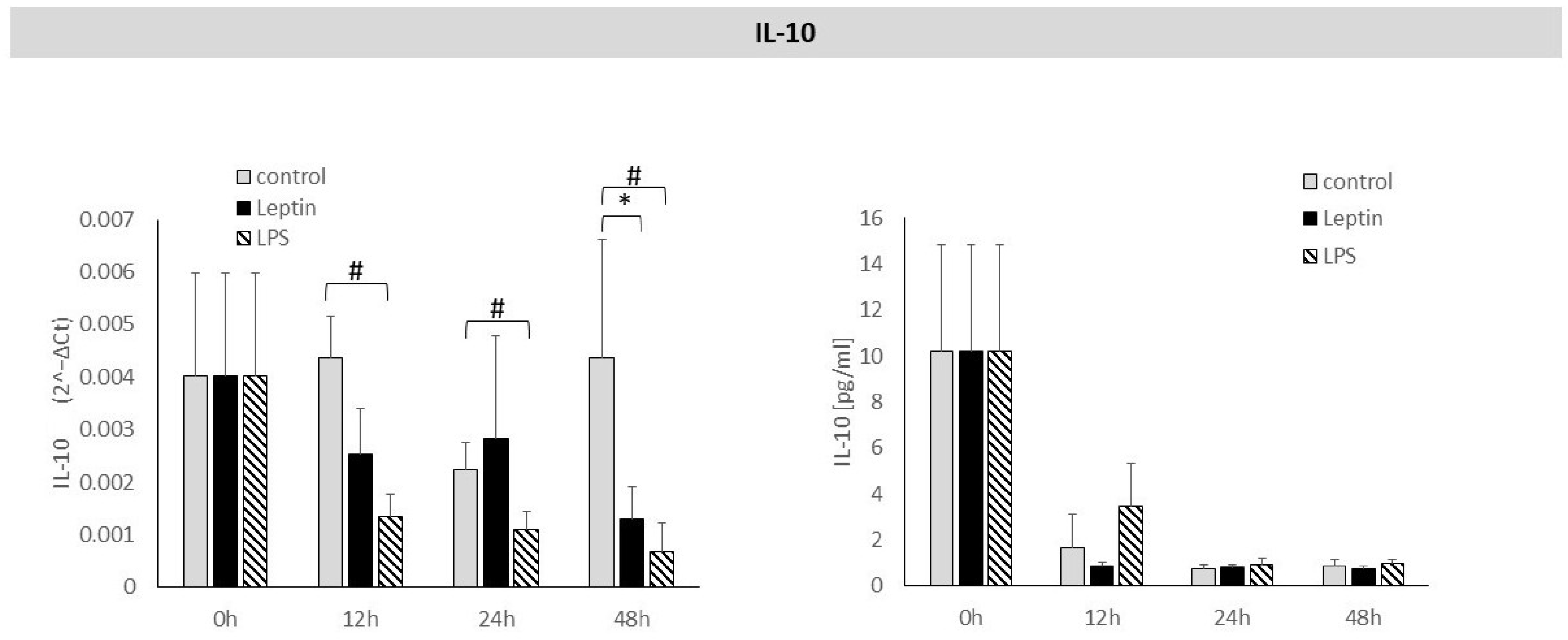
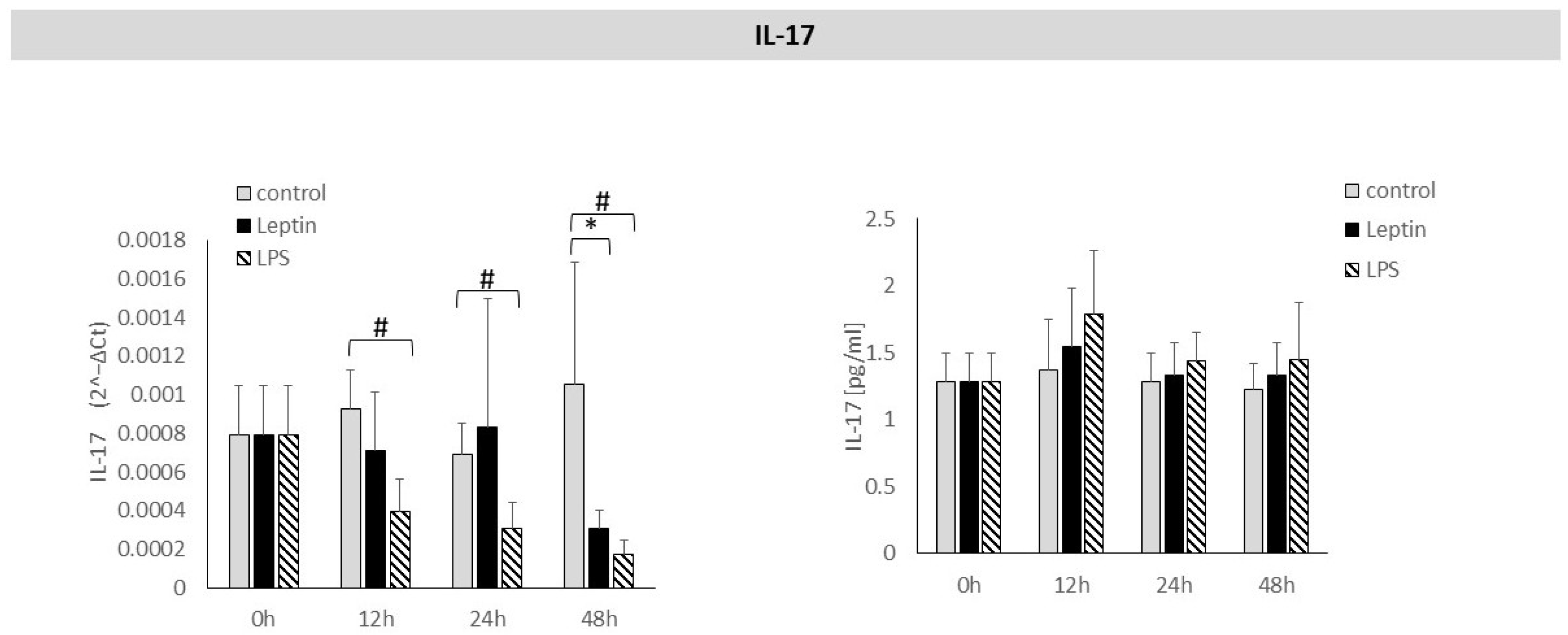
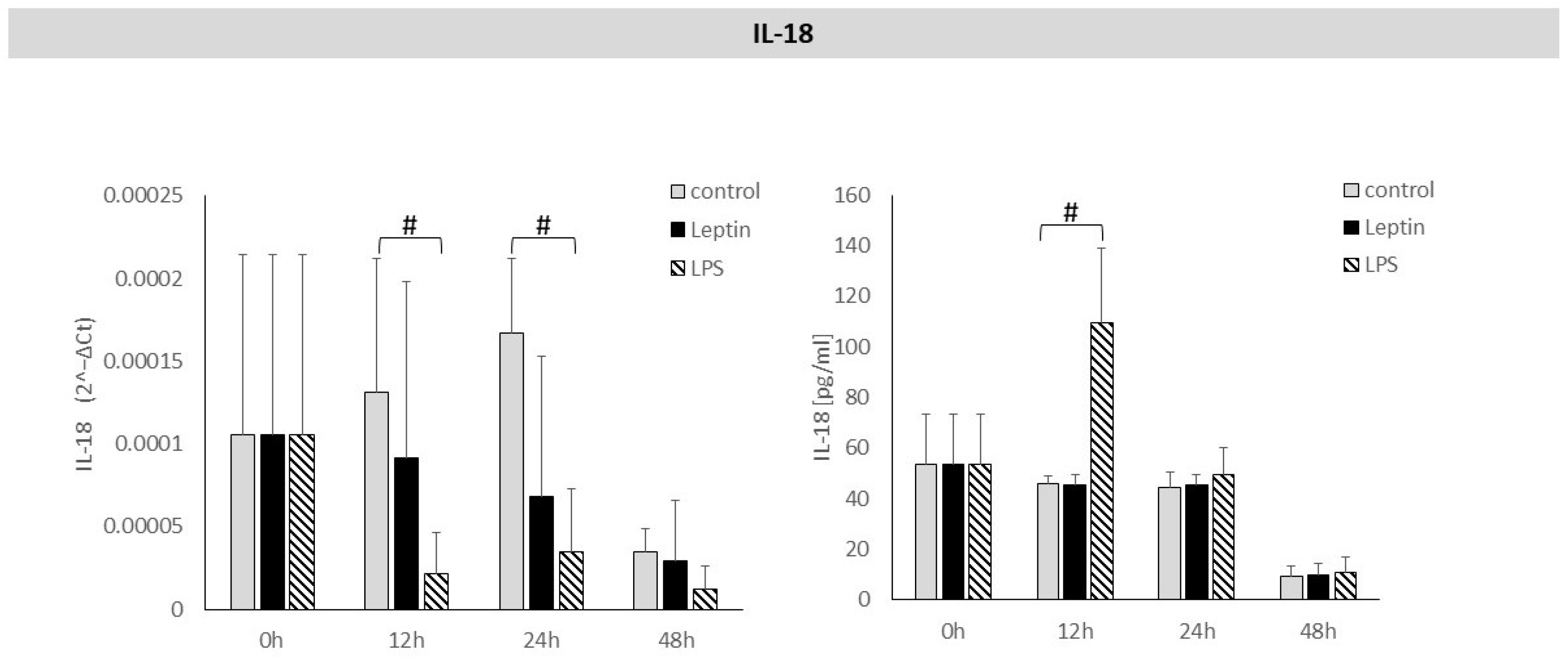
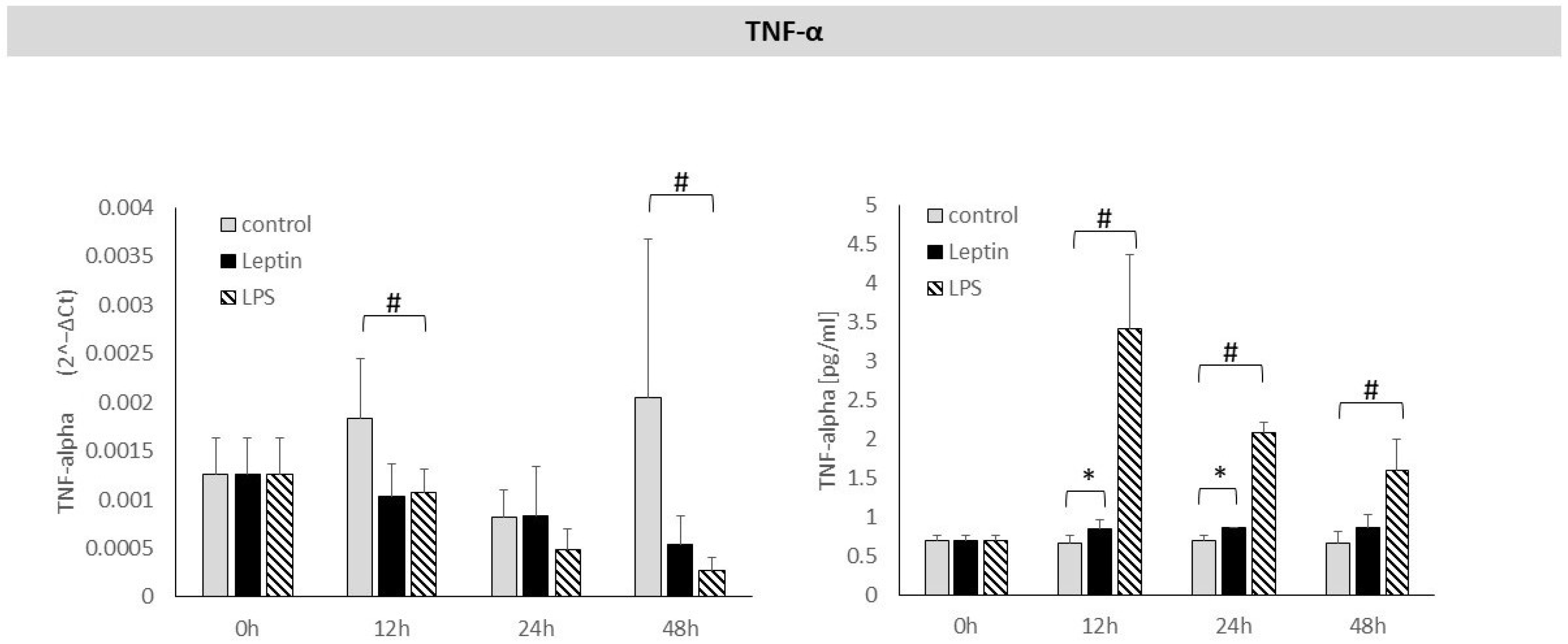
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| hPDLC | Human Periodontal Ligament Cells |
| IL | Interleukin |
| IFN-γ | Interferon gamma |
| LPS | Lipopolysaccharides |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| PCR | Polymerase Chain Reaction |
| RA | Rheumatoid Arthritis |
| RANKL | Receptor Activator for Nuclear Factor κ B Ligand |
| RNA | Ribonucleic Acid |
| RT-qPCR | Reverse Transcription Quantitative Polymerase Chain Reaction |
| TNF-α | Tumor Necrosis Factor Alpha |
| TGF-β1 | Transforming Growth Factor Beta-1 |
References
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell. Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef]
- Lopes, M.E.S.; Marcantonio, C.C.; Salmon, C.R.; Mofatto, L.S.; Junior, F.H.N.; Eick, S.; Deschner, J.; Cirelli, J.A.; Nogueira, A.V.B. Effects of periodontal disease on the proteomic profile of the periodontal ligament. J. Proteom. 2025, 314, 105384. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lambris, J.D. Complement and dysbiosis in periodontal disease. Immunobiology 2012, 11, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Kosho, M.X.F.; Verhelst, A.R.E.; Teeuw, W.J.; van Bruchem, S.; Nazmi, K.; Gerdes, V.E.A.; Loos, B.G. The Prevalence of Metabolic Syndrome and Undiagnosed Diabetes in Periodontitis Patients and Non-Periodontitis Controls in a Dental School. J. Clin. Med. 2024, 13, 7512. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zeng, C.; Chen, X.; Sun, J.; Jin, F.; Chen, Z.; Song, J. Relationship between three body obesity indicators, WWI, BMI, WtHR, and periodontitis. Sci. Rep. 2025, 15, 697. [Google Scholar] [CrossRef]
