Fruits and Seeds as Indicators of the Genetic Diversity of Hymenaea martiana (Fabaceae) in Northeast Brazil
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection Location
2.2. Physical Characterization of Fruits and Seeds
2.3. Seedling Emergence Test
2.4. Initial Growth and Seedling Biomass
2.5. Experimental Design and Statistical Analysis
2.5.1. Univariate Analysis
2.5.2. Genetic Parameters
- (a)
- Phenotypic variance:
- (b)
- Environmental variance:
- (c)
- Genetic variance:
- (d)
- Heritability in the broad sense:
- (e)
- Coefficient of genetic variation:
- (f)
- Coefficient of environmental variation:
- (g)
- Ratio
2.5.3. Genetic Diversity
3. Results
3.1. ANOVA and Genetic Parameters
3.2. Fruit Characterization
3.3. Physical Characterization of Seeds
3.4. Physiological Quality of Seeds and Seedling Performance
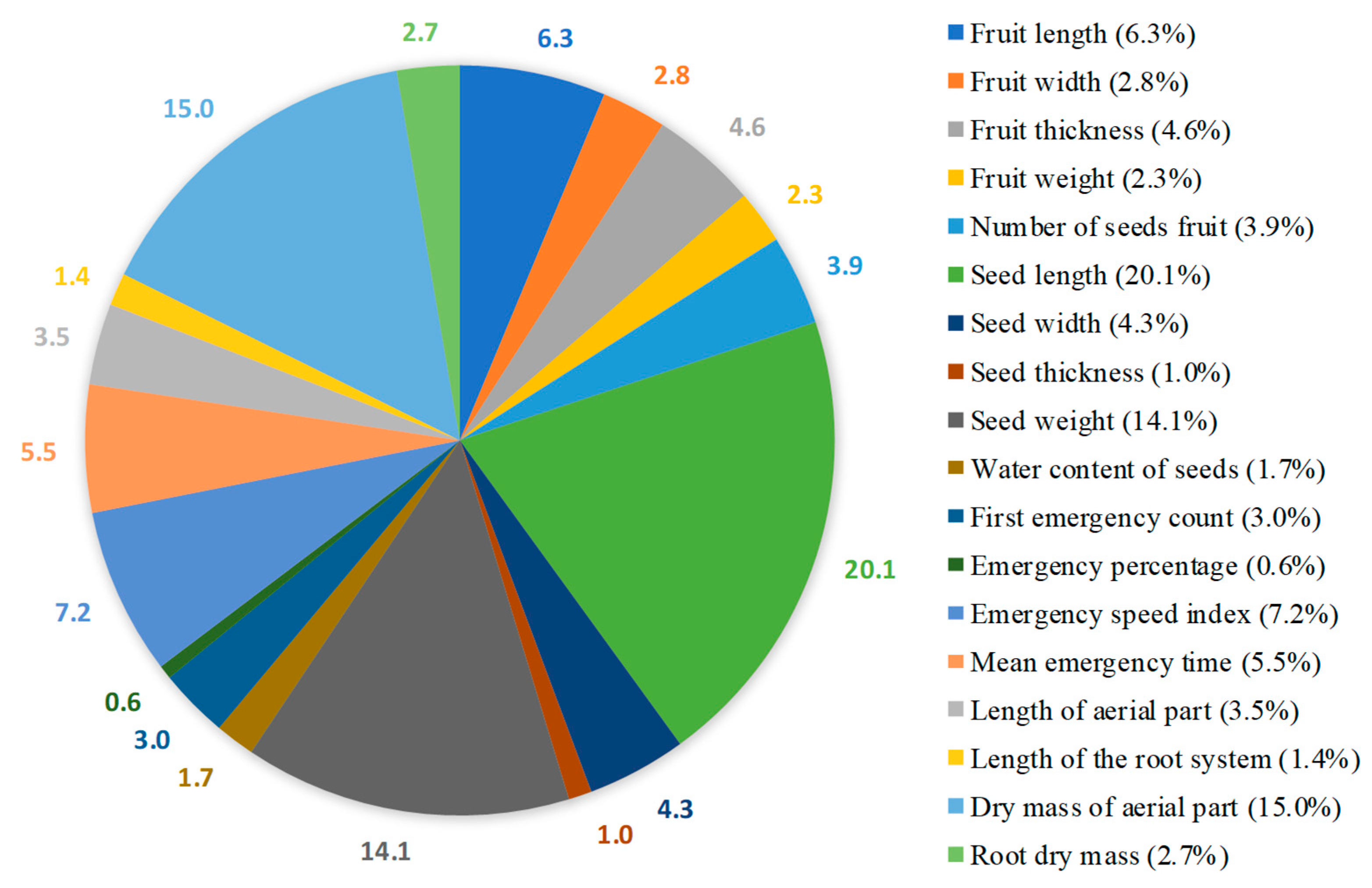
3.5. Cluster Analysis and Contribution of Traits
4. Discussion
4.1. Analysis of Phenotypic Variability and Genetic Parameters
4.2. Fruits and Seeds as Sources of Diversity and Added Value
4.3. Seed Vigor and Early Seedling Performance
4.4. Phenotypic Grouping
4.5. Characteristics Determining Genetic Divergence
4.6. Implications for Conservation and Sustainable Use
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1
| Group * | Averages | Mother Plants ** |
| Fruit length (mm) | ||
| Group 1 (a) | 153.27 | 23 |
| Group 2 (b) | 134.52 | 152 |
| Group 3 (c) | 119.55–124.42 | 76, 9, 59 and 68 |
| Group 4 (d) | 115.45–116.32 | 56, 74, 153 and 8 |
| Group 5 (e) | 107.10–112.30 | 21, 88, 17, 5, 62, 143, 129, 126, 33, 65, 71, 86 and 75 |
| Group 6 (f) | 99.20–105.67 | 131, 101, 100, 107, 134, 43, 69, 142, 39, 123, 29, 66, 85, 48, 125 and 40 |
| Group 7 (g) | 92.82–98.42 | 136, 106, 111, 27, 70, 16, 114, 36, 132, 102, 51, 80, 2, 141, 121, 31, 79, 22, 42, 34, 44, 26, 133, 146, 120, 95, 63, 64, 130, 99, 117 and 67 |
| Group 8 (h) | 86.50–92.17 | 15, 81, 135, 3, 4, 156, 119, 50, 25, 41, 104, 108, 60, 32, 103, 122, 61, 115, 28, 137, 148, 24, 72, 38, 20, 37, 96 and 154 |
| Group 9 (i) | 78.80–86.02 | 7, 124, 97, 11, 151, 160, 105, 157, 116, 149, 147, 84, 10, 55, 6, 46, 35, 57, 118, 30, 19, 49, 18, 77, 113, 12, 47, 87, 144, 128, 90, 109, 158 and 45 |
| Group 10 (j) | 71.82–77.45 | 159, 110, 140, 150, 127, 78, 82, 89, 145, 73, 112, 14, 98 and 1 |
| Group 11 (k) | 59.42–70.12 | 52, 139, 94, 54, 93, 155, 138, 91, 53, 83, 92, 13 and 58 |
| Fruit width (mm) | ||
| Group 1 (a) | 58.15–62.50 | 66, 39, 74, 152 and 151 |
| Group 2 (b) | 52.50–57.55 | 68, 88, 5, 56, 59, 8, 23, 100, 17, 9 and 48 |
| Group 3 (c) | 47.72–51.80 | 117, 49, 143, 16, 65, 113, 125, 35, 76, 36, 137, 102, 71, 142, 153, 126, 31, 21, 86, 85, 77, 44, 101, 75, 129 and 42 |
| Group 4 (d) | 43.62–47.17 | 80, 27, 38, 62, 149, 109, 78, 41, 114, 120, 20, 123, 19, 29, 84, 2, 11, 60, 106, 40, 10, 43, 131, 130, 45, 50, 33, 63, 26, 95, 132, 104, 108, 148, 18, 107, 79, 32 and 67 |
| Group 5 (e) | 37.80–43.22 | 4, 3, 118, 83, 93, 103, 13, 55, 91, 145, 111, 57, 1, 82, 128, 127, 64, 58, 6, 136, 150, 119, 90, 122, 51, 34, 133, 158, 25, 28, 98, 146, 47, 24, 30, 134, 121, 12, 81, 99, 37, 89, 115, 69, 46, 96, 61, 22 and 87 |
| Group 6 (f) | 33.27–37.37 | 70, 138, 154, 54, 52, 147, 73, 156, 140, 116, 97, 112, 7, 124, 92, 144, 157, 105, 14, 53, 110, 141 and 15 |
| Group 7 (g) | 29.65–31.92 | 132, 139, 160 and 72 |
| Group 8 (h) | 26.90–28.57 | 94, 159 and 155 |
| Fruit thickness (mm) | ||
| Group 1 (a) | 35.50–39.80 | 79, 100, 117, 50, 35, 63, 143, 101, 125, 48 and 23 |
| Group 2 (b) | 32.00–34.90 | 137, 1, 19, 135, 121, 115, 18, 98, 90, 112, 130, 74, 60, 111, 87, 17, 114, 129, 42, 47, 59, 44, 109,85, 113, 6 and 43 |
| Group 3 (c) | 29.50–31.80 | 34, 122, 64, 75, 142, 84, 58, 56, 41, 128, 7, 14, 11, 39, 31, 95, 120, 2, 134, 110, 20, 78, 93, 51, 73, 108, 83, 80, 106, 92, 148, 65, 10, 57, 102, 26, 127 and 88 |
