Seasonal Spatial Distribution of Metapenaeopsis provocatoria longirostris in the Southern Yellow and East China Seas and Habitat Area Variation Prediction Under Climate Scenarios
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
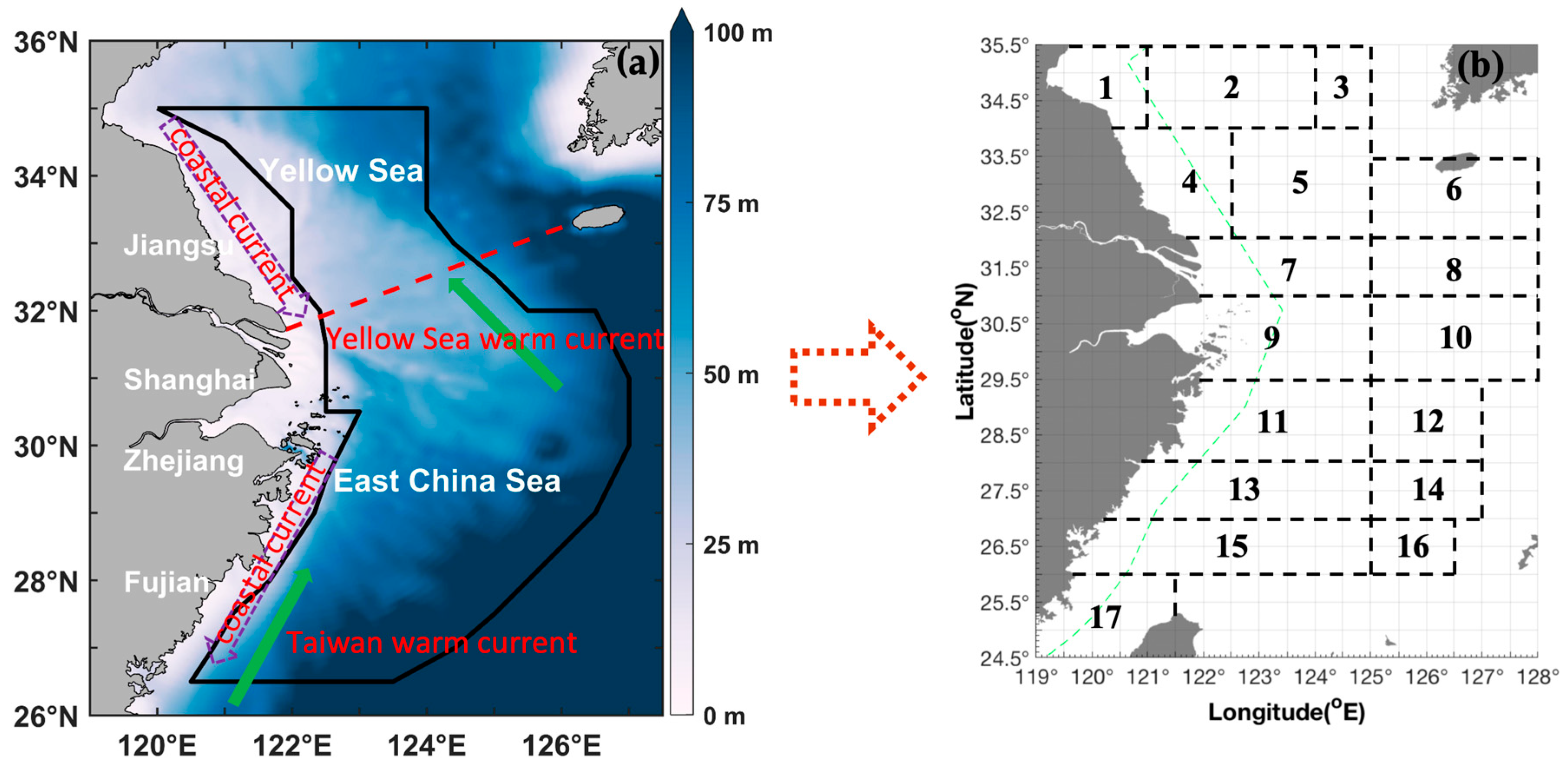
2.1. Geographic Characteristics of the Study Region and Survey Procedures
2.2. Ensemble Model and Climate Scenarios
3. Results and Discussion
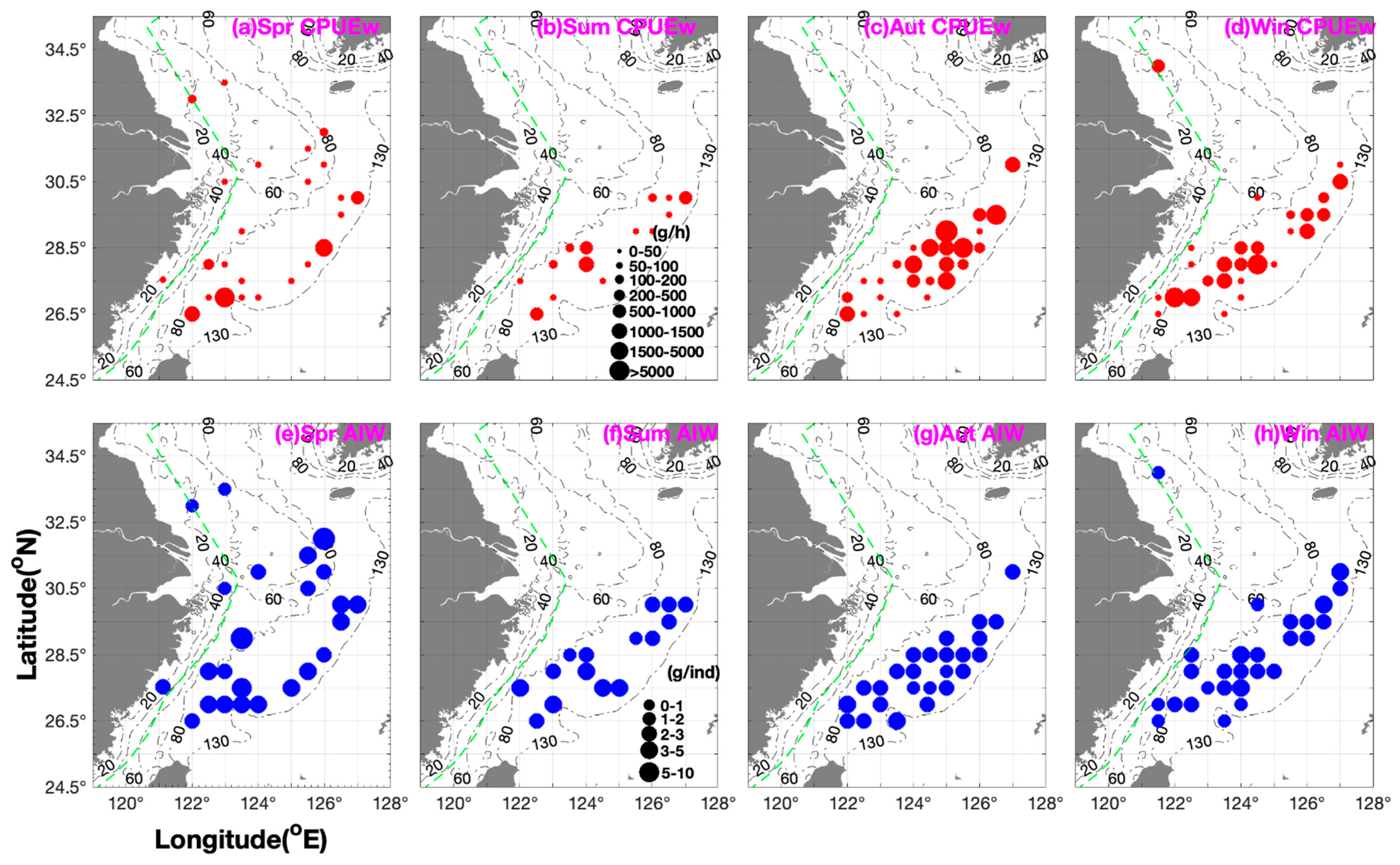
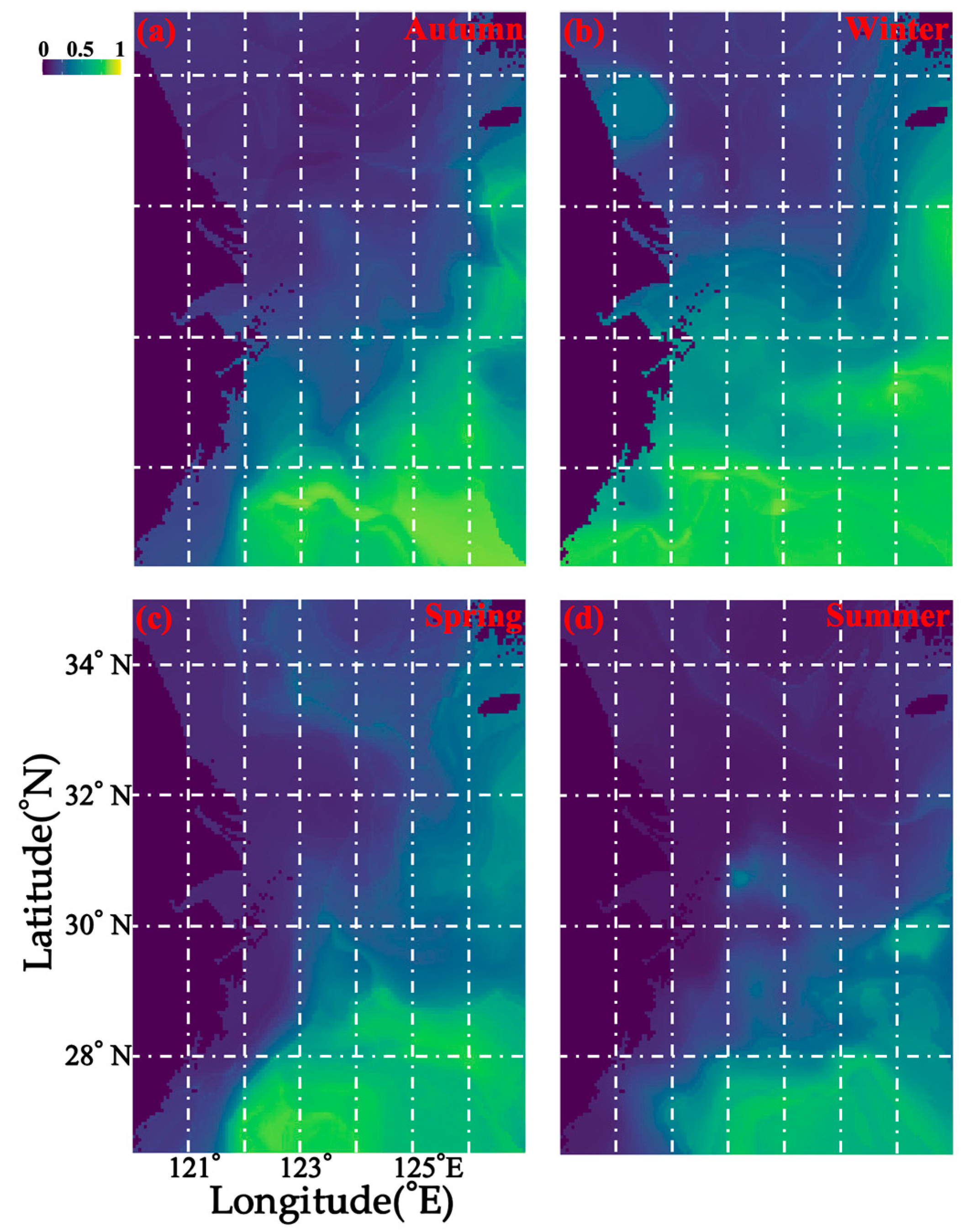
3.1. Seasonal Distribution and Characteristics of M. provocatoria longirostris
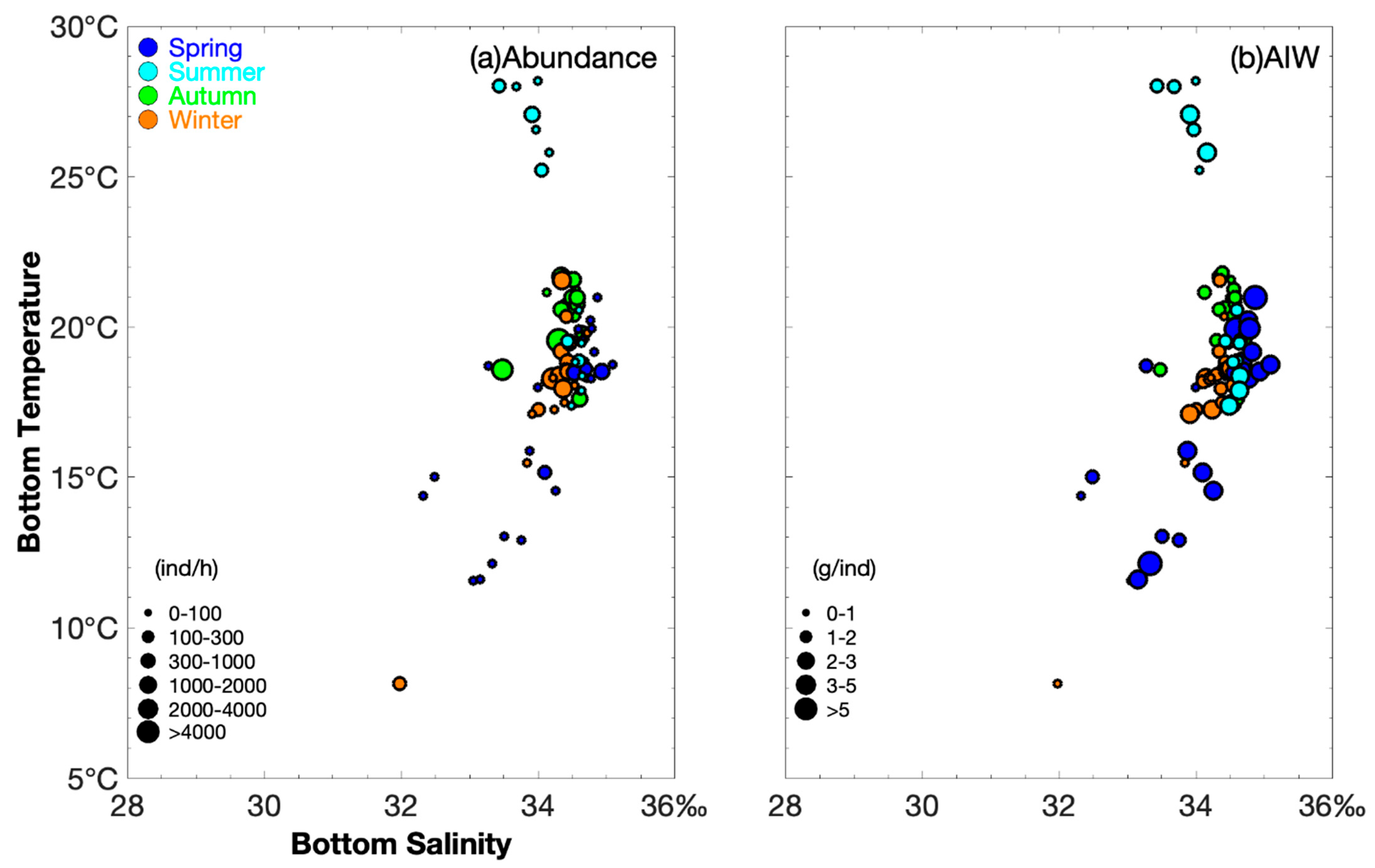
3.2. Seasonal Variations in Biomass and Abundance Under Varying Environmental Conditions
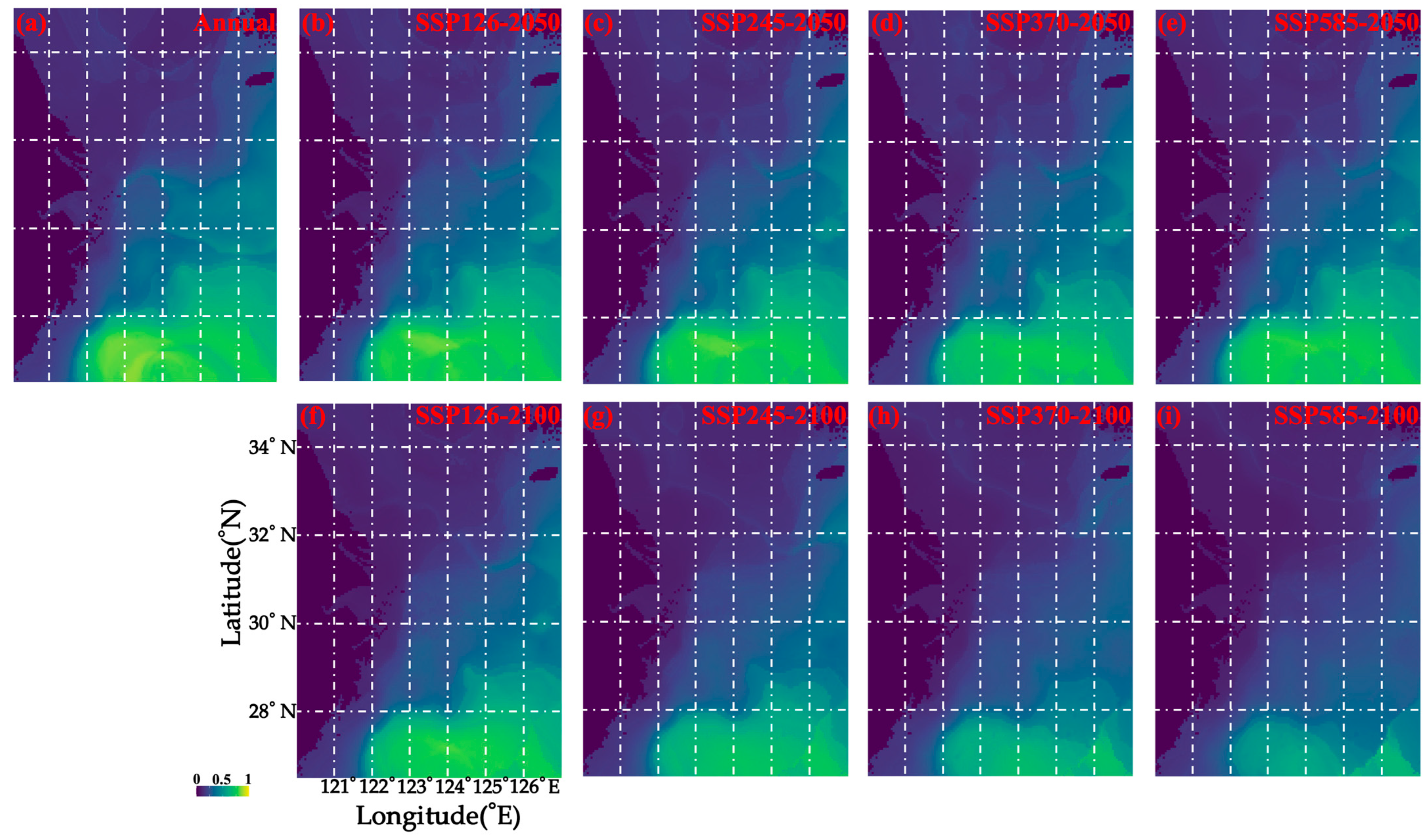
3.3. Prediction of Habitat Loss Under Different Climate Change Scenarios and Across Seasons
3.4. Resource Conservation and Fisheries Management Strategies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC (Intergovernmental Panel on Climate Change). Summary for policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013; pp. 1–30. [Google Scholar]
- Belkin, I.M.; Lee, M.A. Long-term variability of sea surface temperature in Taiwan strait. Clim. Change 2014, 124, 821–834. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Z.N.; Liu, X.S.; Hua, E. Decadal changes in sublittoral macrofaunal biodiversity in the Bohai Sea, China. Mar. Pollut. Bull. 2012, 64, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.A.; Jiang, Y.; Liu, M. Population structure and reproductive dynamics of the ridged swimming crab Charybdis natator in the southern Taiwan Strait of China: Significant changes within 25 years. Front. Mar. Sci. 2023, 10, 1056640. [Google Scholar] [CrossRef]
