Cornelian Cherry (Cornus mas) Fruit Extract Administration in Sleep Deprived Wistar Rats—Friend or Foe?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cornus Mas Fruit Extract–Preparation, Characterisation and In Vitro Antioxidant Activity
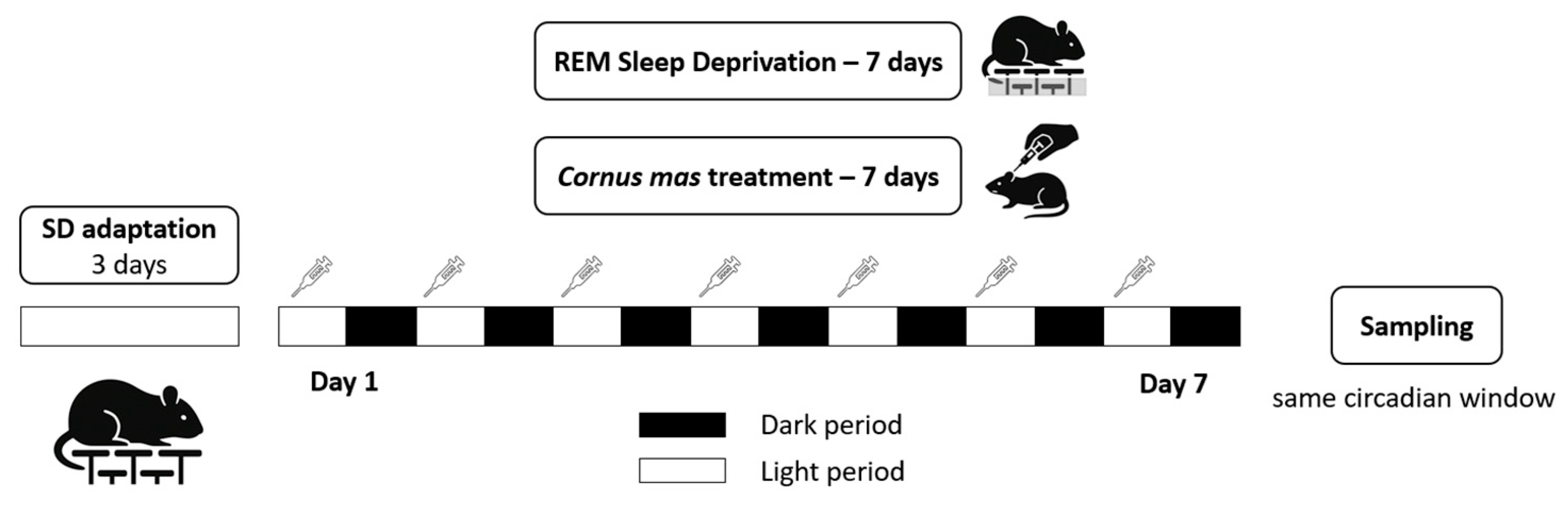
2.2. Experimental Animals and Study Design