| Group 4 (d) | 26.90–29.42 | 53, 68, 15, 140, 30, 152, 16, 86, 37, 151, 28, 67, 55, 139, 9, 138, 52, 8, 76, 107, 149, 69, 77, 96, 24, 131, 36, 62, 158, 22, 126, 27, 21, 124, 123, 136 and 38 |
| Group 5 (e) | 22.17–26.77 | 25, 71, 103, 147, 144, 33, 66, 150, 29, 13, 40, 157, 146, 89, 133, 5, 99, 54, 91, 81, 145, 61, 82, 3, 4, 12 and 104 |
| Group 6 (f) | 18.65–21.92 | 154, 70, 116, 141, 49, 105, 119, 32, 153, 132, 97, 118 and 72 |
| Group 7 (g) | 14.07–18.10 | 159, 160, 155, 46, 94, 156 and 45 |
| Fruit weight (g) | ||
| Group 1 (a) | 140.92 | 23 |
| Group 2 (b) | 123.11–128.52 | 68 and 152 |
| Group 3 (c) | 103.85–112.89 | 48, 74 and 153 |
| Group 4 (d) | 93.31–98.28 | 8, 88, 39, 59, 141 and 9 |
| Group 5 (e) | 67.42–83.91 | 102, 35, 40, 101, 154, 72, 108, 146, 69, 66, 43, 107, 61, 42, 63, 78, 17, 21, 67, 31, 103, 64, 156, 76, 36, 71, 44, 75, 123, 142, 80, 100, 143, 70, 5, 62, 85, 33, 56, 109, 129 and 86 |
| Group 6 (f) | 47.28–66.35 | 160, 81, 149, 99, 157, 25, 18, 11, 96, 147, 84, 15, 119, 28, 3, 4, 37, 128, 133, 24, 111, 34, 132, 113, 134, 144, 16, 120, 97, 90, 10, 145, 49, 77, 38, 135, 151, 117, 6, 27,12, 2, 118, 22, 105, 98, 122, 47, 60, 158, 115, 26, 79, 30, 20, 51, 114, 148, 29, 116, 104, 106, 131, 95, 19, 41, 127, 87, 32, 65, 45, 50, 130, 126, 137 and 121 |
| Group 7 (g) | 37.74–45.46 | 112, 92, 73, 89, 93, 1, 46, 82, 13, 124, 150, 57, 7, 136, 155, 14, 55 and 159 |
| Group 8 (h) | 17.94–35.87 | 139, 54, 52, 53, 138, 58, 91, 94, 83, 140 and 110 |
| Number of seeds per fruit | ||
| Group 1 (a) | 9.50 | 23 |
| Group 2 (b) | 5.75–6.50 | 76, 71, 103, 86, 59 and 154 |
| Group 3 (c) | 4.75–5.25 | 40, 72, 36, 141, 125, 62, 146, 115, 114 and 31 |
| Group 4 (d) | 3.75–4.50 | 116, 42, 81, 60, 6, 131, 35, 144, 33, 128, 121, 68, 69, 75, 61, 157, 156, 153, 95, 105, 143, 88, 160, 158, 85, 5, 8, 9, 70, 152, 90, 109, 51, 74, 107, 110, 20, 48, 80 and 123 |
| Group 5 (e) | 2.75–3.50 | 124, 151, 26, 29, 126, 1 9, 91, 11, 140, 12, 77, 148, 14, 37, 56, 50, 94, 67, 78, 21, 120, 43, 119, 113, 106, 30, 136, 10, 101, 46, 15, 84, 117, 111, 41, 145, 39, 118, 98, 134, 132, 22, 66, 34, 17, 28, 142, 18, 27, 135, 133, 63, 64, 102, 92, 159, 47, 147, 97 and 96 |
| Group 6 (f) | 1.50–2.50 | 89, 93, 53, 73, 127, 58, 65, 82, 138, 79, 16, 139, 150, 149, 112, 137, 99, 52, 155, 130, 129, 38, 49, 54, 25, 108, 7, 104, 1, 3, 4, 87, 83, 122, 13, 45, 44, 100, 57, 55, 32, 24 and 2 |
Appendix A.2
| Group * | Averages | Mother Plants ** |
| Seed length (mm) | ||
| Group 1 (a) | 29.22–29.67 | 35, 5 and 21 |
| Group 2 (b) | 28.05–28.75 | 67, 2, 39, 36, 48 and 68 |
| Group 3 (c) | 26.70–27.82 | 33, 133, 152, 20, 69, 76, 60, 56, 77, 102, 104, 106, 59, 29, 70, 86, 84, 66, 153, 75, 137, 74 and 65 |
| Group 4 (d) | 25.50–26.42 | 72, 109, 22, 32, 10, 42, 19, 88, 11, 143, 142, 71, 16, 100, 41, 116, 17, 156, 31, 4, 3, 78, 119, 12, 125, 23, 63, 118, 37, 146, 62, 141 and 61 |
| Group 5 (e) | 24.60–25.42 | 40, 27, 28, 151, 101, 117, 114, 107, 120, 24, 155, 58, 158, 113, 159, 134, 79, 135, 38, 9, 26, 44, 126 and 49 |
| Group 6 (f) | 23.80–24.52 | 147, 45, 121, 111, 148, 8, 64, 81, 47, 13, 94, 103, 30, 25, 154, 122, 131, 115, 18, 50 and 127 |
| Group 7 (g) | 22.95–23.70 | 95, 150, 15, 90, 132, 85, 7, 96, 149, 34, 98, 136, 52, 99 and 123 |
| Group 8 (h) | 21.45–22.75 | 6, 93, 57, 73, 140, 97, 82, 55, 112, 138, 87, 80, 53, 14, 46, 110, 124, 105, 108, 128, 139, 145, 91 e 160 |
| Group 9 (i) | 20.30–21.35 | 43, 144, 130, 92, 89, 54, 129, 83 and 157 |
| Group 10 (j) | 18.90–19.75 | 1 and 51 |
| Seed width (mm) | ||
| Group 1 (a) | 23.60 | 125 |
| Group 2 (b) | 22.37 | 35 |
| Group 3 (c) | 20.65–21.78 | 158, 36, 106, 109, 11, 58, 10, 74, 2, 19, 48 and 12 |
| Group 4 (d) | 19.72–20.55 | 3, 4, 134, 137, 154, 111, 21, 49, 47, 7, 115, 39, 84, 156, 143, 44, 153, 142, 135, 126, 114, 23, 65, 63, 60, 56, 94, 68, 118, 141, 103, 121 and 13 |
| Group 5 (e) | 19.00–19.67 | 151, 5, 22, 57, 76, 81, 69, 123, 83, 73, 53, 80, 37, 112, 95, 67, 122, 138, 77, 127, 110, 104, 75, 107, 15, 88, 133, 59, 101, 62, 42, 146, 155, 117, 50 and 116 |