- Li, W.; Zhu, W.; Hou, J.; Huang, B.; Liu, K.; Meng, H. Leptin and its receptor expression in dental and periodontal tissues of primates. Cell Tissue Res. 2014, 355, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Gollapudi, S.; Su, H.; Gupta, S. Leptin activates human B cells to secrete TNF-a, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J. Clin. Immunol. 2011, 31, 472–478. [Google Scholar] [CrossRef]
- Ahuja, C.R.; Kolte, A.P.; Kolte, R.A.; Gupta, M.; Chari, S. Effect of non-surgical periodontal treatment on gingival crevicular fluid and serum leptin levels in periodontally healthy chronic periodontitis and chronic periodontitis patients with type 2 diabetes mellitus. J. Investig. Clin. Dent. 2019, 10, e12420. [Google Scholar] [CrossRef]
- Gualillo, O.; Eiras, S.; Lago, F.; Diéguez, C.; Casanueva, F.F. Elevated serum leptin concentrations induced by experimental acute inflammation. Life Sci. 2000, 67, 2433–2441. [Google Scholar] [CrossRef]
- Anton, D.; Sufaru, I.G.; Martu, M.A.; Mocanu, R.; Laza, G.M.; Maris, M.; Martu, S.; Lazar, L. Pathophysiological aspects in the interaction between periodontal disease and diabetes. Rom. J. Med. Dent. Educ. 2020, 9, 57–62. [Google Scholar]
- Guo, Y.; Xu, C.; Wu, X.; Zhang, W.; Sun, Y.; Shrestha, A. Leptin regulates OPG and RANKL expression in gingival fibroblasts and tissues of chronic periodontitis patients. Int. J. Med. Sci. 2021, 18, 2431. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, B.; Liu, K.; Hou, J.; Meng, H. Upregulated leptin in periodontitis promotes inflammatory cytokine expression in periodontal ligament cells. J. Periodontol. 