Annual Variations and Influencing Factors of Zooplankton Community Structure in the Coastal Waters of Northern Shandong Peninsula, China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
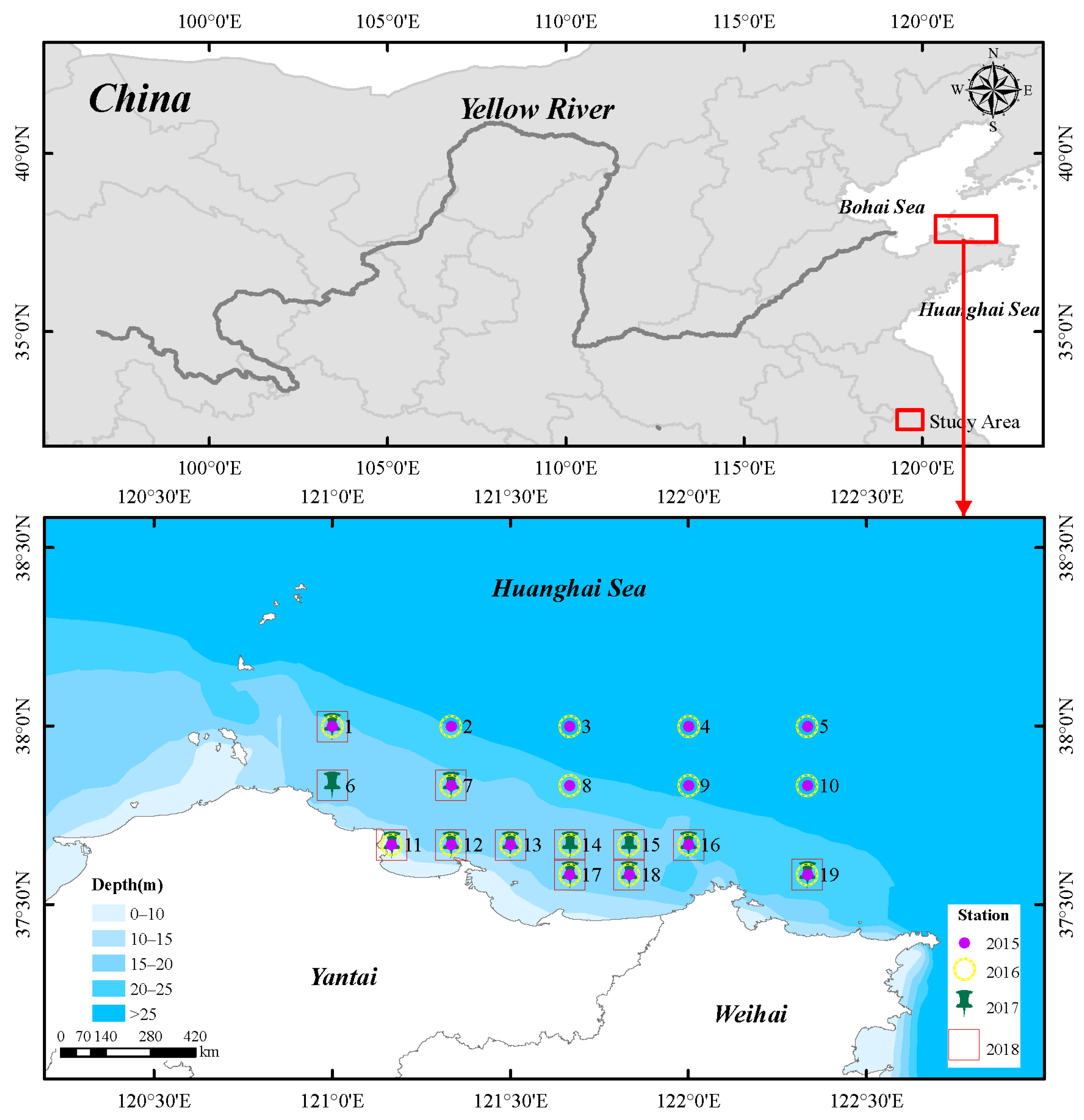
2.1. Study Area and Stations
2.2. Data Sources
2.2.1. Biological Data
2.2.2. Environmental Factor Data
2.3. Data Analysis
2.3.1. Dominant Species
2.3.2. Biodiversity
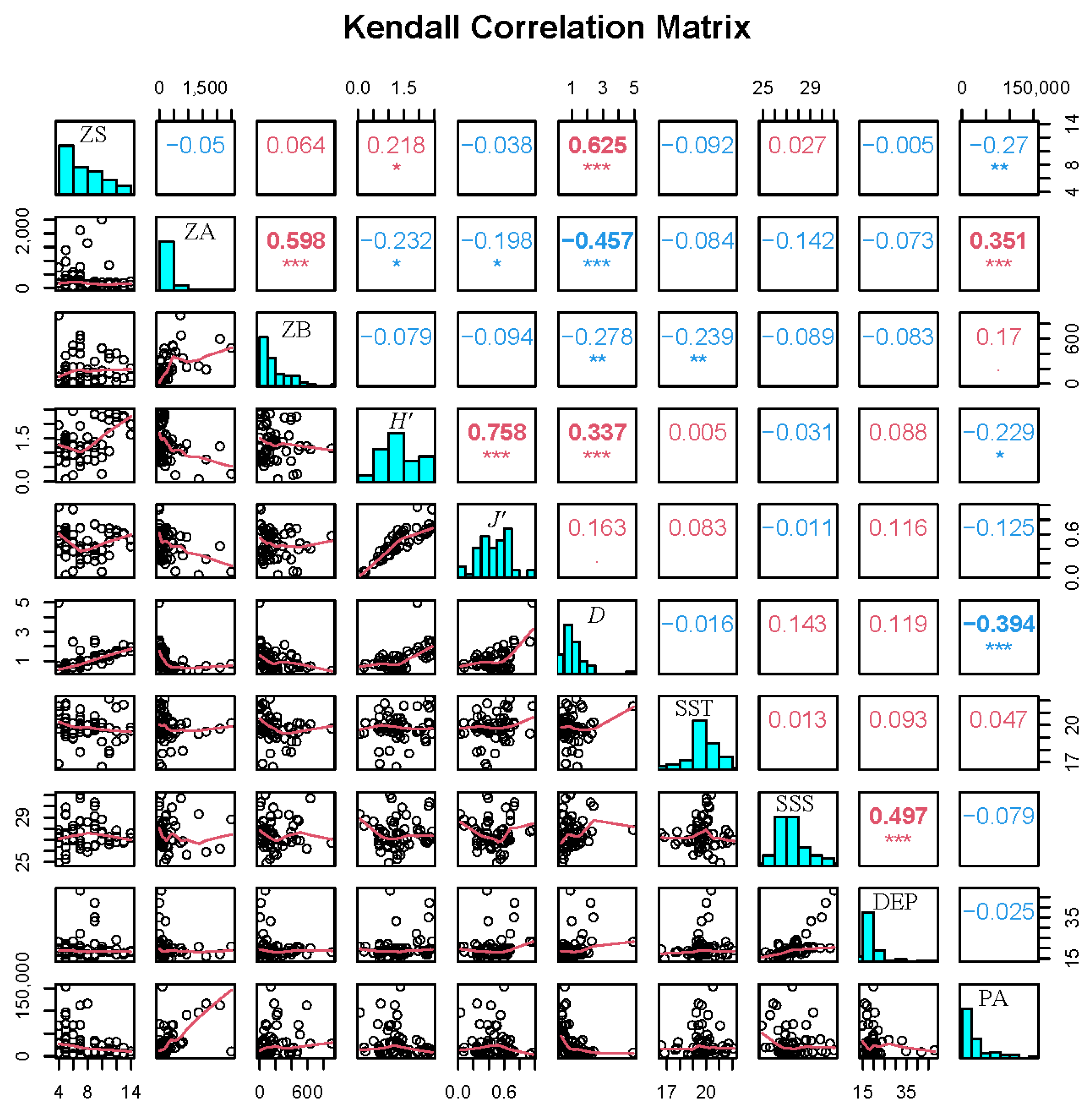
2.3.3. Correlation of Environmental Factors
3. Results
3.1. Species Composition and Dominant Species of Zooplankton
3.2. Annual Variation in Zooplankton
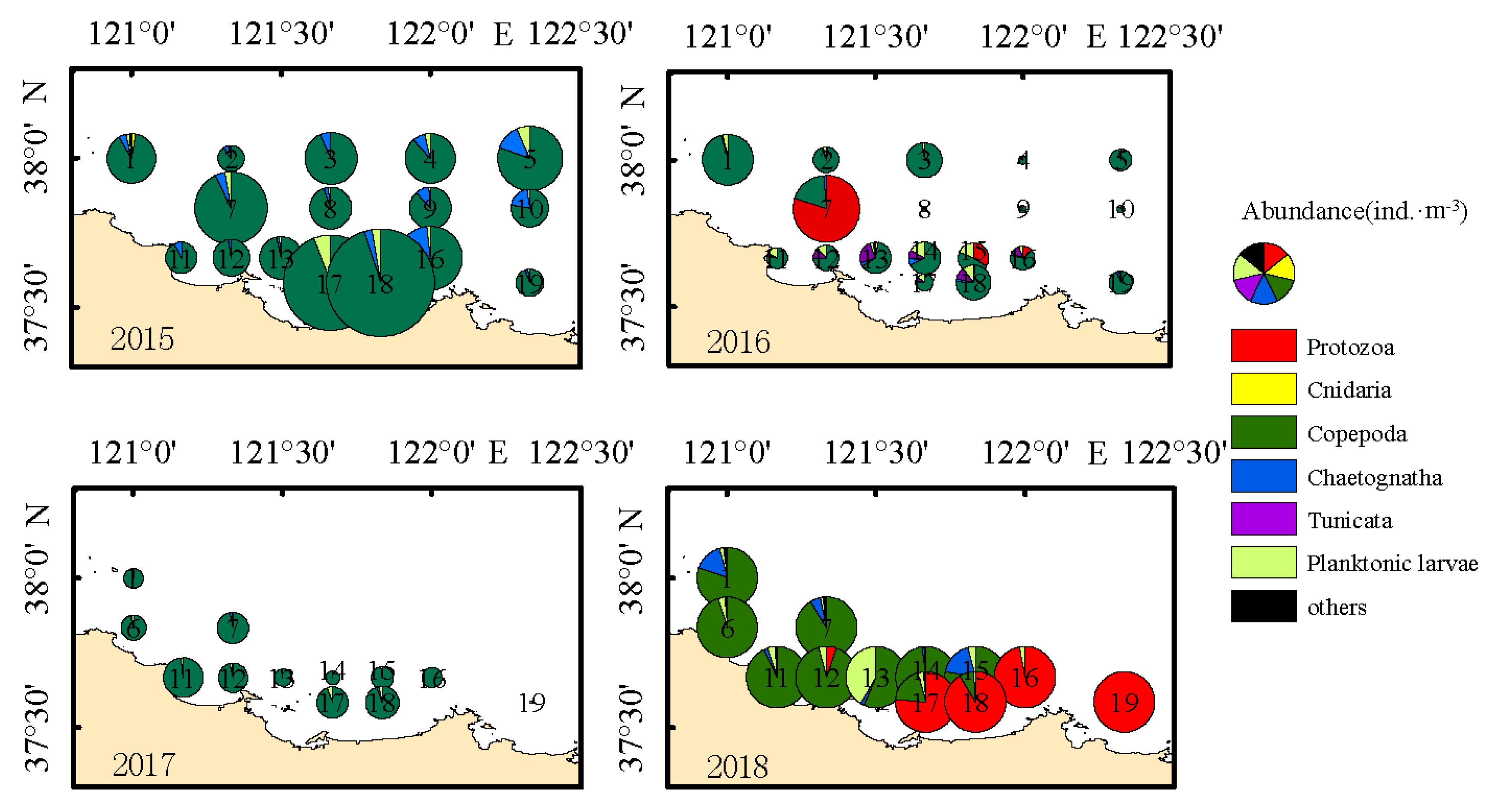