| Group 6 (f) | 17.82–18.95 | 86, 97, 132, 91, 6, 150, 128, 9, 92, 33, 25, 139, 124, 66, 40, 72, 31, 30, 105, 45, 136, 52, 149, 147, 98, 102, 78, 24, 38, 131, 140, 64, 16, 113, 18, 108, 17, 152, 90, 34, 28, 148, 70, 41, 120, 61, 85, 26 and 29 |
| Group 7 (g) | 15.77–17.75 | 89, 93, 130, 82, 157, 160, 46, 99, 32, 96, 159, 20, 71, 55, 8, 14, 129, 51, 79, 145, 54, 144, 100, 119, 1, 43, 87 and 27 |
| Seed thickness (mm) | ||
| Group 1 (a) | 13.92–22.37 | 138, 59, 79, 55, 140, 132, 124, 14, 74, 17, 41, 95, 1, 28, 131, 117, 66, 65, 104, 45, 139, 78, 136, 39, 7, 150, 5, 34, 118, 35, 13, 144, 63, 127, 155, 113, 33, 10, 15, 29, 137, 135, 88, 120, 101, 112, 111, 37, 133, 57, 107, 77, 108, 44, 151, 156, 67, 21, 16, 126, 3, 4, 141, 48, 73, 148, 50, 54, 134, 53, 56, 49, 2, 19, 68, 12, 122, 11, 106, 94, 149, 58 and 36 |
| Group 2 (b) | 10.15–13.85 | 89, 23, 103, 92, 110, 115, 154, 93, 42, 90, 128, 87, 114, 71, 43, 157, 158, 31, 160, 60, 27, 86, 159, 51, 47, 109, 62, 32, 97, 72, 121, 105, 81, 82, 102, 98, 40, 70, 30, 8, 6, 9, 22, 83, 24, 61, 69, 20, 76, 25, 147, 116, 119, 26, 91, 80, 129, 123, 64, 75, 46, 125, 38, 130, 145, 85, 146, 18, 96, 100, 52, 99, 152, 153 and 84 |
| Seed weight (g) | ||
| Group 1 (a) | 6.36–6.85 | 138, 58, 19, 125, 21, 106, 12, 11, 68, 2 and 67 |
| Group 2 (b) | 5.85–6.29 | 65, 156, 94, 29, 153, 35, 5, 118, 39, 3, 4, 56, 74, 141, 10 e 48 |
| Group 3 (c) | 5.47–5.73 | 17, 75, 88, 134, 44, 122, 61, 133, 63, 77 and 126 |
| Group 4 (d) | 5.03–5.42 | 38, 116, 101, 22, 151, 66, 155, 120, 107, 111, 41, 123, 36, 142, 104, 70, 16, 100, 146, 84, 59, 37, 127, 76, 33, 13, 78, 152 and 50 |
| Group 5 (e) | 4.64–4.98 | 149, 34, 42, 147, 45, 7, 60, 24, 27, 25, 64, 85, 131, 53, 62, 86, 117, 28, 95, 31, 26, 143, 15, 148, 69, 113, 72, 109 and 43 |
| Group 6 (f) | 4.17–4.61 | 32, 83, 98, 139, 124, 119, 114, 140, 80, 18, 135, 49, 136, 71, 121, 150, 108, 52, 144, 40, 47, 20, 81, 79, 102, 30, 9, 112, 73 and 57 |
| Group 7 (g) | 3.66–4.15 | 54, 46, 128, 129, 157, 55, 90, 97, 159, 99, 14, 115, 103, 6, 96, 137, 8, 105, 154, 145, 132 and 23 |
| Group 8 (h) | 3.22–3.58 | 92, 160, 93, 158, 130, 87, 1, 82 and 110 |
| Group 9 (i) | 2.62–3.07 | 89, 51 and 91 |
| Water content of seeds (%) | ||
| Group 1 (a) | 14.70–16.96 | 73, 98, 118, 119 and 41 |
| Group 2 (b) | 12.59–14.11 | 91, 138, 75, 48, 111, 106, 25, 9, 134, 74, 69, 7, 110, 114, 137, 68, 77, 51, 19, 125, 115, 136, 58, 76, 63, 78, 131, 2, 105, 86, 87, 15, 133, 18, 64, 21, 16 and 132 |
| Group 3 (c) | 10.85–12.48 | 79, 53, 54, 121, 82, 33, 12, 103, 70, 104, 61, 81, 31,109, 42,40, 101, 30, 117, 116, 27, 11, 36, 6, 38, 20, 14, 141, 102, 100, 8, 60, 57, 67, 26, 112, 3, 4, 13,52, 5, 80. 23, 92, 113, 130, 66, 65, 37, 135, 90, 62, 59 and 56 |
| Group 4 (d) | 9.09–10.77 | 140, 127, 129, 46, 126, 157, 45, 55, 139, 160, 108, 95, 89, 94, 123, 156, 29, 1, 88, 50, 84, 72, 159, 158, 154, 47, 28, 145, 24, 10, 39, 71, 17, 32 and 22 |
| Group 5 (e) | 5.73–8.93 | 153, 148, 43, 128, 96, 147, 120, 44, 35, 155, 149, 83 e 107, 150, 144, 49, 34, 146, 85, 122, 99, 124, 151, 97, 93 and 152 |
Appendix A.3
| Group * | Averages | Mother Plants ** |
| First emergence count (%) | ||
| Group 1 (a) | 77.0–94.0 | 124, 111, 150, 40, 157, 86, 158, 159, 138, 152, 106, 97, 120, 141, 96, 103 and 160 |
| Group 2 (b) | 64.0–75.0 | 122, 72, 105, 32, 119, 19, 144, 151, 74, 54, 56, 57, 55, 156, 136, 128,110, 1, 42, 114, 78, 148 and 81 |
| Group 3 (c) | 51.0–61.0 | 60, 35, 155, 108, 82, 58, 116, 113, 135, 133, 59, 125, 52, 146, 93, 121, 112, 2, 48, 23, 73, 94, 104, 99 and 41 |
| Group 4 (d) | 40.0–50.0 | 123, 145, 118, 131, 77, 153, 126, 101, 91, 49, 154, 149, 134, 140, 53, 79, 107, 26, 44, 84, 80, 83, 34, 85 and 147 |
| Group 5 (e) | 26.0–38.0 | 29, 98, 20, 95, 39, 43, 63, 100, 132, 33 and 47 |
