Effects of Water Temperature and Photoperiod on the Antioxidant Status and Intestinal Microbiota in Larval Spotted Mandarin Fish, Siniperca scherzeri, in the Yalu River
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Breeding Management
2.2. Ethical Approval
2.3. Analysis of Oxidative Stress Parameters
2.4. Extraction and Sequencing of Microbial DNA
2.5. Processing of Sequencing Data
2.6. Statistical Analyses
3. Results
3.1. Activities of Antioxidant Enzymes
3.2. Evaluation of Richness and Diversity of Bacterial Communities in Fish Guts
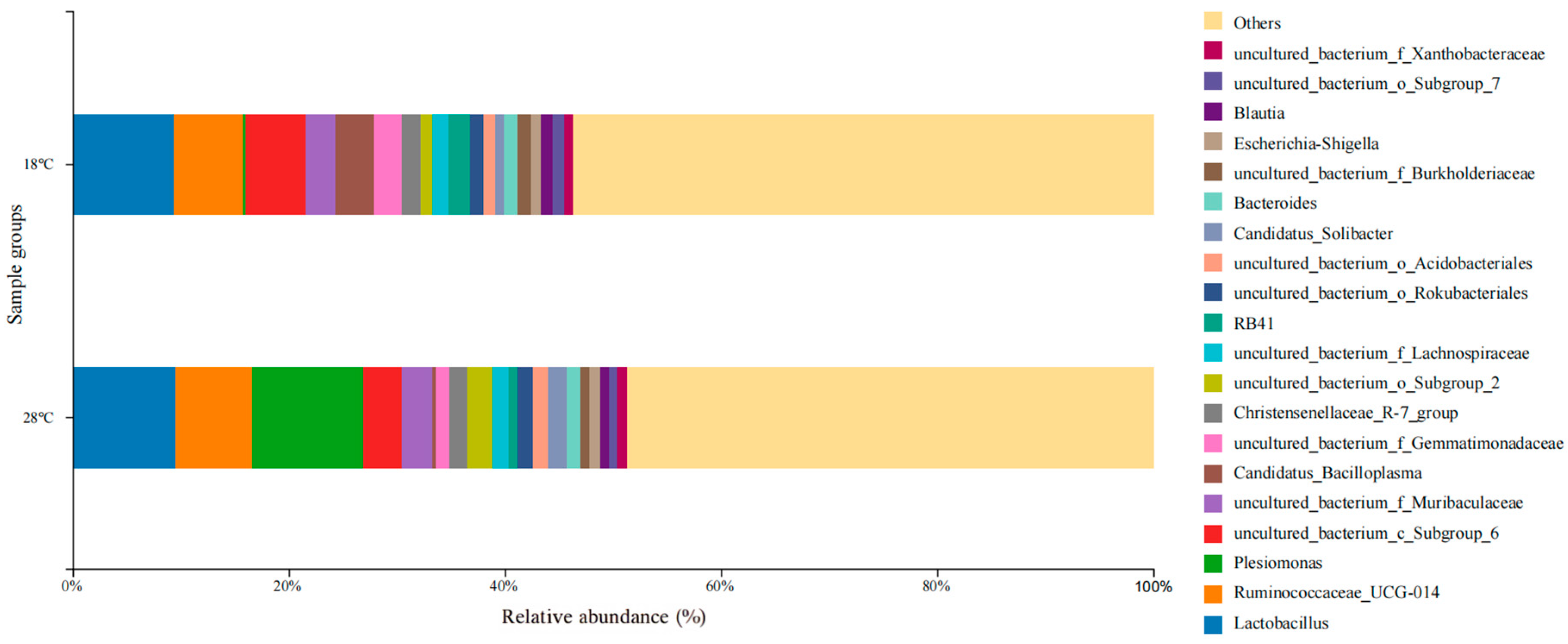
3.3. Composition, Relevance, and Species Abundance of Microbial Communities in Samples
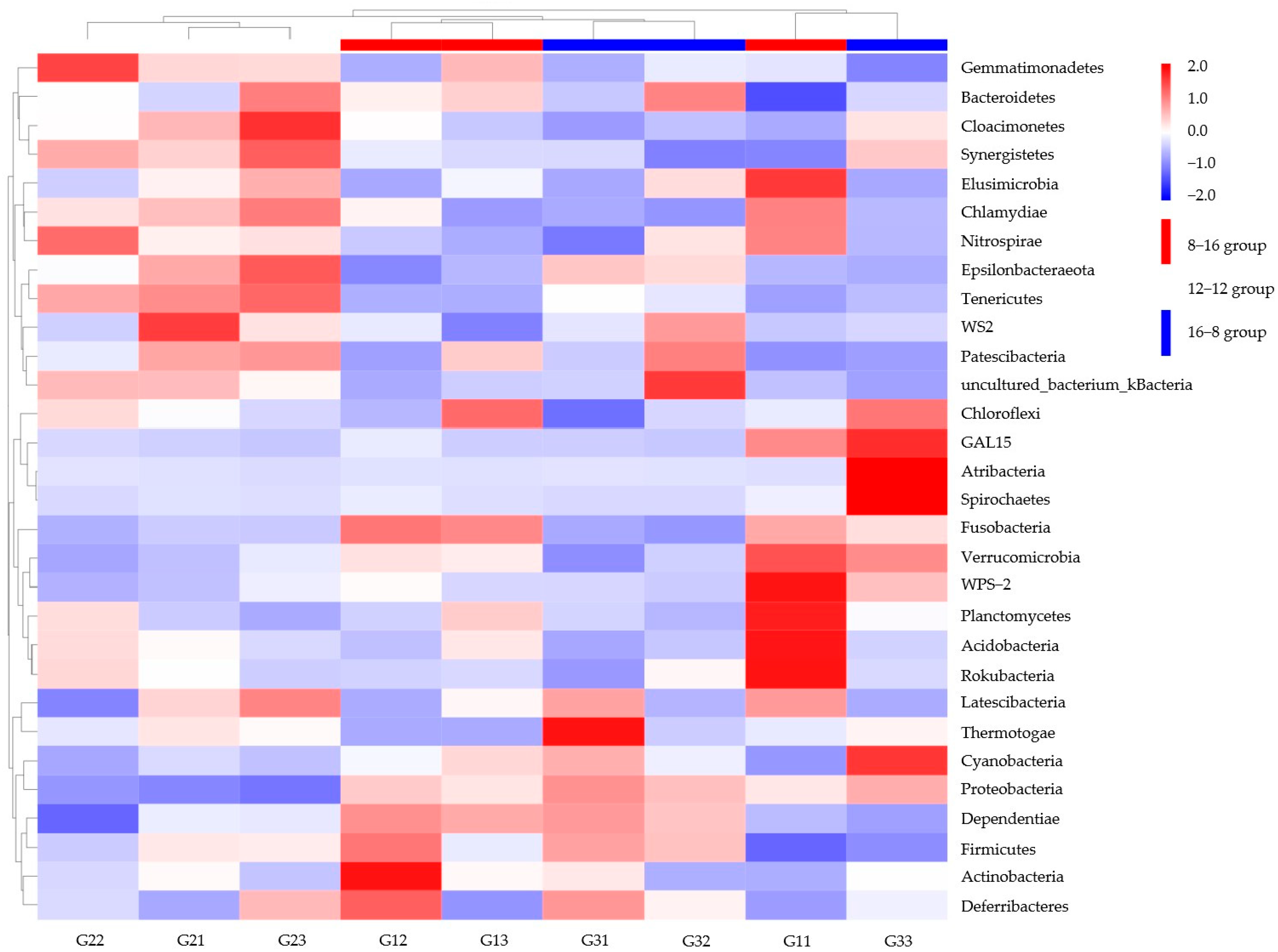
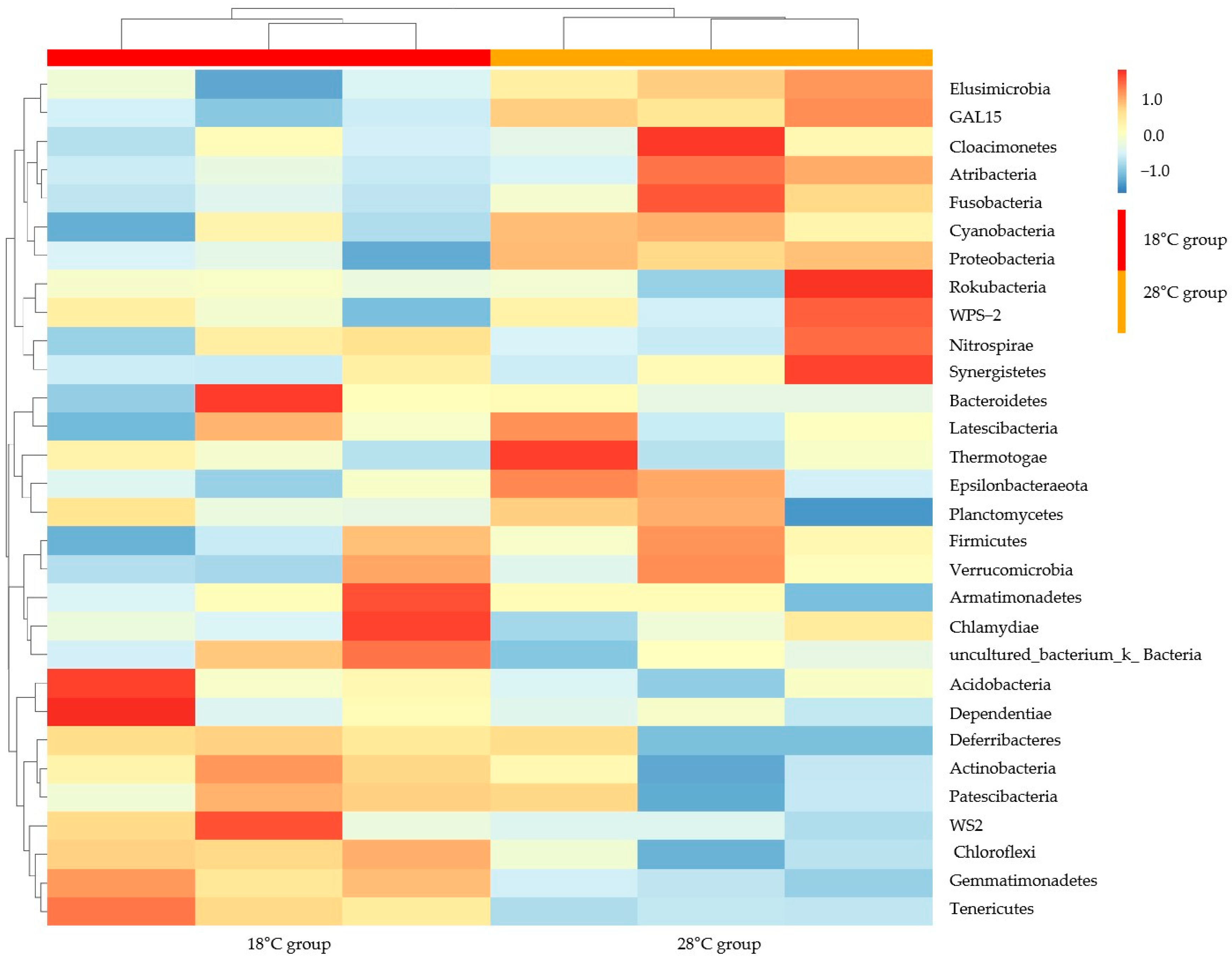
3.4. Similarity Analysis for Microbial Populations in Photoperiod Samples and Water Temperature Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, C.; Choi, J.; Choi, Y.; Kim, B.; Kim, J. Effects of green wavelength light on antioxidant and non-specific immune responses of the olive flounder Paralichthys olivaceus maintained at different stocking densities. Aquac. Eng. 2019, 84, 23–28. [Google Scholar] [CrossRef]
- Wen, X.; Chu, P.; Xu, J.; Wei, X.; Fu, D.; Wang, T.; Yin, S. Combined effects of low temperature and salinity on the immune response, antioxidant capacity and lipid metabolism in the pufferfish (Takifugu fasciatus). Aquaculture 2021, 531, 735866. [Google Scholar] [CrossRef]
- Sepulveda, J.; Moeller, A. The effects of temperature on animal gut microbiomes. Front. Microbiol. 2020, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Mccarthy, I.; Moksness, E.; Pavlov, D. The effects of temperature on growth rate and growth efficiency of juvenile common wolffish. Aquac. Int. 1998, 6, 207–218. [Google Scholar] [CrossRef]
- Booth, D.J.; Poulos, D.E.; Poole, J.; Feary, D.A. Growth and temperature relationship for juvenile fish species in seagrass beds: Implications of climate change. J. Fish Biol. 2014, 84, 231–236. [Google Scholar] [CrossRef]
- Symons, C.; Schulhof, M.; Cavalheri, H.; Shurin, J. Antagonistic effects of temperature and dissolved organic carbon on fish growth in California mountain lakes. Oecologia 2018, 189, 231–241. [Google Scholar] [CrossRef]
- Bartolini, T.; Butail, S.; Porfiri, M. Temperature influences sociality and activity of freshwater fish. Environ. Biol. Fishes 2015, 98, 825–832. [Google Scholar] [CrossRef]
- Herbing, I. Effects of temperature on larval fish swimming performance: The importance of physics to physiology. J. Fish Biol. 2002, 61, 865–876. [Google Scholar] [CrossRef]
- Moriarty, M.; Murray, A.; Berx, B.; Christie, A.; Munro, L.; Wallace, I. Modelling temperature and fish biomass data to predict annual Scottish farmed salmon, Salmo salar L. losses: Development of an early warning tool. Prev. Vet. Med. 2020, 178, 104985. [Google Scholar] [CrossRef]