2015, 86, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, B.V.; Pradeep, A.R. Leptin levels in gingival crevicular fluid in periodontal health and disease. J. Periodontal Res. 2007, 42, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Kandaswamy, E.; Lee, C.T.; Gururaj, S.B.; Shivanaikar, S.; Joshi, V.M. Association of adipokine levels with obesity in periodontal health and disease: A systematic review with meta-analysis and meta-regression. J. Periodontal Res. 2024, 59, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sánchez, C.; De Avila, J.; Ramos-Casallas, A.; Chila-Moreno, L.; Delgadillo, N.A.; Chalem-Choueka, P.; Pacheco-Tena, C.; Bello-Gualtero, J.M.; Bautista-Molano, W. High Levels of Leptin and Adipsin Are Associated with Clinical Activity in Early Rheumatoid Arthritis Patients with Overweight and Periodontal Infection. Diagnostics 2023, 13, 1126. [Google Scholar] [CrossRef]
- Purwar, P.; Khan, M.A.; Gupta, A.; Mahdi, A.A.; Pandey, S.; Singh, B.; Dixit, J.; Rai, P. The effects of periodontal therapy on serum and salivary leptin levels in chronic periodontitis patients with normal body mass index. Acta Odontol. Scand. 2015, 73, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Jain, H.; Mulay, S. Relationship between periodontitis and systemic diseases: Leptin, a new biomarker? Indian J. Dent. Res. 2014, 25, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Ebert, T.; Klöting, N.; Kolb, M.; Gericke, M.; Jeromin, F.; Jessnitzer, B.; Lössner, U.; Burkhardt, R.; Stumvoll, M. Leptin decreases circulating inflammatory IL-6 and MCP-1 in mice. BioFactors 2019, 45, 43–48. [Google Scholar] [CrossRef]
- Lord, G.M.; Matarese, G.; Howard, J.K.; Baker, R.J.; Bloom, S.R.; Lechler, R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998, 394, 897–901. [Google Scholar] [CrossRef]
- Han, Y.; Huang, Y.; Gao, P.; Yang, Q.; Jia, L.; Zheng, Y.; Li, W. Leptin aggravates periodontitis by promoting M1 polarization via NLRP3. J. Dent. Res. 2022, 101, 675–685. [Google Scholar] [CrossRef]