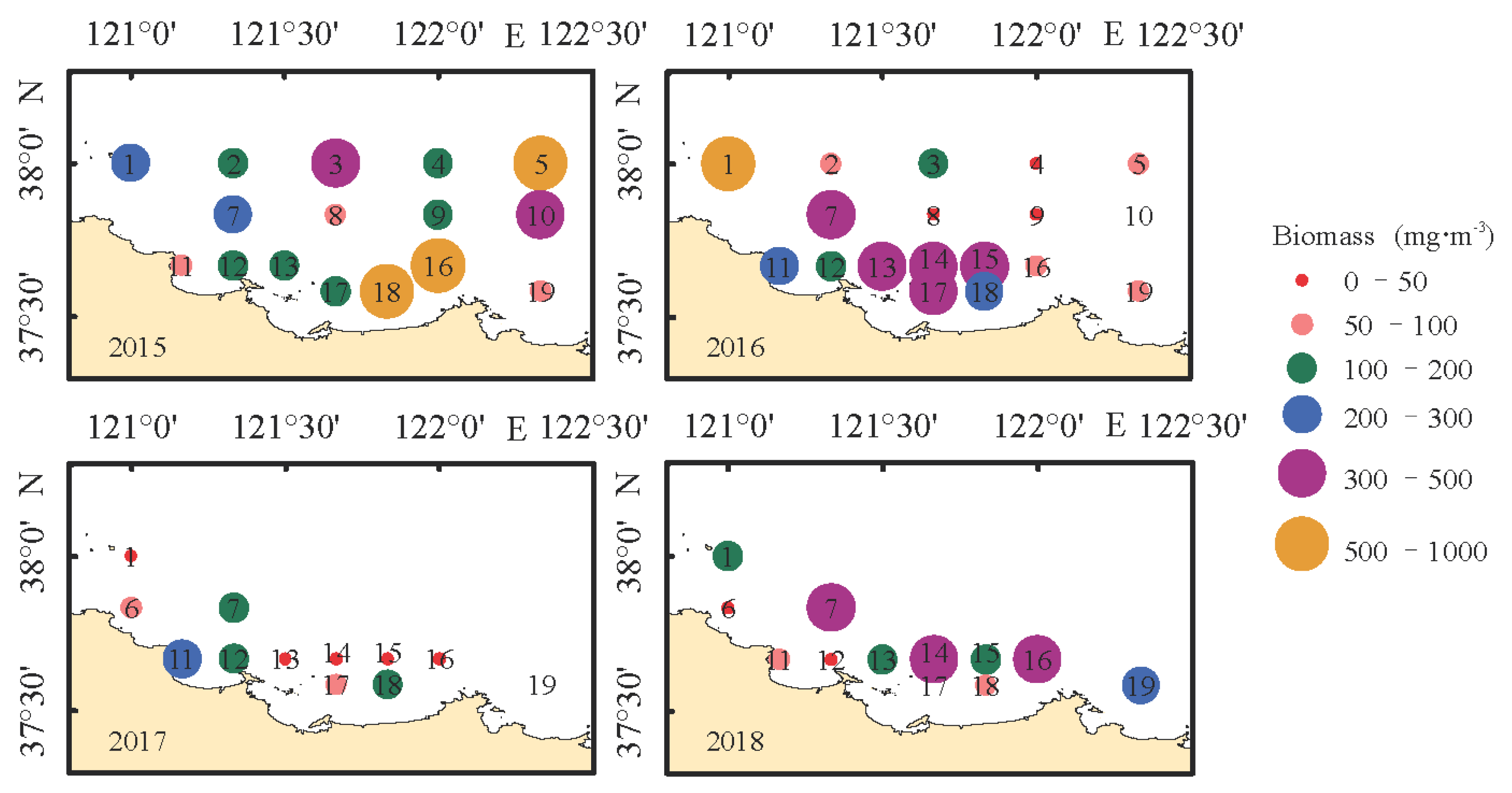
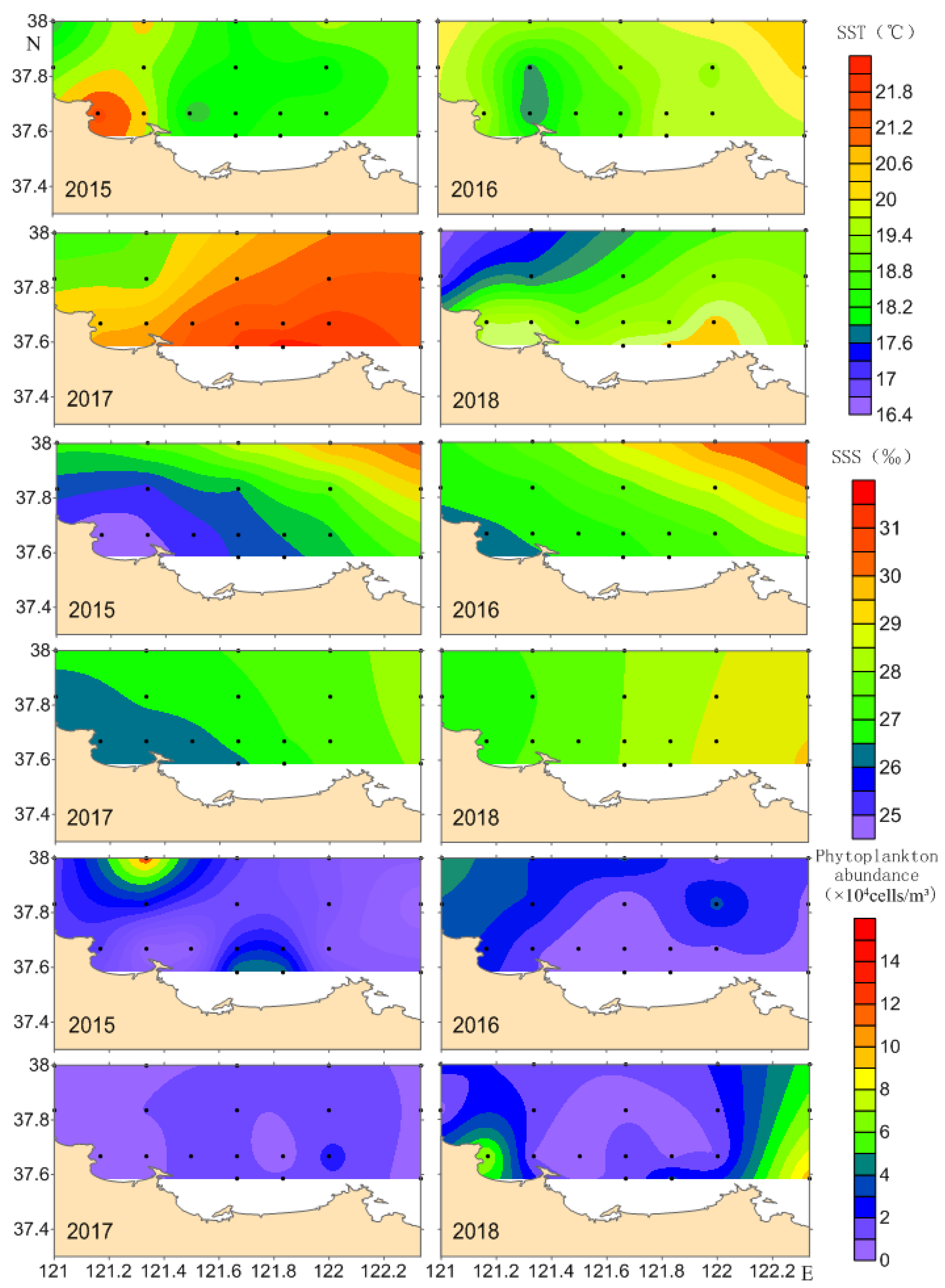
3.3. Horizontal Distribution of Zooplankton
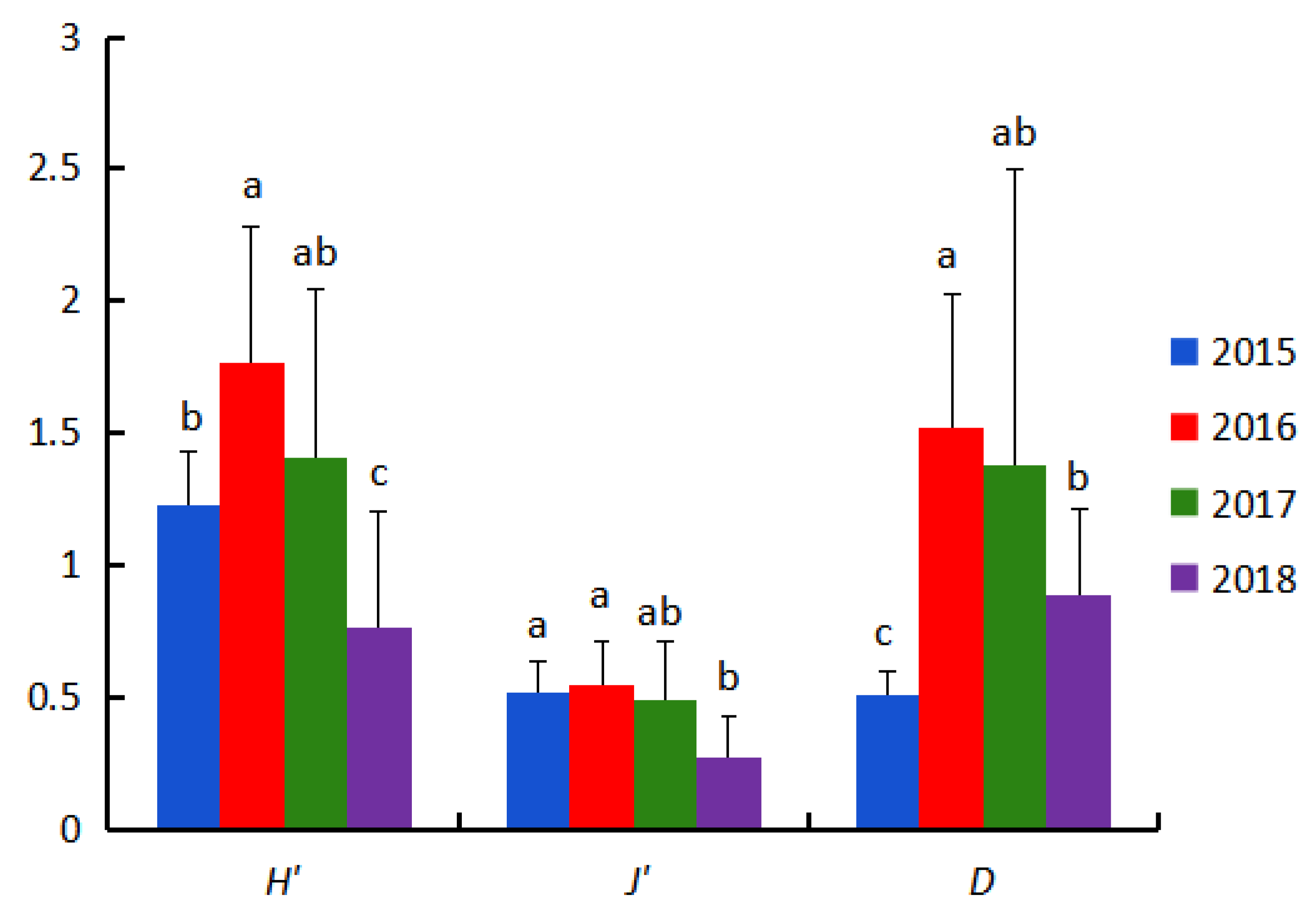
3.4. Community Structure Analysis
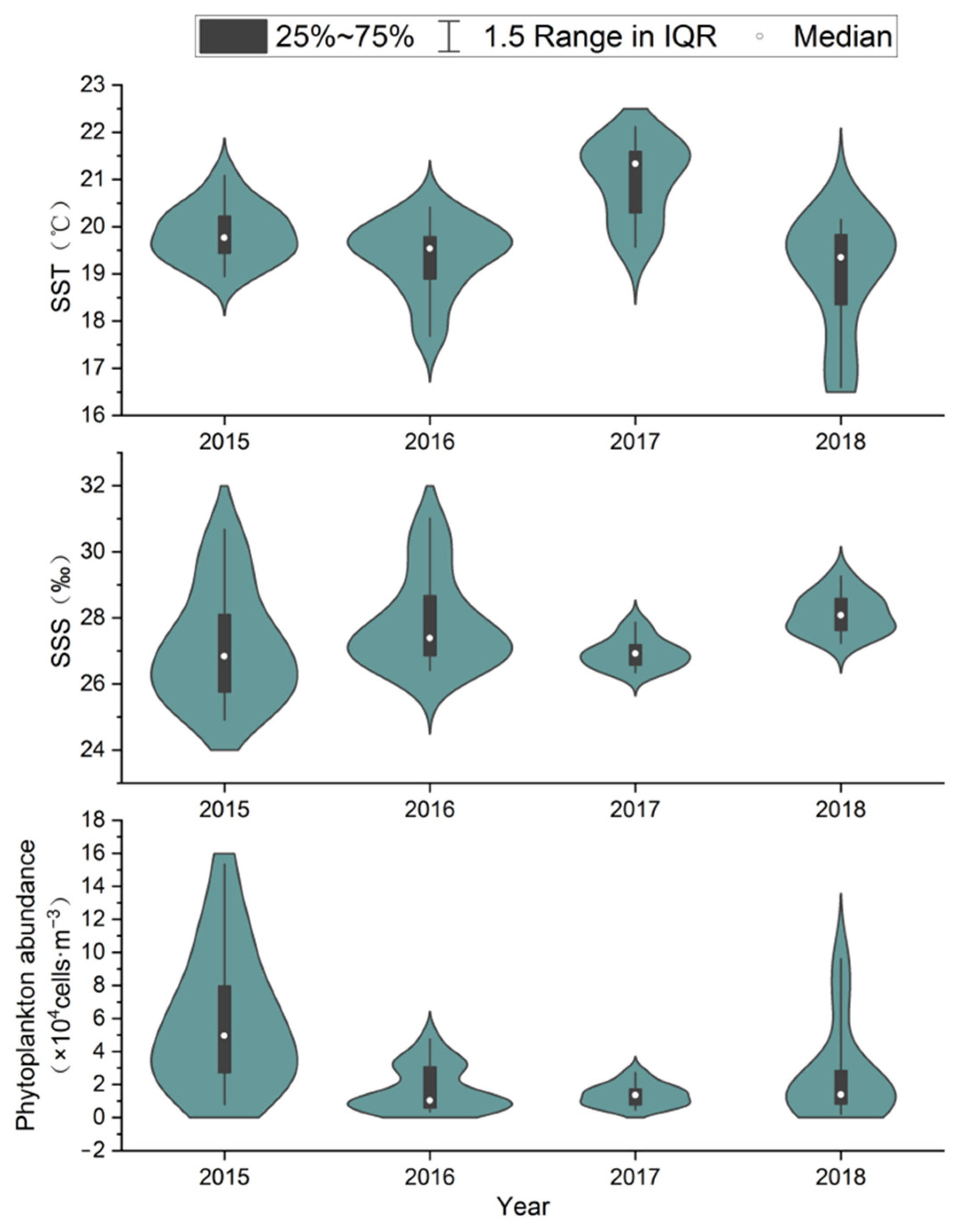
3.5. Analysis of Environmental Parameter
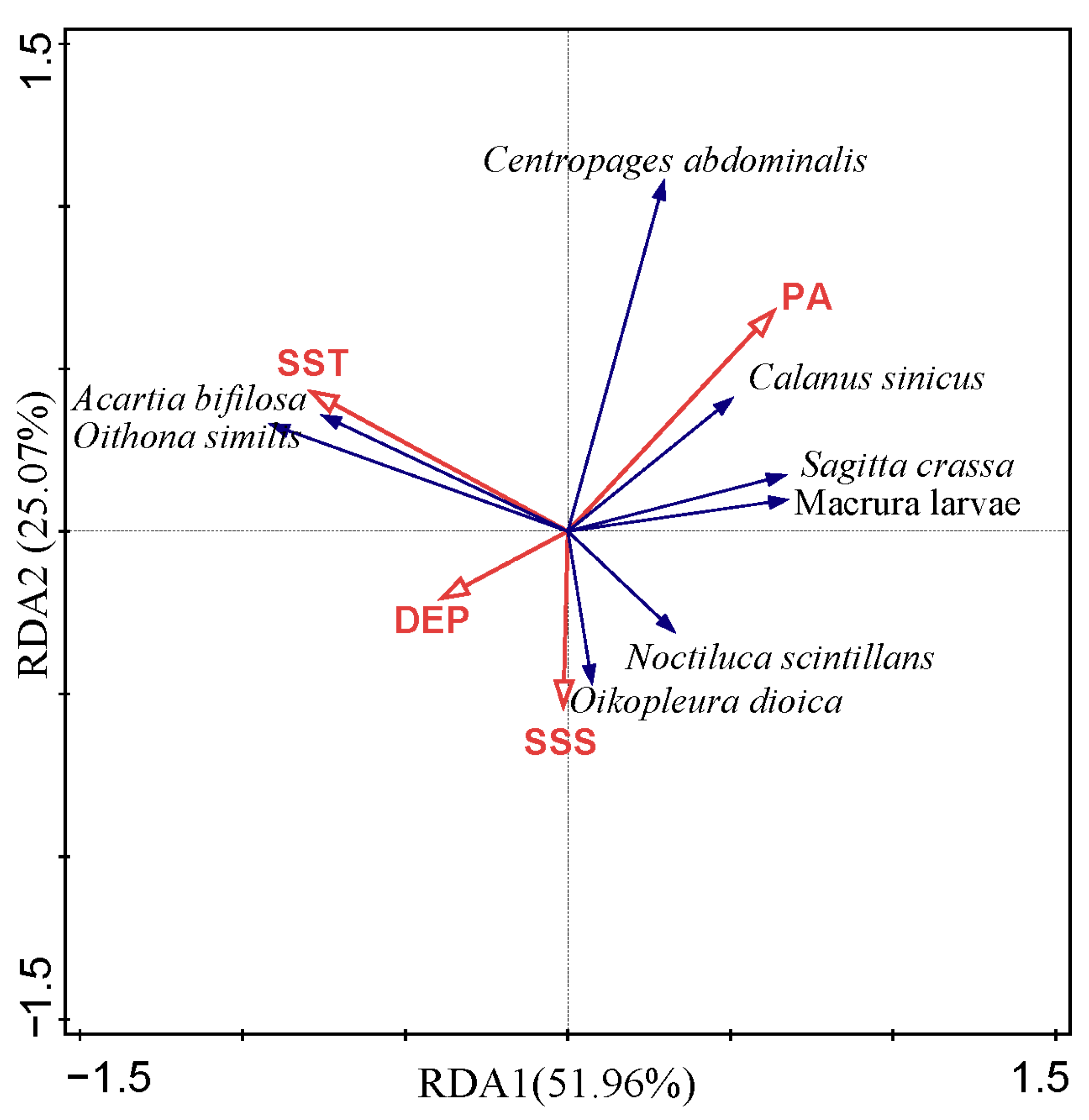
3.6. Relationships Between Zooplankton and Environmental Factors
4. Discussion
4.1. Species Composition
4.2. Horizontal Distribution and Annual Variation in Zooplankton
4.3. Biodiversity and Stability of Community Structure