- Zhou, X.; Niu, C.; Li, Q. Effects of light on feeding behavior, growth and survival of aquatic animals. Acta Hydrobiol. Sin. 2000, 24, 178–181. [Google Scholar] [CrossRef]
- Malinovskyi, O.; Rahimnejad, S.; Stejskal, V.; Boňko, D.; Stará, A.; Velíšek, J.; Policar, T. Effects of different photoperiods on growth performance and health status of largemouth bass (Micropterus salmoides) juveniles. Aquaculture 2022, 548, 737631. [Google Scholar] [CrossRef]
- Ottinger, M.; Clauss, K.; Kuenzer, C. Aquaculture: Relevance, distribution, impacts and spatial assessments-a review. Ocean Coast. Manag. 2016, 119, 244–266. [Google Scholar] [CrossRef]
- Choi, Y.; Choi, J.; Yang, S.; Kim, B.; Choi, C. The effect of green and red light spectra and their intensity on the oxidative stress and non-specific immune responses in gold-striped amberjack, Seriola lalandi. Mar. Freshw. Behav. Physiol. 2016, 49, 223–234. [Google Scholar] [CrossRef]
- Jung, S.; Choi, Y.; Kim, N.; Choi, J.; Kim, B.; Choi, C. Effects of melatonin injection or green-wavelength LED light on the antioxidant system in goldfish (Carassius auratus) during thermal stress. Fish Shellfish Immunol. 2016, 52, 157–166. [Google Scholar] [CrossRef]
- Wei, H.; Cai, W.; Liu, H.; Han, D.; Zhu, X.; Yang, Y.; Xie, S. Effects of photoperiod on growth, lipid metabolism and oxidative stress of juvenile gibel carp (Carassius auratus). J. Photochem. Photobiol. B Biol. 2019, 198, 111552. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Liu, H.; Xia, Y.; Liu, H.; Han, D.; Zhu, X.; Yang, Y.; Jin, J.; Xie, Q. Effects of light intensity on phototaxis, growth, antioxidant and stress of juvenile gibel carp (Carassius auratus gibelio). Aquaculture 2019, 501, 39–47. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, M.; Li, X.; Song, C.; Liu, Y. Physiological metabolism of Haliotis discus hannai Ino under different light qualities and cycles. Aquac. Res. 2016, 48, 3340–3350. [Google Scholar] [CrossRef]
- Wang, M.; Huang, H.; Hu, Z. Effect of disinfectants on hatching of fertilized Siniperca scherzeri eggs. Fujian J. Agric. Sci. 2018, 33, 346–350. [Google Scholar]
- Huang, S.; Pan, Q.; An, M.; Yu, K.; Li, S.; Chen, D.; Ma, X. Morphological differences among three wild populations of spotted mandarinfish Siniperca scherzeri in Guizhou Province. Fish. Sci. 2020, 39, 350–358. [Google Scholar]
- Jiang, X.H.; Wang, X.B.; Kou, L.X.; Wei, H.X.; Liu, Y.; Zhang, T. A comparative study on the nutrient and fatty acid composition in muscle of Siniperca scherzeri in upstream and downstream area of Yalu River. J. Fish. Res. 2023, 45, 569–576. [Google Scholar]