- Kang, B.; Liu, M.; Huang, X.X.; Li, J.; Yan, Y.R.; Han, C.C.; Chen, S.B. Fisheries in Chinese seas: What can we learn from controversial official fisheries statistics? Rev. Fish Biol. Fish. 2018, 28, 503–519. [Google Scholar] [CrossRef]
- Liu, M.; Lin, B. Chinese Red Swimming Crab (Monomia haanii) Fishery Improvement Project (FIP) in Zhangzhou City, Fujian Province, China (January–December 2019); Xiamen University: Xiamen, China, 2020; p. 93. [Google Scholar]
- Green, B.S.; Gardner, C.; Hochmuth, J.D.; Linnane, A. Environmental effects on fished lobsters and crabs. Rev. Fish Biol. Fish. 2014, 24, 613–638. [Google Scholar] [CrossRef]
- Shields, J.D. Reproductive ecology and fecundity of cancer crabs. In Crustacean Egg Production, Crustacean Issues; Wenner, A., Kuris, A., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1991; Volume 7, pp. 193–213. [Google Scholar]
- De Young, C. (Ed.) Review of the State of World Marine Capture Fisheries Management: Indian Ocean; FAO Fisheries Technical Paper No. 488; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2006; pp. 1–458. [Google Scholar]
- De Grave, S.; Fransen, C. Carideorum Catalogus: The Recent Species of the Dendrobranchiate, Stenopodidean, Procarididean and Caridean Shrimps (Crustacea: Decapoda). Zool. Meded. 2011, 85, 195–589. [Google Scholar]
- Chen, H.S.; Chen, C.Y.; Chen, M.H. Life history tactics of southern velvet shrimp Metapenaeopsis palmensis (Crustacea, Decapoda) in the waters off southwestern Taiwan. Hydrobiologia 2014, 741, 177–191. [Google Scholar] [CrossRef]