4.4. Limitation Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sommer, U. Trophic cascades in marine and freshwater plankton. Int. Rev. Hydrobiol. 2008, 93, 506–516. [Google Scholar] [CrossRef]
- Beaugrand, G.; Brander, K.M.; Lindley, J.A.; Souissi, S.; Reid, P.C. Plankton effect on cod recruitment in the North Sea. Nature 2003, 426, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Zhu, M.M.; Song, Q.Q.; Wang, X.X.; Li, F.; Zhang, X.M.; Pang, Z.W.; Su, B. The water-sediment segulation scheme on the community structure of ichthyoplankton in the Yellow River estuary. Acta Hydrobiol. Sin. 2024, 48, 488–503. [Google Scholar]
- Hutchings, J.A.; Reynolds, J.D. Marine fish population collapses: Consequences for recovery and extinction risk. BioScience 2004, 54, 297–309. [Google Scholar] [CrossRef]
- Bian, X.D.; Wan, R.J.; Jin, X.S.; Shan, X.J.; Guan, L.S. Ichthyoplankton succession and assemblage structure in the Bohai Sea during the past 30 years since the 1980s. Prog. Fish. Sci. 2018, 39, 1–15. [Google Scholar]
- Zhang, Y.X.; Bian, X.D.; Shan, X.J.; Jin, X.S.; Wang, H.B. Community structure and suitable habitat for the early life stages of marine fish in the Yantai-Weihai offshore waters. Prog. Fish. Sci. 2022, 43, 148–167. [Google Scholar]
- Zhang, Y.Q.; Qiu, S.Y. A preliminary study on fishery resources in Shandong coastal areas. J. Yantai Univ. (Nat. Sci. Eng.) 2019, 32, 61–67+102. [Google Scholar]