- Hao, C.; Shi, Y.; Peng, G.; Ding, S.; Wang, C.; Zheng, X.; Liu, G. Studies on techniques of the artificial breeding and seedling cultivation of Siniperca scherzeri. J. Aquac. 2018, 39, 13–15. [Google Scholar]
- Luo, X.; Liang, X.; Yi, J.; Tian, C.; Yang, M.; Fang, L.; Dou, Y.; Shi, J. Study on artificial breeding of Siniperca scherzeri (Steindachner) in reservior cages and its embyonic development. Chin. J. Fish. 2014, 27, 22–29. [Google Scholar]
- Pereira, L.; Amanajás, R.; Oliveira, A.; Silva, M.; Val, A. Health of the Amazonian fish tambaqui (Colossoma macropomum): Effects of prolonged photoperiod and high temperature. Aquaculture 2021, 541, 736836. [Google Scholar] [CrossRef]
- Bizarro, Y.; Navarro, F.; Filho, O.; Navarro, R. Photoperiodic effects in blood glucose, cortisol, hematological parameters and reproductive indexes of gift lineage reversed male tilapia. Biosci. J. 2019, 35, 1915–1922. [Google Scholar] [CrossRef]
- Bowman, J.S.; Rasmussen, S.; Blom, N.S.; Deming, J.W.; Rysgaard, S.; Sicheritz-Pontén, T. Microbial community structure of Arctic multiyear sea ice and surface seawater by 454 sequencing of the 16S RNA gene. ISME J. 2011, 6, 11–20. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric-estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Pitta, D.; Pinchak, W.; Dowd, S.; Osterstock, J.; Gontcharova, V.; Youn, E.; Dorton, K.; Yoon, I.; Min, B.; Fulford, J. Rumen bacterial diversity dynamics associated with changing from bermudagrass hay to grazed winter wheat diets. Microb. Ecol. 2010, 59, 511–522. [Google Scholar]
- Shannon, C. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pandya, P.; Singh, K.; Parnerkar, S.; Tripathi, A.; Mehta, H.; Rank, D.; Kothari, R.; Joshi, C. Bacterial diversity in the rumen of Indian Surti buffalo (Bubalus bubalis), assessed by 16S rDNA analysis. J. Appl. Genet. 2010, 51, 395–402. [Google Scholar] [CrossRef]
- Bassano, B.; Iacobuzio, R.; Pennati, R.; Parolini, M.; Saino, N. Melanin-based skin coloration predicts antioxidant capacity in the Brown trout (Salmo trutta). Physiol. Biochem. Zool. 2018, 91, 1026–1035. [Google Scholar]
- Velisek, J.; Stara, A.; Li, Z.; Silovska, S.; Turek, J. Comparison of the effects of four anaesthetics on blood biochemical profiles and oxidative stress biomarkers in rainbow trout. Aquaculture 2011, 310, 369–375. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Ma, X.; Cheng, K.; Wu, G. Effects of dietary starch and lipid levels on the protein retention and growth of largemouth bass (Micropterus salmoides). Amino Acids 2020, 52, 999–1016. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shi, C.; Zhou, Y.; Chen, Y.; Lin, S.; Tang, R. Optimum dietary fiber level could improve growth, plasma biochemical indexes and liver function of largemouth bass, Micropterus salmoides. Aquaculture 2020, 518, 734661. [Google Scholar] [CrossRef]