| Group 6 (f) | 15.0–25.0 | 66, 13, 18, 127, 4, 36, 27, 22, 16, 71, 25, 115, 51, 17, 8, 90, 117, 92, 3, 69, 15, 14, 64, 129, 102, 38, 46, 9, 45, 76, 31, 37 and 75 |
| Group 7 (g) | 0.0–14.0 | 11, 12, 7, 88, 137, 24, 10, 28, 67, 89, 68, 65, 62, 130, 50, 143, 87, 21, 142, 5, 30, 139, 70, 61 and 8 |
| Emergence percentage (%) | ||
| Group 1 (a) | 74.0–96.0 | 99, 1, 54, 6, 125, 19, 148, 132, 140, 91, 93, 134, 40, 86, 150, 157, 123, 151, 159, 135, 105, 78, 97, 104, 107, 73, 108, 138, 80, 111, 120, 124, 81, 158, 106, 152, 96, 103, 160 and 141 |
| Group 2 (b) | 83.0–73.0 | 83, 147, 3, 58, 82, 16, 43, 149, 77, 26, 84, 13, 116, 155, 4, 59, 28, 146, 44, 121, 113, 60, 52, 35, 49, 61, 85, 17, 2, 45, 27, 41, 129, 48, 75, 131, 23, 34, 130, 119, 118, 112, 122, 74, 5, 127, 136, 126, 70, 57, 144, 94, 100, 22, 32, 156, 72, 71, 38, 42, 56, 110, 109, 114, 128, 55 and 133 |
| Group 3 (c) | 31.0–50.0 | 88, 95, 9, 20, 37, 65, 63, 30, 33, 25, 47, 64, 24, 29, 98, 46, 18, 137, 153, 115, 50, 154, 14, 90, 69, 39, 66, 53, 145, 79, 101, 15, 68 and 139 |
| Group 4 (d) | 8.0–30.0 | 87, 21, 7, 10, 11, 89, 62, 36, 8, 51, 102, 92, 67, 76, 31, 12, 117, 142 and 143 |
| Emergence speed index | ||
| Group 1 (a) | 1.23–1.24 | 103, 160 and 120 |
| Group 2 (b) | 1.04–1.14 | 152, 96, 97, 157, 86, 158, 106, 74, 159 and 141 |
| Group 3 (c) | 0.89–1.02 | 6, 104, 57, 73, 1, 132, 134, 133, 150, 151, 42, 144, 78, 40, 124, 136, 111, 148, 81, 105 and 138 |
| Group 4 (d) | 0.65–0.88 | 82, 35, 71, 85, 113, 38, 34, 127, 155, 58, 100, 126, 122, 118, 52, 112, 75, 91, 60, 140, 94, 121, 146, 59, 99, 131, 2, 93, 32, 41, 48, 128, 125, 72, 123, 23, 56, 109, 80, 114, 119, 107, 19, 135, 110, 156, 108, 54 and 55 |
| Group 5 (e) | 0.52–0.64 | 26, 16, 28, 3, 4, 13, 43, 154, 53, 77, 61, 79, 259, 83, 27, 17, 5, 45, 130,84, 129, 49, 116, 70, 44, 147 and 22 |
| Group 6 (f) | 0.39–0.51 | 95, 46, 18, 29, 50, 115, 14, 63, 90, 66, 68, 33, 139, 39, 153, 101, 15, 47, 98, 145 and 69 |
| Group 7 (g) | 0.26–0.38 | 76, 143, 88, 142, 117, 31, 30, 9, 37, 24, 65, 137, 20, 25 and 64 |
| Group 8 (h) | 0.09–0.25 | 87, 7, 21, 11, 10, 89, 51, 67, 62, 36, 8, 12, 102 and 92 |
| Mean emergence time (days) | ||
| Group 1 (a) | 28.3–29.7 | 28, 24, 5, 12, 11 and 137 |
| Group 2 (b) | 26.2–27.9 | 14, 142, 66, 50, 89, 129, 17, 16, 13, 18, 45, 143, 38, 10, 7, 30, 26, 4, 27, 88, 71, 61, 139, 70, 68, 130, 22 and 67 |
| Group 3 (c) | 24.8–26.0 | 108, 37, 15, 107, 126, 43, 25, 77, 39, 123, 140, 3, 91, 64, 127, 115, 46, 80, 90 and 100 |
| Group 4 (d) | 23.5–24.6 | 35, 94, 149, 51, 104, 118, 85, 53, 73, 99, 65, 44, 117, 122, 29, 9, 93, 135, 101, 34 and 49 |
| Group 5 (e) | 22.4–23.3 | 6, 78, 20, 96, 151, 128, 112, 116, 36, 113, 83, 111, 81, 76, 92, 145, 125, 124 and 84 |
| Group 6 (f) | 21.3–22.3 | 155, 141, 131, 134, 138, 31, 87, 69, 21, 105, 114, 56, 98, 102, 62, 75, 153, 19, 150, 72, 32, 152 and 109 |
| Group 7 (g) | 19.6–21.0 | 154, 119, 42, 146, 121, 148, 57, 95, 157, 58, 63, 41, 133, 97, 40, 48, 23, 2, 156, 1, 33, 106, 54, 147, 8, 60, 79, 55, 82, 110, 158, 132 and 52 |
| Group 8 (h) | 18.5–19.3 | 136, 86, 160, 159, 144, 103, 47 and 59 |
| Group 9 (i) | 17.1 | 120 |
| Group 10 (j) | 15.5 | 74 |
| Length of aerial part (cm) | ||
| Group 1 (a) | 23.40–27.42 | 36, 156, 96, 54, 55, 104, 77, 133, 56, 52 and 74 |
| Group 2 (b) | 20.75–22.95 | 101, 33, 31, 119, 141, 86, 128, 122, 134, 97, 144, 73, 32, 102, 2, 121, 42, 53, 29, 59, 120, 58, 106, 99, 40, 131, 158 and 103 |
| Group 3 (c) | 18.75–20.50 | 160, 154, 157, 148, 123, 81, 82, 41, 1, 94, 107, 152, 91, 47, 75, 63, 17, 108, 159, 153 and 19 |
| Group 4 (d) | 14.75–18.47 | 100, 145, 68, 7, 98, 85, 105, 113, 46, 14, 45, 37, 80, 25, 8, 139, 49, 83, 48, 22, 79, 110, 69, 51, 142, 92, 124, 151, 95, 27, 43, 140, 87, 84, 93, 9, 44, 132, 109, 114, 35, 78, 150, 38, 57, 125, 149, 21, 111, 88, 62, 60, 155, 138, 146, 20, 72, 34, 136, 76, 127, 116, 147 and 65 |
| Group 5 (e) | 12.17–14.55 | 115, 30, 90, 71, 129, 64, 4, 15, 16, 130, 18, 135, 28, 70, 70, 118, 50, 26, 11, 61, 3, 6, 5, 143 and 66 |