- Rahman, M.; Ohtomi, J. Reproductive biology of the deep-water velvet shrimp Metapenaeopsis sibogae (De Man, 1907)(Decapoda: Penaeidae). J. Crustac. Biol. 2017, 37, 743–752. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ohtomi, J. Biometric relationships of the deep-water velvet shrimp Metapenaeopsis sibogae (De Man, 1907)(Crustacea: Decapoda: Penaeidae) in Kagoshima Bay, Japan. Zool. Ecol. 2018, 28, 365–375. [Google Scholar] [CrossRef]
- Tzeng, T.; Chiu, C.; Yeh, S. Growth and mortality of the red-spot prawn (Metapenaeopsis barbata) in the northeastern coast off Taiwan. J. Fish. Soc. Taiwan 2005, 32, 229. [Google Scholar]
- Muralidharan, A.; Chakraborty, R.D. Insights into ovarian maturation and reproductive traits of the deep-water penaeid shrimp Metapenaeopsis andamanensis (Wood-Mason in Wood-Mason and Alcock, 1891) from southwestern India. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2024, 209, 104345. [Google Scholar] [CrossRef]
- Song, H.; Xue, L.; He, Z. Study on the fishery biological characteristics of Metapenaeopsis longirostris in the East China Sea. Mar. Fish. Res. 2008, 29, 9–14. (In Chinese) [Google Scholar]
- Mohale, H.P.; Jawahar, P.; Jayakumar, N.; Karuppasamy, K.; Narsale, S.A.; Kadam, R.V.; Gavhane, S.V. Fishery, Biology, and Dynamics of the Metapenaeopsis andamanensis (Wood-Mason, 1891) from the Southeast Coast of Tamil Nadu. Thalass. Int. J. Mar. Sci. 2025, 41, 1–16. [Google Scholar] [CrossRef]
- Muralidharan, A.; Chakraborty, R.D.; Chakraborty, K.; Dhara, S. Trophic ecology and diet of the deep-sea penaeid shrimp Metapenaeopsis andamanensis (Wood-Mason in Wood-Mason and Alcock, 1891) by fatty acid signatures and stomach content analysis. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2023, 200, 104135. [Google Scholar] [CrossRef]