- Hamed, H.S.; Abdel-Tawwab, M. Dietary pomegranate (Punica granatum) peel mitigated the adverse effects of silver nanoparticles on the performance, haemato-biochemical, antioxidant, and immune responses of Nile tilapia fingerlings. Aquaculture 2021, 540, 736742. [Google Scholar] [CrossRef]
- Li, Z.; Li, P.; Shi, Z. Chronic exposure to tributyltin induces brain functional damage in juvenile common carp (Cyprinus carpio). PLoS ONE 2015, 10, e0123091. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.; He, S.; Li, L. Effects of long-term low-concentration nitrite exposure and detoxification on growth performance, antioxidant capacities, and immune responses in Chinese perch (Siniperca chuatsi). Aquaculture 2021, 533, 736123. [Google Scholar] [CrossRef]
- Li, Z.; Chen, L.; Wu, Y.; Li, P.; Li, Y.; Ni, Z. Effects of mercury on oxidative stress and gene expression of potential biomarkers in larvae of the Chinese rare minnow Gobiocypris rarus. Arch. Environ. Contam. Toxicol. 2014, 67, 245–251. [Google Scholar] [CrossRef]
- Nwani, C.; Lakra, W.; Nagpure, N.; Kumar, R.; Kushwaha, B.; Srivastava, S. Toxicity of the herbicide atrazine: Effects on lipid peroxidation and activities of antioxidant enzymes in the freshwater fish Channa Punctatus (Bloch). Int. J. Environ. Res. Public Health 2010, 7, 3298–3312. [Google Scholar] [CrossRef]
- Pandey, S.; Parvez, S.; Sayeedl, I.; Haque, R.; Bin-Hafeez, B.; Raisuddin, S. Biomarkers of oxidative stress: A comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci. Total Environ. 2003, 309, 105–115. [Google Scholar]
- Van der Oost, R.; Beyer, J.; Vermeulen, N. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Maheshwari, D.; Kumar, M.; Verma, S.; Singh, V.; Singh, S. Antioxidant and hepatoprotective activities of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves. Food Chem. Toxicol. 2011, 49, 2422–2428. [Google Scholar] [CrossRef]
- Shaban, N.; El-Kersh, M.; El-Rashidy, F.; Habashy, N. Protective role of Punica granatum (pomegranate) peel and seed oil extracts on diethylnitrosamine and phenobarbital-induced hepatic injury in male rats. Food Chem. 2013, 141, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, P.; Wang, W.; Li, Z. Response of growth performance, serum biochemical parameters, antioxidant capacity, and digestive enzyme activity to different feeding strategies in common carp (Cyprinus carpio) under high-temperature stress. Aquaculture 2022, 548, 737636. [Google Scholar] [CrossRef]
- Yang, S.; Yan, T.; Zhao, L.; Wu, H.; Du, Z.; Yan, T.; Xiao, Q. Effects of temperature on activities of antioxidant enzymes and Na+/K+-ATPase, and hormone levels in Schizothorax prenanti. J. Therm. Biol. 2018, 72, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Stanic, B.; Andric, N.; Zoric, S.; Grubor-Lajsic, G.; Kovacevic, R. Assessing pollution in the Danube River near Novi Sad (Serbia) using several biomarkers in sterlet (Acipenser ruthenus L.). Ecotoxicol. Environ. Saf. 2006, 65, 395–402. [Google Scholar] [CrossRef]