| Group 6 (f) | 10.02–11.92 | 10, 117, 67, 137, 39, 13, 12, 126, 24, 89 and 112 |
| Group 7 (g) | 4.72 | 23 |
| Length of the root system (cm) | ||
| Group 1 (a) | 23.77–24.72 | 19, 103, 119 and 1 |
| Group 2 (b) | 20.42–22.30 | 131, 102, 33, 121, 160, 125, 53, 133 and 23 |
| Group 3 (c) | 19.97–16.75 | 43, 83, 97, 84, 4, 129, 80, 122, 57, 17, 58, 94, 127, 147, 132, 140, 128, 51, 120, 81, 90 and 2 |
| Group 4 (d) | 13.45–16.50 | 18, 54, 155, 105, 139, 150, 107, 5, 55, 126, 146, 159, 118, 74, 85, 156, 72, 13, 112, 34, 108, 7, 50, 148, 49, 79, 71, 26, 136, 135, 70, 100, 154, 44, 37, 25, 114, 96, 113, 64, 35, 93, 89, 9, 142, 109, 92, 27, 14, 24, 124, 6, 77, 111, 3, 78, 145, 45, 144, 20, 86, 115, 141, 101, 8, 41, 42, 32, 40, 106, 134, 66, 82, 38 and 110 |
| Group 5 (e) | 8.00–13.22 | 10, 21, 104, 123, 30, 95, 88, 117, 39, 62, 29, 87, 65, 63, 60, 48, 130, 31, 11, 68, 36, 91, 52, 16, 56, 138, 99, 157, 67, 69, 75, 12, 152, 47, 149, 153, 158, 116, 143, 59, 76, 15, 28, 61, 46, 73, 137, 98, 151 and 22 |
| Dry mass of aerial part (g seedling−1) | ||
| Group 1 (a) | 16.12 | 34 |
| Group 2 (b) | 12.77 | 23 |
| Group 3 (c) | 0.85–1.67 | 20, 149, 29, 152, 62, 44, 159, 61, 99, 95, 31, 65, 21, 69, 97, 111, 108, 114, 71, 53, 36, 85, 70, 146, 127, 145, 148, 128, 96, 144, 72, 116, 60, 136, 47, 113, 77, 52, 101, 122, 41, 84, 76, 104, 32, 35, 42, 94, 48, 57, 119, 125, 131, 40, 158, 134, 54, 153, 73, 156, 56, 56, 58, 59, 86, 63, 103, 2, 19, 75, 121, 33, 106, 79, 141,133, 120 and 74 |
| Group 4 (d) | 0.23–0.84 | 24, 13, 89, 67, 137, 7, 30, 98, 66, 105, 26, 117, 90, 28, 16, 130, 3, 110, 139, 6, 5, 11, 12, 100, 50, 126, 143, 115, 27, 140, 22, 135, 88, 49, 46, 9, 14, 118, 93, 82, 129, 45, 112, 102, 18, 93, 82, 129, 45, 112, 102, 18, 15, 38, 25, 51, 80, 43, 87, 4, 39, 151, 107, 8, 91, 160, 157, 17, 68, 92, 37, 124, 138, 123, 78, 109, 1, 147, 10, 83, 154, 55, 132, 150, 81, 64, 155 and 142 |
| Root dry mass (g seedling−1) | ||
| Group 1 (a) | 1.31 | 23 |
| Group 2 (b) | 0.63–0.80 | 2, 87, 119, 19, 34 and 102 |
| Group 3 (c) | 0.41–0.56 | 94, 113, 103, 73, 114, 59, 132, 68, 116, 40, 14, 72, 48, 147, 36, 44, 86, 120, 33, 42, 41, 121, 141, 106 and 58 |
| Group 4 (d) | 0.28–0.40 | 122, 81, 20, 61,75, 37, 109, 8, 54, 92, 112, 130, 71, 51, 39, 142, 60, 85, 143, 25, 83, 118, 88, 35, 91, 156, 9, 77, 101, 4, 84, 70, 127, 145, 104, 47, 80, 125, 56, 74, 96, 66, 136, 43, 134, 160, 155, 131, 154, 57, 144, 153, 133, 148 and 63 |
| Group 5 (e) | 0.15–0.27 | 7, 89, 139, 82, 124, 49, 46, 98, 55, 22, 90, 117, 45, 110, 30, 93, 28, 126, 18, 100, 69, 26, 95, 29, 79, 12, 52, 32, 13, 78, 99, 11, 137, 123, 50, 27, 157, 159, 128, 151, 53, 149, 67, 76, 115, 6, 111, 16, 62, 3, 107, 150, 129, 65, 38, 146, 64, 24, 31, 1, 21, 15, 5, 105, 138, 158, 152, 97, 140, 10, 108, 17 and 135 |
References
- Pinto, R.B.; Tozzi, A.M.G.A.; Mansano, V.F. Hymenaea in Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. 2020. Available online: https://floradobrasil.jbrj.gov.br/FB22971 (accessed on 27 February 2025).
- Cipriano, J.; Martins, L.; Deus, M.S.M.; Peron, A.P. O gênero Hymenaea e suas espécies mais importantes do ponto de vista econômico e medicinal para o Brasil. Cad. Pesq. 2014, 26, 41–51. [Google Scholar]
- Judd, W.S.; Campbell, C.S.; Kellogg, E.A.; Stevens, P.F.; Donoghue, M.J. Sistemática Vegetal. Um Enfoque Filogenético; Artmed: Porto Alegre, Brazil, 2009; p. 632. [Google Scholar]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil, 3rd ed.; Instituto Plantarum: Nova Odessa, Brazil, 2009. [Google Scholar]
- IUCN. Hymenaea martiana. The IUCN Red List of Threatened Species. 2018. Available online: https://www.iucnredlist.org/species/144314391/149054864 (accessed on 7 July 2025).
- Reflora. Hymenaea in Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. 2025. Available online: https://floradobrasil.jbrj.gov.br/FB83203 (accessed on 20 April 2025).