- Costalago, D.; Navarro, J.; Alvarez-Calleja, I.; Palomera, I. Ontogenetic and seasonal changes in the feeding habits and trophic levels of two small pelagic fish species. Mar. Ecol. Prog. Ser. 2012, 460, 169–181. [Google Scholar] [CrossRef]
- Chandravanshi, S.; Jayakumar, N.; Durairaja, R.; Aanand, S.; Suresh, E. Feeding aspects and reproductive biology of Smalltooth emperor, Lethrinus microdon (Valenciennes, 1830) in the Gulf of Mannar along the Indian Southeast Coast. Indian J. Anim. Res. 2024, 1, 1–10. [Google Scholar] [CrossRef]
- Fang, J.; Li, Y.; Sun, S.; Deng, J. Analysis of runoff change characteristics at Datong station of Yangtze River. Water Resour. Power 2011, 29, 9–12. [Google Scholar]
- Xu, M.; Liu, Y.; Song, X.; Yang, L. Changes in Seasonal Spatial Distribution Patterns of Euprymna berryi and Euprymna morsei: The Current and Predictions Under Climate Change Scenarios. Biology 2025, 14, 327. [Google Scholar] [CrossRef]
- Yang, L.; Xu, M.; Cui, Y.; Liu, S. Seasonal spatial distribution patterns of AmphiOctopus ovulum in the East China Sea: Current status future projections under various climate change scenarios. Front. Mar. Sci. 2025, 12, 1573253. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Liu, S.; Tian, Y.; Liu, Y.; Alabia, I.D.; Cheng, J.; Ito, S. Development of a prey-predator species distribution model for a large piscivorous fish: A case study for Japanese Spanish mackerel Scomberomorus niphonius and Japanese anchovy Engraulis japonicus. Deep. Sea Res. Part II 2023, 207, 105227. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, J.N.; Ma, C.W.; Cha, H.K. Growth and reproduction of the kishi velvet shrimp, Metapenaeopsis dalei (Rathbun, 1902) (Decapoda, Penaeidae) in the western sea of Korea. Crustaceana 2005, 78, 947–963. [Google Scholar] [CrossRef]
- Cao, L.; Chen, Y.; Dong, S.; Hanson, A.; Huang, B.O.; Leadbitter, D.; Little, D.C.; Pikitch, E.K.; Qiu, Y.; Sadovy de Mitcheson, Y.; et al. Opportunity for marine fisheries reform in China. Proc. Natl. Acad. Sci. USA 2017, 114, 435–442. [Google Scholar] [CrossRef]
- Liang, C.; Pauly, D. Growth and mortality of exploited fishes in China’s coastal seas and their uses for yield-per-recruit analyses. J. Appl. Ichthyol. 2017, 33, 746–756. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, M.; Sadovy de Mitcheson, Y.; Cao, L.; Leadbitter, D.; Newton, R.; Little, D.; Li, S.; Yang, Y.; Chen, X.; et al. Fishing for feed in China: Facts, impacts and implications. Fish Fish. 2020, 21, 47–62. [Google Scholar] [CrossRef]
- Yang, B.; Herrmann, B.; Yan, L.; Li, J.; Wang, T. Comparing size selectivity and exploitation pattern of diamond-mesh codends for southern velvet shrimp (Metapenaeopsis palmensis) in shrimp trawl fishery of the south China Sea. PeerJ 2021, 9, e12436. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
| Mean Value | Total Value | Environmental Variable | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | B% | N | N% | AIW | B | B% | N | N% | AIW | AIW% | SBT (°C) | SBS (‰) | Depth (m) | |
| Spring | ||||||||||||||
| (5) | 36.6 | 3.3% | 47.3 | 8.9% | 0.7 | 73.2 | 1.5% | 94.7 | 4.3% | 1.5 | 0.8% | 11.6–14.4 | 32.3–33.1 | 20–45 |