- Aliko, V.; Qirjo, M.; Sula, E.; Morina, V.; Faggio, C. Antioxidant defense system, immune response and erythron profile modulation in gold fish Carassius auratus, after acute manganese treatment. Fish Shellfish Immunol. 2018, 76, 101–109. [Google Scholar] [CrossRef]
- Sun, X.L.; Yang, S.Y.; Chen, C.X.; Wang, Q.K.; Yu, X.Q.; Hu, J.C.; Xing, K.Z. Effece of photoperiod on growth and antioxidant indices in half-smooth tongue-sole (Cynoglossus semilaevis). Chin. J. Fish. 2012, 25, 23–27. [Google Scholar]
- Guo, K.; Ruan, G.; Fan, W.; Fang, L.; Wang, Q.; Luo, M.; Yi, T. The effect of nitrite and sulfide on the antioxidant capacity and microbial composition of the intestines of red swamp crayfish Procambarus clarkii. Fish Shellfish Immunol. 2020, 96, 290–296. [Google Scholar] [CrossRef]
- Ma, H.; Wei, P.; Li, X.; Liu, S.; Tian, Y.; Zhang, Q.; Liu, Y. Effects of photoperiod on growth, digestive, metabolic and non-special immunity enzymes of Takifugu rubripes larvae. Aquaculture 2021, 542, 736840. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, D.; Xu, C.; Wang, F.; Liu, W. Effects of light intensity on growth, immune responses, antioxidant capability and disease resistance of juvenile blunt snout bream Megalobrama amblycephala. Fish Shellfish Immunol. 2015, 47, 674–680. [Google Scholar] [CrossRef]
- Chang, C.; Mayer, M.; Rivera-Ingraham, G.; Blondeau-Bidet, E.; Wu, W.; Lorin-Nebel, C.; Lee, T. Effects of temperature and salinity on antioxidant responses in livers of temperate (Dicentrarchus labrax) and tropical (Chanos Chanos) marine euryhaline fish. J. Therm. Biol. 2021, 99, 103016. [Google Scholar] [CrossRef] [PubMed]
- Barda, I.; Purina, I.; Rimsa, E.; Balode, M. Seasonal dynamics of biomarkers in infaunal clam Macoma balthica from the Gulf of Riga (Baltic Sea). J. Mar. Syst. 2014, 129, 150–156. [Google Scholar] [CrossRef]
- Nash, S.; Johnstone, J.; Rahman, M. Elevated temperature attenuates ovarian functions and induces apoptosis and oxidative stress in the American oyster, Crassostrea virginica: Potential mechanisms and signaling pathways. Cell Stress Chaperone 2019, 24, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, C.; Madeira, D.; Mendonca, V.; Dias, M.; Roma, J.; Diniz, M. Effect of increasing temperature in the differential activity of oxidative stress biomarkers in various tissues of the Rock goby, Gobius paganellus. Mar. Environ. Res. 2014, 97, 10–14. [Google Scholar] [CrossRef]