- Gazzaneo, L.R.S.; Lucena, R.F.P.; Albuquerque, U.P. Knowledge and use of medicinal plants by local specialists in an region of Atlantic Forest in the state of Pernambuco (Northeastern Brazil). J. Ethnobiol. Ethnomed. 2005, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.S.; Sá, G.B.; Dantas, M.K.L.; Almeida, M.G.V.M. Plantas medicinais comercializadas na feira livre do município de Patos, Paraíba. Rev. Verd. Agroecol. Desenvolv. 2019, 14, 150–155. [Google Scholar] [CrossRef]
- Silva, C.P.; Manolio, S.F.R.A.; Sampaio, G.R.; Barros, M.C.S.; Nascimento, T.P.; Cameron, L.C.; Ferreira, M.S.L.; Gomes, J.A.A. Identification and action of phenolic compounds of Jatobá-do-cerrado (Hymenaea stignocarpa Mart.) on αamylase and α-glucosidase activities and flour effect on glycemic response and nutritional quality of breads. Food Res. Int. 2019, 116, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.G.S.; Veras, B.O.; Silva, A.P.S.A.; Araújo, A.D.; Barbosa, D.C.S.; Silva, T.C.M.; Ribeiro, E.R.F.R.; Maia, M.M.L.; Souza-Júnior, U.P.; Lima, V.L.M.; et al. Photoprotective activity and HPLC-MS-ESI-IT profile of flavonoids from the barks of Hymenaea martiana Hayne (Fabaceae): Development of topical formulations containing the hydroalcoholic extract. Biotechnol. Biotechnol. Equip. 2021, 35, 504–516. [Google Scholar] [CrossRef]
- Silva, J.M.D.S.; Rolim, L.A.; Almeida, J.R.D.S.; Feitosa, T.A.; Nery, D.A.; Souza Araújo, C.; Oliveira, A.P. Chemometric studies in Hymenaea martiana. Phytochem Lett. 2024, 61, 158–165. [Google Scholar] [CrossRef]
- Valls, J.F.M. Caracterização de recursos genéticos vegetais. In Recursos Genéticos Vegetais; Nass, L.L., Ed.; Embrapa Recursos Genéticos e Biotecnologia: Brasília, Brazil, 2007; pp. 281–342. [Google Scholar]
- Spigler, R.B.; Theodorou, K.; Chang, S.M. Inbreeding depression and drift load in small populations at demographic disequilibrium. Evolution 2017, 71, 81–94. [Google Scholar] [CrossRef]
- Penha, J.S.; Lopes, A.C.A.; Gomes, R.L.F.; Pinheiro, J.B.; Assunção-Filho, J.R.; Silvestre, E.A.; Viana, J.P.G.; Martínez-Castillo, J. Estimation of natural outcrossing rate and genetic diversity in lima bean (Phaseolus lunatus L. var. lunatus) from Brazil using SSR markers: Implications for conservation and breeding. Genet. Resour. Crop Evol. 2017, 64, 1355–1364. [Google Scholar] [CrossRef]
- Cruz, C.D.; Ferreira, F.M.; Pessoni, L.A. Biometria Aplicada ao Estudo da Diversidade Genética, 2nd ed.; UFV: Viçosa, Brazil, 2020. [Google Scholar]
- Silva-Júnior, A.L.; Cabral, R.L.R.; Sartori, L.; Miranda, F.D.; Caldeira, M.V.W.; Moreira, S.O.; Godinho, T.O.; Oliveira, F.S. Molecular markers applied to the genetic characterization of Dalbergia nigra: Implications for conservation and management. Trees 2022, 36, 1539–1557. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Chauhan, B.S. Genetic diversity, conservation, and utilization of plant genetic resources. Genes 2023, 14, 174. [Google Scholar] [CrossRef]
- Gao, S.; Ren, Y.; Masabni, J.; Zou, F.; Xiong, H.; Zhu, J. Influence of geographical and climatic factors on Quercus variabilis Blume fruit phenotypic diversity. Diversity 2021, 13, 329. [Google Scholar] [CrossRef]
- Rajarajan, K.; Uthappa, A.R.; Handa, A.K.; Chavan, S.B.; Vishnu, R.; Shrivastava, A.; Handa, A.; Rana, M.; Sahu, S.; Kumar, N.; et al. Genetic diversity and population structure of Leucaena leucocephala (Lam.) de Wit genotypes using molecular and morphological atributes. Genet. Resour. Crop Evol. 2022, 69, 71–83. [Google Scholar] [CrossRef]
- Silva, J.N.; Pádua, G.V.G.; Rodrigues, C.M.; Silva, J.H.C.S.; Bernardo, M.K.F.; Farias, G.E.S.; Gomes, C.L.S.; Alves, E.U. Caracterização morfofisiológica de frutos e sementes de espécies florestais: Um estudo de revisão. In Ciências Agrárias e Ambientais: Desafios e Perspectivas, 1st ed.; Abreu, K.G., Silva, J.N., Silva, J.M., Silva, J.H.B., Sousa, A.S., Santos, J.P.O., Eds.; Editora Itacaiúnas: Ananindeua, Brazil, 2023. [Google Scholar]
- Capucho, H.L.V.; Lopez, M.T.G.; Lima Junior, M.J.V.; Valente, M.S.F.; Mendes, A.M.S.; Muniz, G.I.B. Genetic Parameters in seed characters of Ormosia discolor under different ambient conditions. Floresta 2021, 51, 492–501. [Google Scholar] [CrossRef]
- Silva-Junior, I.; Lima-Junior, M.D.J.V.; Mendes, A.M.D.S.; Bastos, L.L.D.S.; Menezes, V.D.S. Parâmetros de qualidade de sementes para escolha de matrizes de Handroanthus serratifolius (Vahl) S. Grose na Amazônia Ocidental. Ciênc. Florest. 2024, 34, e84834. [Google Scholar] [CrossRef]
- Sousa, M.B.; Silva, C.L.; Santos, P.C.S.; Silva, D.Y.B.O.; Nonato, E.R.L.; Chaves, L.F.C.; Farias, S.G.G.; Gallo, R. Genetic divergence analysis in Parkia platycephala Benth.: Fruit and seed morphometry as indicators within and among populations. Discov. For. 2025, 1, 17. [Google Scholar] [CrossRef]
- Francisco, P.R.M.; Santos, D. Climatologia do Estado da Paraíba; EDUFCG: Campina Grande, Brazil, 2017. [Google Scholar]
- Arruda, L.V.; Rodrigues, L.P.M.; Silva, I.C.; Souza, R.S. Configuração geoambiental e dinâmica do espaço agrário atual do brejo paraibano (PB), Paraíba, Brasil. Ciênc. Geogr. 2022, 26, 72–102. [Google Scholar] [CrossRef]
- Nascimento, R.L.X.; Souza, C.C.; Grassi, G.; Oliveira, M.A.N. Caderno de Caracterização: Estado da Paraíba; Codevasf: Brasília, Brazil, 2022. [Google Scholar]
- Beltrão, B.A.; Rocha, D.E.G.A.; Mascarenhas, J.C.; Souza-Junior, L.C.; Pires, S.T.M.; Carvalho, V.G.D. Projeto Cadastro de Fontes de Abastecimento por Água Subterrânea. Diagnóstico do Município de Jaçanã, Estado do Rio Grande do Norte; CPRM/PRODEEM: Recife, Brazil, 2005. [Google Scholar]
- Nascimento, R.L.X.; Souza, C.C.; Oliveira, M.A.N. Caderno de Caracterização: Estado do Rio Grande do Norte; Codevasf: Brasília, Brazil, 2021. [Google Scholar]
- Silva, J.N. Caracterização Morfofisiológica de Frutos e Sementes de Hymenaea sp. Master’s Thesis, Universidade Federal da Paraíba, Areia, Brazil, 2021. [Google Scholar]
- Brasil, Ministério da Agricultura, Pecuária e Abastecimento. Regras para Análise de Sementes; Secretaria de Defesa Agropecuária MAPA/ACS: Brasília, Brazil, 2009. [Google Scholar]
- Brasil, Ministério da Agricultura, Pecuária e Abastecimento. Manual de Análise Sanitária de Sementes; Secretaria de Defesa Agropecuária MAPA/ACS: Brasília, Brazil, 2009. [Google Scholar]
- Maguire, J.D. Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Edmond, J.B.; Drapala, W.J. The effects of temperature, sand and soil, and acetone on germination of okra seeds. HortScience 1957, 71, 428–434. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 30 June 2024).