| (8) | 37.2 | 3.4% | 5.4 | 1% | 5.5 | 74.5 | 1.5% | 10.8 | 0.5% | 11 | 6% | 11.6–12.1 | 33.2–33.3 | 55–81 |
| (9) | 16.1 | 1.4% | 10 | 1.9% | 1.4 | 32.2 | 0.7% | 20 | 0.9% | 2.7 | 1.5% | 15–18 | 32.5–34 | 41–49 |
| (10) | 98.8 | 8.9% | 45.5 | 8.6% | 2 | 395.2 | 8.1% | 182.1 | 8.3% | 7.8 | 4.3% | 12.9–15.2 | 33.5–34.3 | 65–97 |
| (12) | 404.3 | 36.4% | 218 | 41% | 4.2 | 1213 | 24.8% | 653.9 | 29.8% | 12.7 | 6.9% | 15.9–19.9 | 33.9–34.6 | 74–107 |
| (13) | 41.6 | 3.7% | 19.8 | 3.7% | 2.4 | 249.6 | 5.1% | 119 | 5.4% | 14.2 | 7.7% | 18.6–20.2 | 33.3–34.8 | 40–104 |
| (15) | 476.7 | 42.9% | 185.7 | 34.9% | 2.5 | 2860 | 58.4% | 1114 | 50.8% | 134.2 | 72.9% | 18.3–21 | 34.7–35.1 | 98–140 |
| Summer | ||||||||||||||
| (10) | 177 | 23.7% | 98 | 20.4% | 1.7 | 531.1 | 24% | 294.1 | 22.4% | 5.2 | 19.9% | 18.9–20.6 | 34.6 | 77–97 |
| (11) | 242.4 | 32.4% | 201.8 | 42.1% | 1.1 | 484.7 | 21.9% | 403.6 | 30.7% | 2.1 | 8.1% | 25.2–28 | 33.4–34.1 | 70–84 |
| (12) | 14.8 | 2% | 7.8 | 1.6% | 1.6 | 44.4 | 2% | 23.4 | 1.8% | 4.8 | 18% | 18.8–28.2 | 33.7–34.5 | 10–117 |
| (13) | 175 | 23.4% | 82.7 | 17.2% | 2.1 | 875.2 | 39.6% | 413.7 | 31.5% | 10.3 | 39.1% | 17.9–27.1 | 33.9–34.6 | 75–101 |
| (15) | 138.5 | 18.5% | 89.5 | 18.7% | 2 | 276.9 | 12.5% | 179 | 13.6% | 3.9 | 14.9% | 17.4–19.5 | 34.4–34.5 | 104–117 |
| Autumn | ||||||||||||||
| (12) | 3665.1 | 86.5% | 3161.6 | 86.9% | 1.4 | 36,650.8 | 88.8% | 31,615.6 | 89% | 13.5 | 38% | 18.5–21.7 | 33.5–34.6 | 82–107 |
| (13) | 406.1 | 9.6% | 342.7 | 9.4% | 1.2 | 3655.2 | 8.9% | 3084 | 8.7% | 10.6 | 29.8% | 18.5–21.8 | 34.4–34.6 | 83–105 |