- Berdjeb, L.; Ghiglione, J.; Jacquet, S.J.A. Bottom-up versus top-down control of hypo- and epilimnion free-living bacterial community structures in two neighboring freshwater lakes. Appl. Environ. Microbiol. 2011, 77, 3591–3599. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Fang, L.; Liang, X.; Guo, W.; Lv, L.; Li, L. Influence of environmental factors and bacterial community diversity in pond water on health of Chinese perch through Gut Microbiota change. Aquac. Rep. 2011, 20, 100629. [Google Scholar] [CrossRef]
- Tianm, C.; Laim, X.; Hem, Y.; Gaom, H. Research progress of gut microbiome of marine fish. J. Anhui Agric. Sci. 2024, 52, 19–22+31. [Google Scholar]
- Puvanendran, V.; Brown, J. Foraging, growth and survival of Atlantic cod larvae reared in different light intensities and photoperiods. Aquaculture 2022, 214, 131–151. [Google Scholar] [CrossRef]
- Liu, H.; Stickney, R.; Dickhoff, W.; McCaughran, D. Effect of environmental factors on egg development and hatching of Pacific Halibut Hippoglossus stenolepis. J. World Aquac. Soc. 2010, 25, 317–321. [Google Scholar] [CrossRef]
- Luan, Y.; Li, M.; Zhou, W.; Yao, Y.; Yang, Y.; Zhang, Z.; Ringø, E.; Olsen, R.; Clarke, J.; Xie, S.; et al. The Fish Microbiota: Research Progress and Potential Applications. Engineering 2023, 29, 137–146. [Google Scholar] [CrossRef]
- Qin, K.; Wu, L.; He, Z. The feeding intensity of fat greenling larval under different illumination. Chin. J. Zool. 1999, 34, 4–8. [Google Scholar]
- Walburn, J.; Wemheuer, B.; Thomas, T.; Copelandm, E.; O’Connor, W.; Booth, M.; Fielder, S.; Egan, S. Diet and diet-associated bacteria shape early microbiome development inyellowtail kingfish (Seriola lalandi). Microb. Biotechnol. 2019, 12, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Ruyet, P.; Mahé, K.; Bayon, N.; Delliou, H. Effects of temperature on growth and metabolism in a Mediterranean population of European sea bass, Dicentrarchus labrax. Aquaculture 2004, 237, 269–280. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, S.; Ka, W.; Gao, P.; Li, Y.; Long, R.; Wang, J. Association of gut microbiotawith metabolism in rainbow trout under acute heat stress. Front. Microbiol. 2022, 13, 846336. [Google Scholar]
- Ye, L.; Amberg, J.; Chapman, D.; Gaikowski, M.; Liu, W. Fish gut microbiota analysis differentiates physiology and behavior of invasive Asian carp and indigenous American fish. ISME J. 2014, 8, 541–551. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Gooneratne, R.; Lai, R.; Zeng, C.; Zhan, F.; Wang, W. The gut microbiome and degradation enzyme activity of wild freshwater fishes influenced by their trophic levels. Sci. Rep. 2016, 6, 24340. [Google Scholar] [CrossRef]
- Brown, R.M.; Wiens, G.D.; Salinas, I. Analysis of the gut and gill microbiome of resistant and susceptible lines of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2019, 86, 497–506. [Google Scholar] [CrossRef]