- Shi, D.; Liu, Y.Y.; Li, W.; Zhang, X.; Sun, X.J.; Xu, L.; Zhang, L.; Chen, Z.B.; Meng, H.X. Association between plasma leptin level and systemic inflammatory markers in patients with aggressive periodontitis. Chin. Med. J. 2015, 128, 528–532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kozak, M.; Poniewierska-Baran, A.; Czerewaty, M.; Łuczkowska, K.; Safranow, K.; Mazurek-Mochol, M.; Machaliński, B.; Pawlik, A. Effect of Adiponectin on the Expression of Selected Cytokines in Periodontal Ligament Cells. Biol. 2025, 14, 321. [Google Scholar] [CrossRef] [PubMed]
- Schröder, A.; Meyer, A.; Spanier, G.; Damanaki, A.; Paddenberg, E.; Proff, P.; Kirschneck, C. Impact of Leptin on Periodontal Ligament Fibroblasts during Mechanical Strain. Int. J. Mol. Sci. 2021, 22, 6847. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.C.; Skelton, A.J.; Todryk, S.M.; Rowan, A.D.; Preshaw, P.M.; Taylor, J.J. Leptin and Pro-Inflammatory Stimuli Synergistically Upregulate MMP-1 and MMP-3 Secretion in Human Gingival Fibroblasts. PLoS ONE. 2016, 11, e0148024. [Google Scholar] [CrossRef]
- Hoareau, L.; Bencharif, K.; Rondeau, P.; Murumalla, R.; Ravanan, P.; Tallet, F.; Delarue, P.; Cesari, M.; Roche, R.; Festy, F. Signaling pathways involved in LPS induced TNFalpha production in human adipocytes. J. Inflamm. 2010, 7, 1. [Google Scholar] [CrossRef]
- Mattioli, B.; Giordani, L.; Quaranta, M.G.; Viora, M. Leptin exerts an anti-apoptotic effect on human dendritic cells via the PI3K-Akt signaling pathway. FEBS Lett. 2009, 583, 1102–1106. [Google Scholar] [CrossRef]
- Purwar, P.; Khan, M.; Mahdi, A.A.; Pandey, S.; Singh, B.; Dixit, J.; Sareen, S. Salivary and serum leptin concentrations in patients with chronic periodontitis. J. Periodontol. 2015, 86, 588–594. [Google Scholar] [CrossRef]