- Jelihovschi, E.G.; Faria, J.C.; Allaman, I.B. ScottKnott: A package for performing the Scott-Knott clustering algorithm in R. TEMA 2014, 15, 3–17. [Google Scholar] [CrossRef]
- Cruz, C.D.; Regazzi, A.J.; Carneiro, P.C.S. Modelos Biométricos Aplicados ao Melhoramento Genético, 3rd ed.; UFV: Viçosa, Brazil, 2004. [Google Scholar]
- Cruz, C.D. Genes Software-extended and integrated with the R, Matlab and Selegen. Acta Sci. Agron. 2016, 38, 547–552. [Google Scholar] [CrossRef]
- Mahalanobis, P.C. On the generalized distance in statistics. Proc. Natl. Acad. Sci. India 1936, 2, 49–55. [Google Scholar]
- Singh, D. The relative importance of characters affecting genetic divergence. Indian J. Genet. Plant Breed 1981, 41, 237–245. [Google Scholar]
- Cruz, C.D.; Regazzi, A.J.; Carneiro, P.C.S. Modelos Biométricos Aplicados ao Melhoramento Genético, 4th ed.; UFV: Viçosa, Brazil, 2012. [Google Scholar]
- Mojena, R. Hierarchical grouping methods and stopping rules: An evaluation. J. Comput. 1977, 20, 359–363. [Google Scholar] [CrossRef]
- Friendly, M.; Fox, J. Candisc: Visualizing Generalized Canonical Discriminant and Canonical Correlation Analysis. R Package Version 0.9-0. 2021. Available online: https://CRAN.R-project.org/package=candisc (accessed on 15 September 2024).
- Silva, A.R.; Azevedo, C.F. biotools: Tools for Biometry and Applied Statistics in Agricultural Science. R Package Version 4.3. 2025. Available online: https://CRAN.R-project.org/package=biotools (accessed on 15 September 2024).
- Kassambara, A. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 15 September 2024).
- Silva, D.Y.B.D.O.; Farias, S.G.G.D.; Araujo, P.C.D.; Sousa, M.B.D.; Silva, R.B.E.; Oliveira, C.V.D.A. Genetic variability of Parkia platycephala populations: Support for defining seed collection areas. Rev. Caatinga 2022, 35, 905–914. [Google Scholar] [CrossRef]
- Sander, N.L.; Silva, C.J.; Duarte, A.V.M.; Zago, W.B.; Galbiati, C.; Viana, I.G.; Arruda, J.C.; Dardengo, J.E.; Poletine, J.P.; Leite, M.H.S.; et al. The influence of environmental features on the morphometric variation in Mauritia flexuosa L.f. fruits and seeds. Plants 2020, 9, 1304. [Google Scholar] [CrossRef] [PubMed]
- Menegatti, R.D.; Mantovani, A.; Navroski, M. Biometric and physiological quality of bracatinga seeds from different mother trees. Floresta Ambient. 2019, 26, e20160359. [Google Scholar] [CrossRef]
- Bezerra, A.C.; Zuza, J.F.C.; Barbosa, L.D.S.; Azevedo, C.F.; Alves, E.U. Biometrics of mulungu seeds from different mother plants in the semi-arid region of Paraíba, Brazil. Rev. Caatinga 2022, 35, 393–401. [Google Scholar] [CrossRef]
- Wang, C.; Gong, H.; Feng, M.; Tian, C. Phenotypic variation in leaf, fruit and seed traits in natural populations of Eucommia ulmoides, a relict Chinese endemic tree. Forests 2023, 14, 462. [Google Scholar] [CrossRef]
- Macedo, M.C.; Scalon, S.P.Q.; Sari, A.P.; Scalon Filho, H.; Rosa, Y.B.C.J.; Robaina, A.D. Biometria de frutos e sementes e germinação de Magonia pubescens St. Hil (Sapindaceae). Rev. Bras. Sementes 2009, 31, 202–211. [Google Scholar] [CrossRef]
- Gonçalves, L.G.V.; Andrade, F.R.; Marimon Junior, B.H.; Schossler, T.R.; Lenza, E.; Marimon, B.S. Biometria de frutos e sementes de mangaba (Hancronia speciosa Gomes) em vegetação natural na região de Mato Grosso, Brasil. Rev. Ciênc. Agrár. 2013, 36, 31–40. [Google Scholar] [CrossRef]
- França-Neto, J.B.; Krzyzanowski, F.C.; Henning, A.A.; Pádua, G.P.; Lorini, I.; Henning, F.A. Tecnologia da Produção de Semente de Soja de Alta Qualidade; Embrapa Soja: Londrina, Brazil, 2016. [Google Scholar]
- Rossi, R.F.; Cavariani, C.B.; França-Neto, J.B. Vigor de sementes, população de plantas e desempenho agronômico de soja. Rev. Ciênc. Agrár. 2017, 60, 215–222. [Google Scholar] [CrossRef][Green Version]
- Krzyzanowski, F.C.; França-Neto, J.B.; Gomes-Junior, F.G.; Nakagawa, J. Testes de vigor baseados no desempenho das plântulas. In Vigor de Sementes: Conceitos e Testes, 2nd ed.; Krzyzanoswki, F.C., Vieira, R.D., França-Neto, J.B., Marcos-Filho, J., Eds.; ABRATES: Londrina, Brazil, 2020. [Google Scholar][Green Version]
- Cruz, C.D.; Carneiro, P.C.S. Modelos Biométricos Aplicados ao Melhoramento Genético, 2nd ed.; UFV: Viçosa, Brazil, 2006. [Google Scholar][Green Version]
- Correa, A.S.A.S.; Luz, P.B.; Rossi, A.A.B.; Silva, S.A.A. Biometria de frutos e sementes e divergência genética entre matrizes de Parkia pendula (will.) Benth. Ex walp. (angelim saia) nativa na Amazônia Matogrossense. Res. Soc. Dev. 2021, 10, e40410817498. [Google Scholar] [CrossRef]
- Alves, F.A.L. Utilização de técnicas de análise multivariadas no estudo da diversidade genética em quixabeira (Sideroxylon obtusifolium). Pesq. Agropec. Pernambuc. 2021, 26, e2351262021. [Google Scholar] [CrossRef]
- Belarmino, K.S.; Rêgo, M.M.; Bruno, R.L.A.; Medeiros, G.D.A.; Andrade, A.P.; Rêgo, E.R. Genetic diversity in a Poincianella pyramidalis (Tul.) LP Queiroz population assessed by RAPD molecular markers. Genet. Mol. Res. 2017, 16, gmr16039663. [Google Scholar] [CrossRef] [PubMed]
- Erickson, V.J.; Halford, A. Seed planning, sourcing, and procurement. Restor. Ecol. 2020, 28 (Suppl. 3), S219–S227. [Google Scholar] [CrossRef]
- Correa, E.; Espitia, M.; Araméndiz, H.; Murillo, O.; Pastrana, I. Variabilidad genética en semillas de árboles individuales de Tectona grandis L.f. en la conformación de lotes mezclados en córdoba, Colombia. Rev. UDCA Act. Divulg. Cient. 2013, 16, 379–389. [Google Scholar] [CrossRef]