- Kuang, T.; He, A.; Lin, Y.; Huang, X.; Zhou, L. Comparative analysis of microbial communities associated with the gill, gut, and habitat of two filter-feeding fish. Aquac. Rep. 2020, 18, 100501. [Google Scholar] [CrossRef]
- Shin, N.; Whon, T.; Bae, J. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Blandford, M.I.; Taylor-Brown, A.; Schlacher, T.A.; Nowak, B.; Polkinghorne, A. Epitheliocystis in fish: An emerging aquaculture disease with a global impact. Transbound. Emerg. Dis. 2018, 65, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, H.; Zhao, L.; Liu, X.; Xiang, P.; Deng, L.; Song, Z. Host habitats influence the gut microbial assembly mechanisms of cold-water fish. Aquac. Rep. 2024, 36, 102094. [Google Scholar] [CrossRef]
- Miyake, S.; Ngugi, D.; Stingl, U. Diet strongly influences the gut microbiota of surgeonfishes. Mol. Ecol. 2015, 24, 656–672. [Google Scholar] [CrossRef]
- Youngblut, N.; Reischer, G.; Walters, W.; Schuster, N.; Walzer, C.; Stalder, G.; Ley, R.; Farnleitner, A. Host diet and evolutionary history explain different aspects of gut microbiome diversity among vertebrate clades. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Wang, M.; Li, Z.; Shi, J.; Zhong, Y.; Zhao, G.; Liu, D. Effects of different light cycles on feeding and growth of juvenile California perch Microperus salmoides. J. Aquac. 2024, 45, 1–4+31. [Google Scholar]
- Qian, Q.; Chen, Z.; Xu, J.; Zhu, Y.; Xu, W.; Gao, X.; Jiang, Q.; Zhang, X. Pathogenicity of Plesiomonas shigelloides causing mass mortalities of largemouth bass (Micropterus salmoides) and its induced host immune response. Fish Shellfish Immunol. 2023, 132, 108487. [Google Scholar] [CrossRef]
- Liu, Z.; Ke, X.; Lu, M.; Gao, F.; Zhu, H.; Wang, M. Identification and pathological observation of a pathogenic Plesiomonas shigelloides strain isolated from cultured tilapia (Oreochromis niloticus). Acta Microbiol. Sin. 2015, 55, 96–106. [Google Scholar]
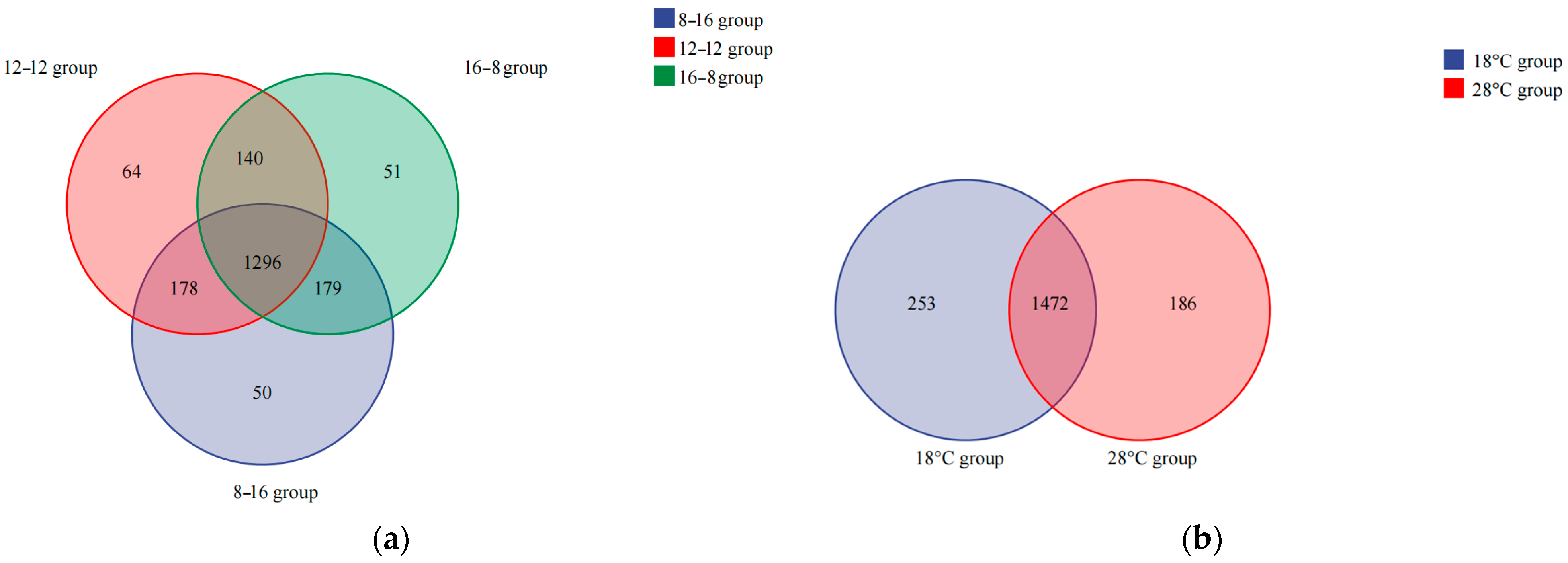
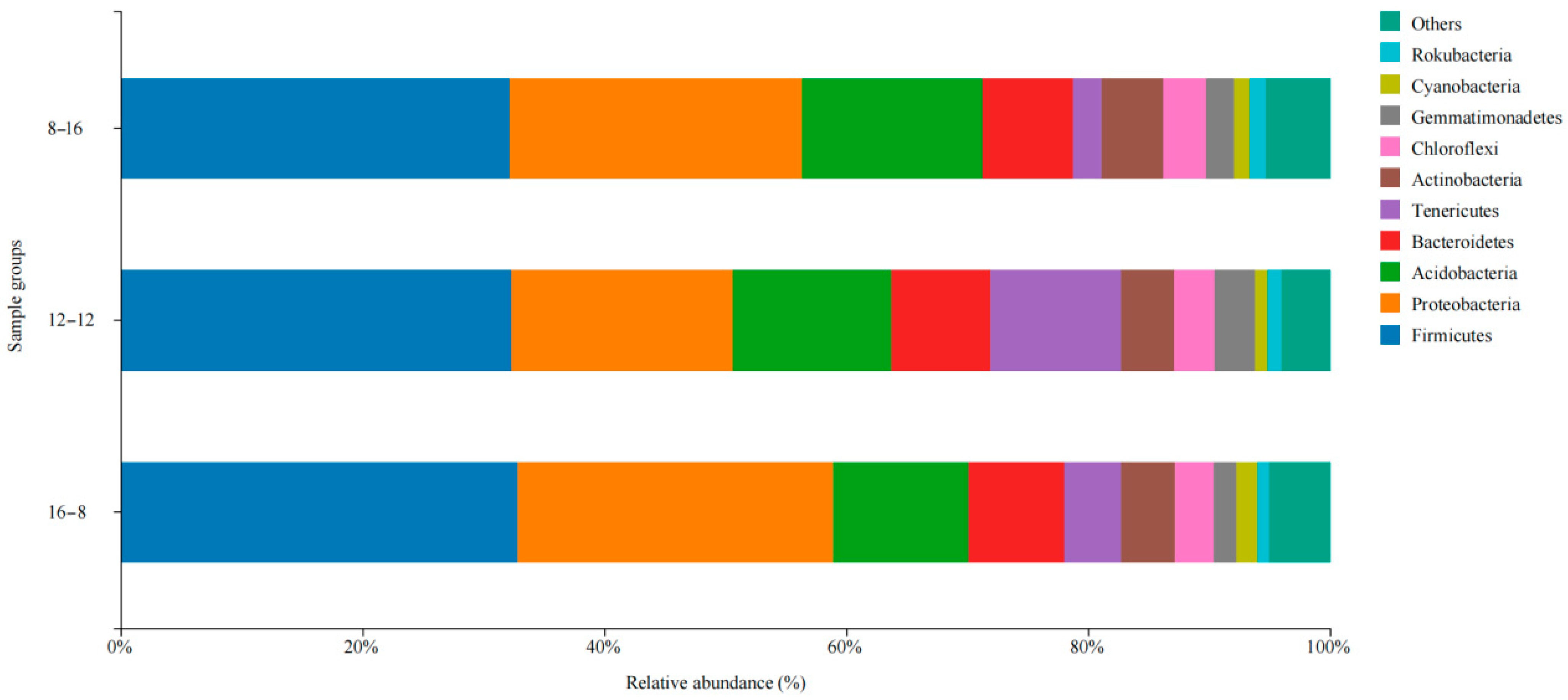
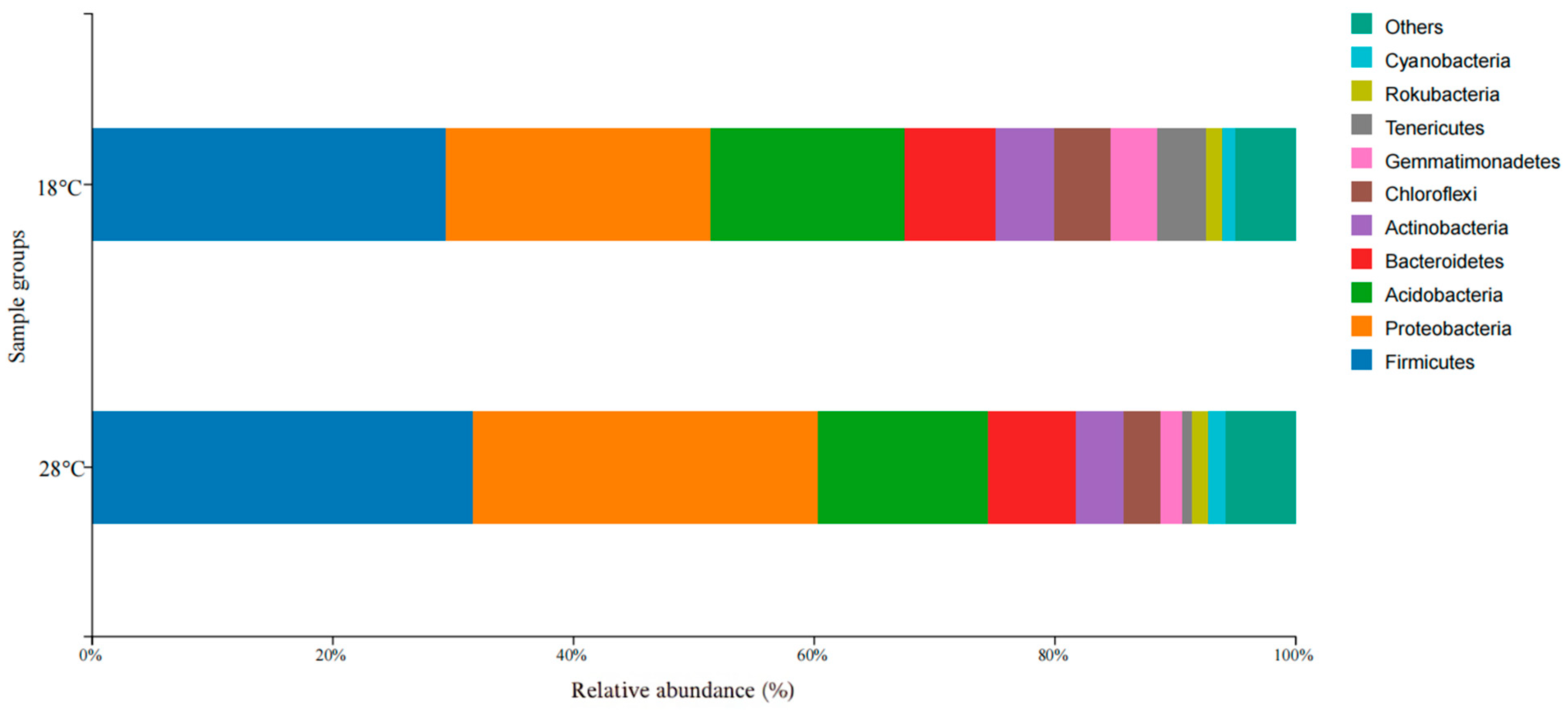
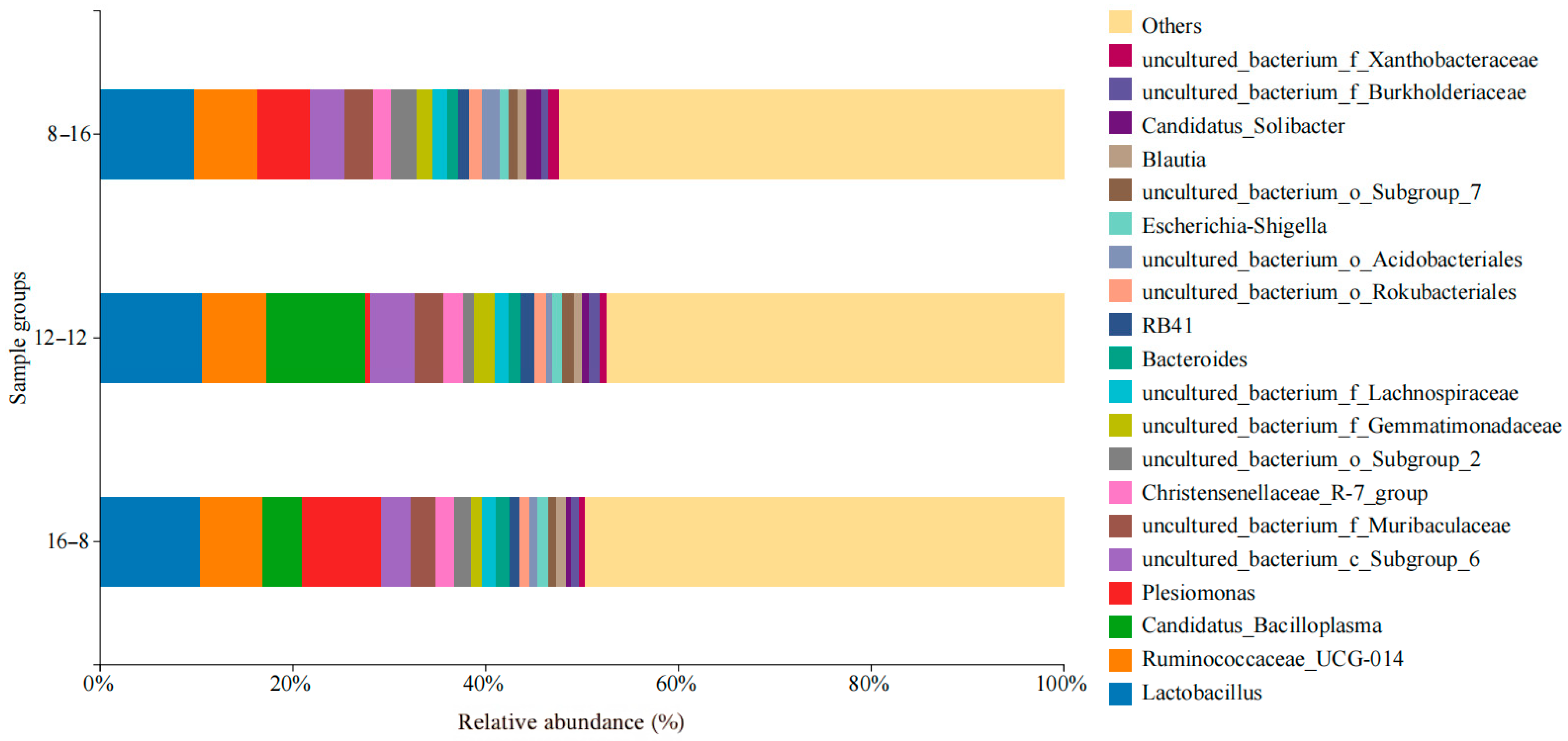
| Condition | Group | Antioxidant Enzymes | |||
|---|---|---|---|---|---|
| MDA (nmol/L) | SOD (U/mL) | GSH-Px (U/mL) | CAT (U/mL) | ||
| 8L: 16D | G11 | 0.365 | 734.031 | 18.301 | 125.771 |
| G12 | 0.345 | 740.151 | 17.545 | 130.157 | |
| G13 | 0.385 | 727.910 | 19.056 | 121.386 | |
| average | 0.365 | 734.031 | 18.301 | 125.771 | |
| S.E.M | 0.020 | 6.121 | 0.755 | 4.385 | |
| 12L: 12D | G21 | 0.573 | 582.091 | 4.995 | 98.350 |
| G22 | 0.565 | 590.157 | 4.335 | 100.157 | |
| G23 | 0.582 | 574.024 | 5.655 | 96.543 | |
| average | 0.573 | 582.091 | 4.995 | 98.350 | |
| S.E.M | 0.009 | 8.066 | 0.660 | 1.807 | |
| 16L: 8D | G31 | 0.419 | 587.520 | 16.467 | 110.905 |
| G32 | 0.445 | 567.429 | 15.575 | 111.175 | |