- Zuo, T.; Wang, R.; Chen, Y.Q.; Gao, S.W.; Wang, K. Net macro-zooplankton community classification on the shelf area of the East China Sea and the Yellow Sea in spring and autumn. Acta Ecol. Sin. 2005, 25, 1531–1540. [Google Scholar]
- Yang, Q.; Wang, Z.L.; Fan, J.F.; Shao, K.S.; Li, H.J. Zooplankton diversity and its variation in the Northern Yellow Sea in the autumn and winter of 1959, 1982 and 2009. Acta Ecol. Sin. 2012, 32, 6747–6754. [Google Scholar] [CrossRef]
- Zou, Y.W.; Yang, Q.; Li, Q.B.; Liu, G.Z.; Guo, H.; Wang, Z.L. Community structure and variation of zooplankton in the Northern Yellow Sea. Mar. Environ. Sci. 2013, 32, 683–687. [Google Scholar]
- Jiang, H.C.; Liu, N.; Gao, J.Q.; Su, B.; Li, J.H.; He, J.L.; Liu, A.Y. Zooplankton community structure in Sishili Bay and its relationship with environmental factors. Acta Ecol. Sin. 2017, 37, 1318–1327. [Google Scholar] [CrossRef]
- Wang, R.; Gao, S.W.; Wang, K.; Zuo, T. Zooplankton indication of the Yellow Sea Warm Current in winter. J. Fish. China 2003, 27, 39–48. [Google Scholar]
- GB/T12763.6-2007; Specifications for Marine Surveys Part 6 Marine Biological Survey. China Standards Press: Beijing, China, 2007; pp. 41–44.