- Meharwade, V.V.; Gayathri, G.V.; Mehta, D.S. Effects of scaling and root planing with or without a local drug delivery system on the gingival crevicular fluid leptin level in chronic periodontitis patients: A clinico-biochemical study. J. Periodontal Implant Sci. 2014, 44, 118–125. [Google Scholar] [CrossRef]
- Boyapati, R.; Chintalapani, S.; Ramisetti, A.; Salavadhi, S.S.; Ramachandran, R. Evaluation of Serum Leptin and Adiponectin in Obese Individuals with Chronic Periodontitis. Contemp. Clin. Dent. 2018, 9, S210–S214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Landman, R.E.; Puder, J.J.; Xiao, E.; Freda, P.U.; Ferin, M.; Wardlaw, S.L. Endotoxin stimulates leptin in the human and nonhuman primate. J. Clin. Endocrinol. Metab. 2003, 88, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Yang, T. Roles of leptinin bone metabolism and bone diseases. J. Bone Miner. Metab. 2015, 33, 474–485. [Google Scholar] [CrossRef]
- Reid, I.R.; Baldock, P.A.; Cornish, J. Effects of leptin on the skeleton. Endocr. Rev. 2018, 39, 938–959. [Google Scholar] [CrossRef]
- Faienza, M.F.; D’Amato, G.; Chiarito, M.; Colaianni, G.; Colucci, S.; Grano, M.; Corbo, F.; Brunetti, G. Mechanisms involved in childhood obesity-related bone fragility. Front. Endocrinol. 2019, 10, 269. [Google Scholar] [CrossRef]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Yong, J.; Groeger, S.E.; Ruf, S.; Ruiz-Heiland, G. Influence of leptin and compression in GAS-6 mediated homeostasis of periodontal ligament cell. Oral Dis. 2023, 29, 1172–1183. [Google Scholar] [CrossRef]
- Zhang, S.; An, N.; Ouyang, X.; Liu, Y.; Wang, X. Role of growth arrest-specific protein 6 in migration and osteogenic differentiation of human periodontal ligament cells. J. Peking Univ. Health Sci. 2020, 53, 9–15. [Google Scholar]