| G33 | 0.394 | 607.611 | 17.359 | 110.634 | |
| average | 0.419 | 587.520 | 16.467 | 110.905 | |
| S.E.M | 0.026 | 20.091 | 0.892 | 0.271 | |
| 18 °C | W11 | 0.497 | 647.671 | 5.590 | 86.694 |
| W12 | 0.512 | 666.177 | 5.468 | 83.290 | |
| W13 | 0.482 | 629.165 | 5.350 | 90.099 | |
| average | 0.497 | 647.671 | 5.468 | 86.694 | |
| S.E.M | 0.015 | 18.506 | 0.118 | 3.405 | |
| 28 °C | W21 | 0.265 | 1029.465 | 11.476 | 146.258 |
| W22 | 0.276 | 1033.157 | 12.173 | 150.459 | |
| W23 | 0.254 | 1025.773 | 10.780 | 142.058 | |
| average | 0.265 | 1029.465 | 11.476 | 146.258 | |
| S.E.M | 0.011 | 3.692 | 0.696 | 4.200 | |
| Group | Observed OTUs | Predicted ACE | OTUs Chao1 | Simpson | Shannon | Good’s Coverage |
|---|---|---|---|---|---|---|
| 8L: 16D | 1161.33 ± 106.82 a | 1210.84 ± 94.81 a | 1286.26 ± 86.91 a | 0.99 ± 0.00 a | 8.39 ± 0.27 a | 0.99 ± 0.00 a |
| 12L: 12D | 1158.00 ± 19.67 a | 1240.04 ± 4.36 a | 1318.67 ± 5.91 a | 0.98 ± 0.00 a | 8.10 ± 0.11 a | 0.99 ± 0.00 a |
| 16L: 8D | 1130.00 ± 82.82 a | 1195.09 ± 46.50 a | 1284.58 ± 50.49 a | 0.98 ± 0.01 a | 8.09 ± 0.35 a | 0.99 ± 0.00 a |
| 18 °C | 1214.33 ± 77.59 a | 1272.36 ± 54.22 a | 1373.37 ± 32.52 a | 0.99 ± 0.00 a | 8.61 ± 0.09 a | 0.99 ± 0.00 a |
| 28 °C | 1147.33 ± 33.56 a | 1193.52 ± 27.17 b | 1268.18 ± 62.31 b | 0.98 ± 0.00 a | 8.23 ± 0.15 b | 0.99 ± 0.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Liang, X.; Wang, Y.; Li, N.; Wang, Y.; Lu, K.; Tian, T.; Li, J.; Xiong, Y.; Li, M.; et al. Effects of Water Temperature and Photoperiod on the Antioxidant Status and Intestinal Microbiota in Larval Spotted Mandarin Fish, Siniperca scherzeri, in the Yalu River. Biology 2025, 14, 1400. https://doi.org/10.3390/biology14101400
Yang J, Liang X, Wang Y, Li N, Wang Y, Lu K, Tian T, Li J, Xiong Y, Li M, et al. Effects of Water Temperature and Photoperiod on the Antioxidant Status and Intestinal Microbiota in Larval Spotted Mandarin Fish, Siniperca scherzeri, in the Yalu River. Biology. 2025; 14(10):1400. https://doi.org/10.3390/biology14101400
Chicago/Turabian StyleYang, Jun, Xufang Liang, Yan Wang, Na Li, Yanjun Wang, Ke Lu, Tao Tian, Jiao Li, Yuyu Xiong, Meixuan Li, and et al. 2025. "Effects of Water Temperature and Photoperiod on the Antioxidant Status and Intestinal Microbiota in Larval Spotted Mandarin Fish, Siniperca scherzeri, in the Yalu River" Biology 14, no. 10: 1400. https://doi.org/10.3390/biology14101400
APA StyleYang, J., Liang, X., Wang, Y., Li, N., Wang, Y., Lu, K., Tian, T., Li, J., Xiong, Y., Li, M., & Gao, Y. (2025). Effects of Water Temperature and Photoperiod on the Antioxidant Status and Intestinal Microbiota in Larval Spotted Mandarin Fish, Siniperca scherzeri, in the Yalu River. Biology, 14(10), 1400. https://doi.org/10.3390/biology14101400