- Sun, R.Y. Principles of Animal Ecology, 2nd ed.; Beijing Normal University Publishing Group: Beijing, China, 1992; pp. 356–357. [Google Scholar]
- Yang, Z.; Ye, J.Q.; Yang, Q.; Guo, H. Zooplankton diversity and its relationships with environmental factors in the Liaohe estuary. Mar. Environ. Sci. 2020, 39, 25–30. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; Plymouth Marine Laboratory: Plymouth, UK, 2001; pp. 62–81. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER: Plymouth, UK, 2006. [Google Scholar]
- Morris, C. Multivariate analysis of ecological data using CANOCO 5, 2nd Edition. Afr. J. Range Forage Sci. 2015, 32, 289–290. [Google Scholar] [CrossRef]
- Tang, Q.S.; Ye, M.Z. Development and Protection of Fishery Resources in Shandong Coastal Waters; Agriculture Press: Beijing, China, 1990; p. 203. [Google Scholar]
- Lin, Y.; Zhou, Q.; Wang, H.J.; Liu, Y.L.; Qiu, J.K. Characteristics of zooplankton community structure and its response to environment factors in the Wenzhou coastal waters during spring and summer. Ocean. Dev. Manag. 2021, 38, 51–59. [Google Scholar]
- Yin, J.Q.; Huang, L.M.; Li, K.Z.; Lian, S.M.; Li, C.L.; Zhang, J.L. Effects of coastal current and upwelling on the distributions of Calanus sinicus on the northwest continental shelf of the South China Sea. Acta Oceanol. Sin. 2013, 35, 143–153. [Google Scholar]
- Bieri, R. The distribution of the planktonic Chaetognatha in the Pacific and their relationship to the water masses. Limnol. Oceanogr. 1959, 4, 1–28. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.R.; Chen, H.J.; Liu, G.X.; Zhuang, Y.Y. Community characteristics of zooplankton in the Yellow Sea and Bohai Sea in spring 2014. Mar. Sci. 2022, 46, 17–29. [Google Scholar]
- Huang, B.B.; Wu, F.X.; Zheng, S.X.; Tian, F.G.; He, J. Relationships between community structure of zooplankton and environmental factors in the east Leizhou Peninsula coastal area. J. Guangdong Ocean. Univ. 2022, 42, 53–61. [Google Scholar]
- Wang, X.X.; Gao, Y.J.; Zuo, M.; Zhang, X.M.; Li, S.W.; Yang, Y.Y.; Xu, B.Q.; Li, F.; Wang, Y.H. Interannual variation and influencing factors of zooplankton in the Yellow River estuary before and after water and sediment discharge regulation from 2011 to 2020. Mar. Sci. 2022, 46, 115–127. [Google Scholar]
- Jin, X.J.; Yuang, T.Z.; He, J.L.; Fu, P.; Song, X.K.; Sun, S.; Wang, L.M.; Yu, X.X.; You, L.P. Species composition, spatial and temporal distribution of zooplankton community in Miaodao Archipelago. Ocean. Dev. Manag. 2023, 40, 133–142. [Google Scholar]
- Li, Y.X.; Wang, R.R.; Wang, W.; Yin, Y.M.; Liu, Z.P.; Dai, Z.; Li, M.H.; Yan, Q.L. Study on the changes in the community structure of marine zooplankton in Dalian bay from 2013 to 2022 and its relationship with environmental factors. Mar. Environ. Sci. 2025, 44, 247–256. [Google Scholar]
- Gao, X.; Li, F.; Lv, Z.B.; Xu, B.Q.; Zhang, L.C. Study of nekton community structure in the offshore of Yantai and Weihai. Mar. Fish. 2019, 41, 179–187. [Google Scholar]
- Yan, L.P.; Hu, F.; Ling, J.Z.; Li, S.F. Study on age and growth of Larimichthys polyactis in the East China Sea. Period. Ocean. Univ. China (Nat. Sci. Ed.) 2006, 36, 95–100. [Google Scholar]
- Hu, C.L.; Zhang, H.L.; Zhang, Y.Z.; Pan, G.L.; Xu, K.D.; Bi, Y.X.; Liang, J.; Wang, H.X.; Zhou, Y.D. Fish community structure and its relationship with environmental factors in the Nature Reserve of Trichiurus japonicus. J. Fish. China 2018, 42, 39–48. [Google Scholar]
- Zhang, T.W.; Zhu, L.Y.; Xu, P.P.; Zhou, H.; Qi, B.J. Seasonal variation and distribution characteristics of noctiluca scintillans in Jiaozhou Bay. Period. Ocean. Univ. China (Nat. Sci. Ed.) 2009, 39, 89–93. [Google Scholar] [CrossRef]
- Sun, H.B.; Wang, X.H.; He, M.; Fang, D.A.; Zhou, T.P. Structure of zooplankton community in rugao section of Yangtze River and its relationship with water environmental factors. Wetl. Sci. 2023, 21, 555–563. [Google Scholar]
- Yang, G.; Li, F.; Lv, Z.B.; Xu, B.Q.; Yuan, X.N.; Wang, X.X.; Chen, W.J. Study on the community structure of crabs in the coastal waters along Shandong Peninsula. Haiyang Xuebao 2017, 39, 48–61. [Google Scholar]
- Liu, W.Y.; Xu, Y.L.; Tie, N.; Gao, R.H.; Zhang, Q.L. Study on the relationship between zooplankton diversity index and environmental factors in the salina wetland in Inner Mongolia. Guangdong Agric. Sci. 2013, 20, 148–150. [Google Scholar]
- Hochreiter, S.; Schmidhuber, J. Long short-term memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Liu, Y.M.; Sun, B.; Su, K.; Zhao, L.; Ji, Y.L. Community structure of zooplankton in the sea area near Rushan Bay in spring and autumn. J. Tianjin Norm. Univ. (Nat. Sci. Ed.) 2023, 43, 15–22+52. [Google Scholar]