| (15) | 164.7 | 3.9% | 136 | 3.7% | 1.9 | 988.4 | 2.4% | 816 | 2.3% | 11.4 | 32.2% | 17.5–19.9 | 34.5–34.7 | 85–135 |
| Winter | ||||||||||||||
| (4) | 299.2 | 12.5% | 300 | 16.4% | 1 | 8.1 | 32 | 15 | ||||||
| (10) | 186 | 7.8% | 123.9 | 6.8% | 1.7 | 744.2 | 6% | 495.7 | 5.2% | 7 | 18.1% | 15.5–19.2 | 33.8–34.3 | 55–100 |
| (11) | 245.3 | 10.3% | 118.7 | 6.5% | 1.9 | 735.8 | 5.9% | 356.2 | 3.8% | 5.8 | 15.2% | 17.1–17.3 | 33.9–34 | 64–92 |
| (12) | 303.5 | 12.7% | 219 | 12% | 1.6 | 1517.3 | 12.2% | 1095.2 | 11.6% | 7.8 | 20.2% | 17.5–18.8 | 34.4–34.6 | 88–114 |
| (13) | 652.7 | 27.4% | 563.9 | 30.9% | 1.5 | 5221.8 | 42.1% | 4511.3 | 47.7% | 12.1 | 31.5% | 18.2–20.4 | 34.1–34.5 | 80–107 |
| (15) | 699.7 | 29.3% | 498.7 | 27.3% | 1 | 4198 | 33.8% | 2992 | 31.7% | 5.7 | 15% | 17.9–21.6 | 34.2–34.7 | 69–145 |
| Factor | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| Mean CPUEw at collection stations (g·h−1) | 195.91 | 147.49 | 1651.78 | 470.98 |
| Value range of CPUEw (g·h−1) | 2–2012 | 1.4–693.6 | 2.8–27,686.26 | 2.8–3099.43 |
| Mean CPUEn at collection stations (ind·h−1) | 87.78 | 87.59 | 1420.62 | 361.13 |
| Value range of CPUEn (ind·h−1) | 0.92–788 | 1–324 | 1–24,575.88 | 4–2674.29 |
| Mean AIW (g·ind−1) | 2.6 | 1.76 | 1.42 | 1.46 |
| Value range of AIW (g·ind−1) | 0.7–8.7 | 0.8–2.4 | 0.7–2.8 | 0.35–2.75 |
| Factor | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| SST (°C) | 13.6–26 | 26.1–29 | 21.9–26.3 | 8.1–22.3 |
| SSS (‰) | 30.5–34.6 | 31.7–34.1 | 33.3–34.4 | 31.9–34.5 |
| SBT (°C) | 11.6–21 | 17.4–28.2 | 17.5–21.8 | 8.1–21.6 |
| SBS (‰) | 32.3–35.1 | 33.4–34.6 | 33.5–34.7 | 32–34.7 |
| Depth (m) | 20–140 | 10–117 | 82–135 | 15–145 |