- Soler-Guilhen, J.H.; Bernardes, C.D.O.; Marçal, T.D.S.; Oliveira, W.B.D.S.; Ferreira, M.F.D.S.; Ferreira, A. Euterpe edulis seed germination parameters and genotype selection. Acta Sci. Agron. 2020, 42, e42461. [Google Scholar] [CrossRef]

| Mother Plants | Collection Location | Coordinates | Region | Precipitation (mm year−1) | Average Temperature (°C) |
|---|---|---|---|---|---|
| 1 to 136 | Areia—PB | 6°57′46″ S 35°41′31″ W | Brejo | >1400 | 21–25 |
| 137 to 140 | Bananeiras—PB | 6°45′0″ S 35°37′58″ W | Brejo | >1400 | 21–25 |
| 141 | Cuité—PB | 6°29′6″ S 36°9′25″ W | Western Curimataú | <400 | >26 |
| 142 to 143 | Marcação—PB | 6°46′12″ S 35°0′54″ W | North coastline | until 1800 | 26 |
| 144 to 156 | Nova Floresta—PB | 6°27′18″ S 36°12′10″ W | Western Curimataú | <400 | >26 |
| 157 to 160 | Jaçanã—RN | 6°25′33″ S 36°12′18″ W | Borborema Potiguar | 500–800 | 25.6 |
| Sources of Variation | Mean Squares 1 | |||||
|---|---|---|---|---|---|---|
| FL (mm) | FWi (mm) | FT (mm) | FWe (g) | NSF | SL (mm) | |
| Mother plants | 837.33 ** | 184.07 ** | 99.76 ** | 1487.68 ** | 5.55 ** | 41.07 ** |
| Residue | 25.52 | 10.14 | 4.65 | 93.24 | 0.33 | 0.62 |
| h2 (%) | 96.95 | 94.49 | 95.34 | 93.73 | 94.06 | 98.47 |
| CVg/CVe | 2.82 | 2.07 | 2.26 | 1.93 | 1.99 | 4.01 |
| CV (%) | 5.54 | 7.29 | 7.53 | 15.61 | 17.08 | 3.23 |
| Sources of Variation | Mean Squares 1 | |||||
| SWi (mm) | ST (mm) | SWe (g) | WCS (%) | FEC (%) | EP (%) | |
| Mother plants | 8.89 ** | 10.84 ** | 6.33 ** | 15.07 ** | 2579.24 ** | 1775.88 ** |
| Residue | 0.62 | 3.07 | 0.11 | 2.27 | 125.08 | 144.89 |
| h2 (%) | 92.95 | 71.66 | 98.29 | 84.91 | 95.15 | 91.84 |
| CVg/CVe | 1.81 | 0.79 | 3.80 | 1.19 | 2.21 | 1.67 |
| CV (%) | 4.15 | 12.51 | 6,57 | 13.49 | 26.61 | 20.48 |
| Sources of Variation | Mean Squares 1 | |||||
| ESI | MET (days) | LAP (cm) | LRS (cm) | DMAP (g seedling−1) | RDM (g seedling−1) | |
| Mother plants | 0.29 ** | 32.55 ** | 61.09 ** | 39.28 ** | 7.84 ** | 0.08 ** |
| Residue | 0.02 | 1.00 | 3.81 | 5.82 | 0.05 | 0.009 |
| h2 (%) | 93.72 | 96.92 | 93.75 | 85.17 | 87.24 | 87.99 |
| CVg/CVe | 0.97 | 2.80 | 1.93 | 1.19 | 1.31 | 1.35 |
| CV (%) | 20.50 | 4.28 | 11.02 | 16.12 | 26.28 | 29.81 |
| Variable | Minimum | Maximum | Average ± SD | Scott–Knott Groups (5%) |
|---|---|---|---|---|
| Fruit length (mm) | 59.4 | 153.3 | 91.12 ± 14.47 | a–k |
| Fruit width (mm) | 26.9 | 62.5 | 43.67 ± 6.78 | a–h |
| Fruit thickness (mm) | 14.1 | 39.8 | 28.61 ± 17.94 | a–g |
| Fruit weight (g) | 17.9 | 140.9 | 61.84 ± 19.28 | a–h |
| Number of seeds per fruit | 1.0 | 9.0 | 3.40 ± 1.14 | a–f |
| Variable | Minimum | Maximum | Average ± SD | Scott–Knott Groups (5%) |
|---|---|---|---|---|
| Seed length (mm) | 18.9 | 29.7 | 24.76 ± 2.16 | a–j |
| Seed width (mm) | 15.8 | 23.6 | 19.01 ± 1.29 | a–g |
| Seed thickness (mm) | 10.1 | 22.4 | 13.93 ± 1.51 | a–b |
| Seed weight (g) | 2.6 | 6.8 | 4.89 ± 0.88 | a–i |
| Water content of seeds (%) | 5.7 | 17.0 | 11.13 ± 1.88 | a–e |
| Variable | Minimum | Maximum | Average ± SD | Scott–Knott Groups (5%) |
|---|---|---|---|---|
| First emergence count (%) | 0 | 94 | 42.00 ± 25.39 | a–g |
| Emergence percentage (%) | 8 | 96 | 59.00 ± 26.07 | a–d |
| Emergence speed index | 0.09 | 1.24 | 0.65 ± 0.27 | a–h |
| Mean emergence time (days) | 16 | 30 | 23.00 ± 2.85 | a–j |
| Length of aerial part (cm) | 4.7 | 27.4 | 17.73 ± 3.91 | a–g |
| Length of the root system (cm) | 8.0 | 24.7 | 14.96 ± 3.14 | a–e |
| Dry mass of aerial part (g seedling−1) | 0.23 | 16.1 | 1.01 ± 1.55 | a–d |
| Root dry mass (g seedling−1) | 0.15 | 1.32 | 0.32 ± 0.14 | a–e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.N.d.; Pádua, G.V.G.d.; Rodrigues, C.M.; Silva, J.H.C.S.; Gomes, C.L.S.; Rodrigues, M.H.B.S.; Bernardo, M.K.F.; Silva, E.L.F.d.; Almeida, L.G.A.d.; Araújo, L.D.A.d.; et al. Fruits and Seeds as Indicators of the Genetic Diversity of Hymenaea martiana (Fabaceae) in Northeast Brazil. Biology 2025, 14, 1418. https://doi.org/10.3390/biology14101418
Silva JNd, Pádua GVGd, Rodrigues CM, Silva JHCS, Gomes CLS, Rodrigues MHBS, Bernardo MKF, Silva ELFd, Almeida LGAd, Araújo LDAd, et al. Fruits and Seeds as Indicators of the Genetic Diversity of Hymenaea martiana (Fabaceae) in Northeast Brazil. Biology. 2025; 14(10):1418. https://doi.org/10.3390/biology14101418
Chicago/Turabian StyleSilva, Joyce Naiara da, Guilherme Vinícius Gonçalves de Pádua, Caroline Marques Rodrigues, João Henrique Constantino Sales Silva, Cosma Layssa Santos Gomes, Marília Hortência Batista Silva Rodrigues, Maria Karoline Ferreira Bernardo, Eduardo Luã Fernandes da Silva, Luís Gustavo Alves de Almeida, Lenyneves Duarte Alvino de Araújo, and et al. 2025. "Fruits and Seeds as Indicators of the Genetic Diversity of Hymenaea martiana (Fabaceae) in Northeast Brazil" Biology 14, no. 10: 1418. https://doi.org/10.3390/biology14101418
APA StyleSilva, J. N. d., Pádua, G. V. G. d., Rodrigues, C. M., Silva, J. H. C. S., Gomes, C. L. S., Rodrigues, M. H. B. S., Bernardo, M. K. F., Silva, E. L. F. d., Almeida, L. G. A. d., Araújo, L. D. A. d., Souza, A. d. G., Nascimento, N. F. F. d., & Alves, E. U. (2025). Fruits and Seeds as Indicators of the Genetic Diversity of Hymenaea martiana (Fabaceae) in Northeast Brazil. Biology, 14(10), 1418. https://doi.org/10.3390/biology14101418