- Nokhbehsaim, M.; Keser, S.; Nogueira, A.V.; Jäger, A.; Jepsen, S.; Cirelli, J.A.; Bourauel, C.; Eick, S.; Deschner, J. Leptin effects on the regenerative capacity of human periodontal cells. Int. J. Endocrinol. 2014, 2014, 180304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| β2-M | 5′-AATGCGGCATCTTCAAACCT-3′ | 5′-TGACTTTGTCACAGCCCAAGA-3′ |
| TNF-α | 5′-GATGATCTGACTGCCTGGGC-3′ | 5′-CACGCTCTTCTGCCTGCTG-3′ |
| IL-1β | 5′-ACAGATGAAGTGCTCCTTCCA-3′ | 5′-GTCGGAGATTCGTAGCTGGAT-3′ |
| IL-6 | 5′-CACTGGTCTTTTGGAGTTTGAG-3′ | 5′-GGACTTTTGTACTCATCTGCAC-3′ |
| IL-8 | 5′-AACCCTCTGCACCCAGTTTTC-3′ | 5′-ACTGAGAGTGATTGAGAGTGGAC-3′ |
| IL-10 | 5′-GGTTGCCAAGCCTTGTCTGA-3′ | 5′-AGGGAGTTCACATGCGCCT-3′ |
| IL-17α | 5′-GAGCCCCAAAAGCAAGAGGAA-3′ | 5′-TGCGGGCATACGGTTTCATC-3′ |
| IL-18 | 5′-ATCGCTTCCTCTCGCAACAA-3′ | 5′-CTTCTACTGGTTCAGCAGCCATCT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozak, M.; Poniewierska-Baran, A.; Czerewaty, M.; Łuczkowska, K.; Mazurek-Mochol, M.; Machaliński, B.; Pawlik, A. Possible Involvement of Leptin in Pathogenesis of Periodontal Disease. Biology 2025, 14, 1454. https://doi.org/10.3390/biology14101454
Kozak M, Poniewierska-Baran A, Czerewaty M, Łuczkowska K, Mazurek-Mochol M, Machaliński B, Pawlik A. Possible Involvement of Leptin in Pathogenesis of Periodontal Disease. Biology. 2025; 14(10):1454. https://doi.org/10.3390/biology14101454
Chicago/Turabian StyleKozak, Małgorzata, Agata Poniewierska-Baran, Michał Czerewaty, Karolina Łuczkowska, Małgorzata Mazurek-Mochol, Bogusław Machaliński, and Andrzej Pawlik. 2025. "Possible Involvement of Leptin in Pathogenesis of Periodontal Disease" Biology 14, no. 10: 1454. https://doi.org/10.3390/biology14101454
APA StyleKozak, M., Poniewierska-Baran, A., Czerewaty, M., Łuczkowska, K., Mazurek-Mochol, M., Machaliński, B., & Pawlik, A. (2025). Possible Involvement of Leptin in Pathogenesis of Periodontal Disease. Biology, 14(10), 1454. https://doi.org/10.3390/biology14101454