- Wang, Z.H.; Wang, K.; Zhao, J.; Zhang, S.Y. Fish community structure and its seasonal change in subtidal sandy beach habitat off southern Gouqi Island. Chin. J. Appl. Ecol. 2011, 22, 1332–1342. [Google Scholar]
- Yang, G.; Li, F.; Wang, X.X.; Yuan, X.N.; Lv, Z.B.; Song, M.Y. Community structure of crabs in the coastal waters along the southern Shandong Peninsula. J. Fish. Sci. China 2017, 24, 862–874. [Google Scholar]
- Sun, S.; Li, J.H.; Jin, Y.; Ma, Y.Q.; Bai, Y.Y.; Tang, X.C.; Liu, Y.H.; Qin, H.W. Distribution features of nutrients and flux between the sediment and water interface in Sishili Bay. Mar. Environ. Sci. 2012, 31, 195–200. [Google Scholar]
- Jiang, J.J.; Liu, D.Y.; Di, B.P.; Dong, Z.J.; Wang, Y.J.; Wang, Y.Q.; Shi, Y.J. Seasonal changes of phytoplankton community and its indication of environment in Sishili Bay, Yantai. Acta Oceanol. Sin. 2011, 33, 151–164. [Google Scholar]
| Groups | Species | Year | |||
|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | ||
| Protozoa | Noctiluca scintillans | √ | √ | ||
| Cnidaria | Rathkea octopunctata | √ | √ | ||
| Euphysora bigelowi | √ | √ | |||
| Clytia folleata | √ | ||||
| Proboscidactyla flavicirrata | √ | ||||
| Obelia spp. | √ | ||||
| Cladocera | Evadne tergestina | √ | √ | ||
| Copepoda | Calanus sinicus | √ | √ | √ | √ |
| Paracalanus parvus | √ | √ | √ | √ | |
| Centropages abdominalis | √ | √ | √ | √ | |
| Eurytemora pacifica | √ | ||||
| Labidocera bipinnata | √ | √ | |||
| Acartia clausi | √ | ||||
| Acartia pacifica | √ | √ | |||
| Acartia bifilosa | √ | √ | √ | ||
| Oithona similis | √ | √ | √ | √ | |
| Corycaeus affinis | √ | √ | √ | ||
| Oithona brevicornis | √ | ||||
| Microsetella norvegica | √ | √ | |||
| Amphipoda | Themisto gracilipes | √ | √ | √ | √ |
| Decapoda | Leptochela gracilis | √ | |||
| Euphausiacea | Euphausia pacifica | √ | |||
| Chaetognatha | Sagitta crassa | √ | √ | √ | √ |
| Tunicata | Oikopleura dioica | √ | √ | ||
| Planktonic larvae | Polychaeta larvae | √ | √ | ||
| Gastropoda larvae | √ | √ | |||
| Bivalvia larvae | √ | √ | √ | √ | |
| Copepoda nauplius larvae | √ | √ | √ | ||
| Gammarus sp. | √ | √ | |||
| Macrura mysis larvae | √ | √ | √ | √ | |
| Macrura zoea larvae | √ | ||||
| Brachyura zoea larvae | √ | √ | √ | √ | |
| Ophiopluteus larvae | √ | √ | |||
| Fish eggs | √ | √ | √ | ||
| Fish larvae | √ | √ | √ | ||
| Dominance | Year | ||||
|---|---|---|---|---|---|
| Species | 2015 | 2016 | 2017 | 2018 | |
| Noctiluca scintillans | - | 0.068 | - | 0.301 | |
| Calanus sinicus | 0.457 | 0.441 | 0.649 | 0.231 | |
| Centropages abdominalis | 0.437 | 0.173 | 0.130 | - | |
| Acartia bifilosa | - | - | 0.044 | - | |
| Oithona similis | - | - | 0.069 | - | |
| Sagitta crassa | 0.066 | - | - | - | |
| Oikopleura dioica | - | 0.033 | - | - | |
| Macrura mysis larvae | - | - | - | 0.022 | |
| Year | Species | Abundance (ind./m3) | Biomass (mg/m3) |
| 2015 | 5.44 ± 1.17 b | 594.36 ± 541.43 a | 290.53 ± 235.52 a |
| 2016 | 9.94 ± 2.44 a | 167.35 ± 194.85 b | 200.31 ± 163.59 ab |
| 2017 | 7.83 ± 2.76 ab | 118.73 ± 77.08 b | 72.08 ± 67.7 b |
| 2018 | 7.25 ± 2.05 ab | 464.59 ± 717.34 ab | 183.82 ± 155.85 ab |
| Axis | Eigenvalue | Pseudo-Canonical Correlation | Cumulative Variable Percentage (%) | Sum of All Eigenvalues | Sum of All Canonical Eigenvalues | |
|---|---|---|---|---|---|---|
| Explained Variation (Cumulative) | Explained Fitted Variation (Cumulative) | |||||
| Axis1 | 0.1170 | 0.6175 | 11.70 | 51.96 | 1.000 | 0.2250 |
| Axis2 | 0.0564 | 0.5080 | 17.34 | 77.03 | ||
| Axis3 | 0.0331 | 0.5388 | 20.65 | 91.76 | ||
| Axis4 | 0.0185 | 0.4102 | 22.51 | 100.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhu, M.; Xu, B.; Yang, Y.; Zhang, X.; Li, S.; Wang, T.; Li, F.; Cui, G.; Zheng, X. Annual Variations and Influencing Factors of Zooplankton Community Structure in the Coastal Waters of Northern Shandong Peninsula, China. Biology 2025, 14, 1386. https://doi.org/10.3390/biology14101386
Wang X, Zhu M, Xu B, Yang Y, Zhang X, Li S, Wang T, Li F, Cui G, Zheng X. Annual Variations and Influencing Factors of Zooplankton Community Structure in the Coastal Waters of Northern Shandong Peninsula, China. Biology. 2025; 14(10):1386. https://doi.org/10.3390/biology14101386
Chicago/Turabian StyleWang, Xiuxia, Mingming Zhu, Bingqing Xu, Yanyan Yang, Xiaomin Zhang, Shaowen Li, Tiantian Wang, Fan Li, Guangxin Cui, and Xiang Zheng. 2025. "Annual Variations and Influencing Factors of Zooplankton Community Structure in the Coastal Waters of Northern Shandong Peninsula, China" Biology 14, no. 10: 1386. https://doi.org/10.3390/biology14101386
APA StyleWang, X., Zhu, M., Xu, B., Yang, Y., Zhang, X., Li, S., Wang, T., Li, F., Cui, G., & Zheng, X. (2025). Annual Variations and Influencing Factors of Zooplankton Community Structure in the Coastal Waters of Northern Shandong Peninsula, China. Biology, 14(10), 1386. https://doi.org/10.3390/biology14101386