| Case | Loss% | Gain% | Gain% − Loss% |
|---|---|---|---|
| SSP126–2050 | −1.67% | 0.37% | −1.3% |
| SSP126–2100 | −13.84% | 0% | 13.84% |
| SSP245–2050 | −3.02% | 0.12% | −2.91% |
| SSP245–2100 | −30.15% | 0% | −30.15% |
| SSP370–2050 | −14.44% | 0% | −14.45% |
| SSP370–2100 | −54.41% | 0% | −54.41% |
| SSP585–2050 | −3.16% | 0% | −3.16% |
| SSP585–2100 | −72.34% | 0% | −72.34% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Liu, Y.; Zhang, H.; Ling, J.; Li, H. Seasonal Spatial Distribution of Metapenaeopsis provocatoria longirostris in the Southern Yellow and East China Seas and Habitat Area Variation Prediction Under Climate Scenarios. Biology 2025, 14, 1328. https://doi.org/10.3390/biology14101328
Xu M, Liu Y, Zhang H, Ling J, Li H. Seasonal Spatial Distribution of Metapenaeopsis provocatoria longirostris in the Southern Yellow and East China Seas and Habitat Area Variation Prediction Under Climate Scenarios. Biology. 2025; 14(10):1328. https://doi.org/10.3390/biology14101328
Chicago/Turabian StyleXu, Min, Yong Liu, Hui Zhang, Jianzhong Ling, and Huiyu Li. 2025. "Seasonal Spatial Distribution of Metapenaeopsis provocatoria longirostris in the Southern Yellow and East China Seas and Habitat Area Variation Prediction Under Climate Scenarios" Biology 14, no. 10: 1328. https://doi.org/10.3390/biology14101328
APA StyleXu, M., Liu, Y., Zhang, H., Ling, J., & Li, H. (2025). Seasonal Spatial Distribution of Metapenaeopsis provocatoria longirostris in the Southern Yellow and East China Seas and Habitat Area Variation Prediction Under Climate Scenarios. Biology, 14(10), 1328. https://doi.org/10.3390/biology14101328