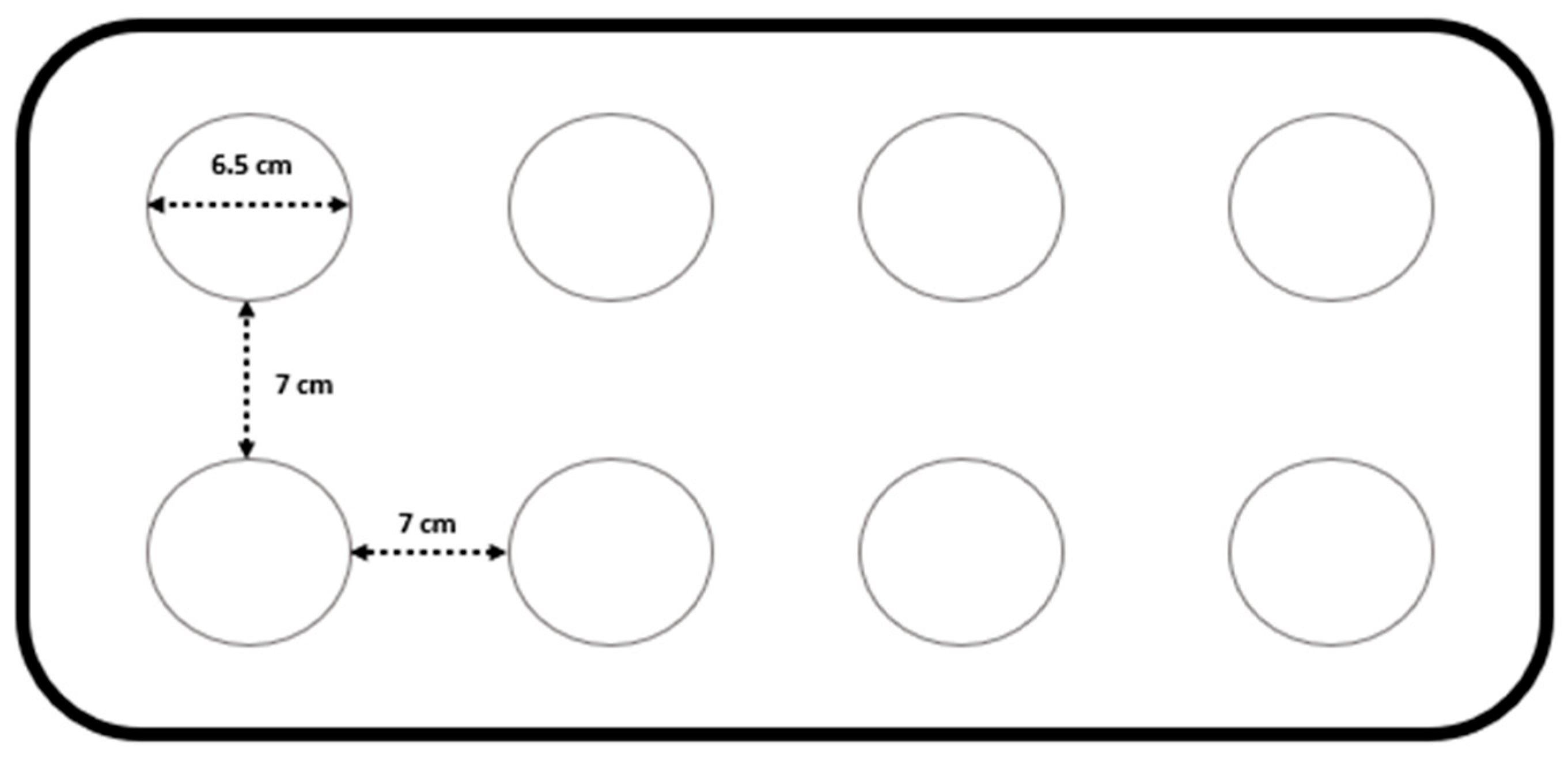
2.3. Induction of Sleep Deprivation
2.4. Sample Collection
2.5. Transmission Electron Microscopy (TEM)
2.6. Oxidative Stress Parameters
2.7. Pro- and Anti-Inflammatory Cytokine Determination
2.8. Statistical Analysis
3. Results
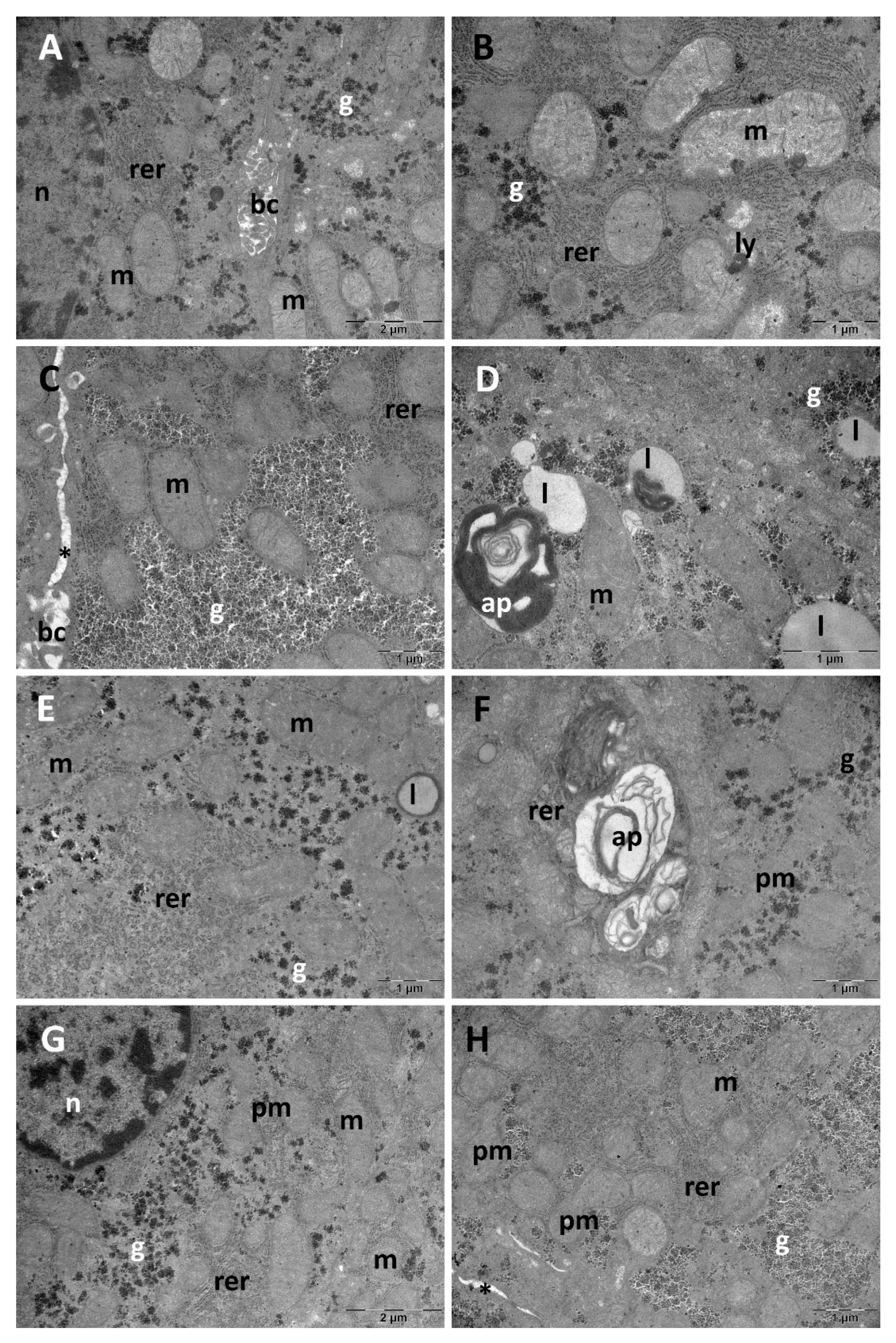
3.1. Liver Ultrastructural Analysis
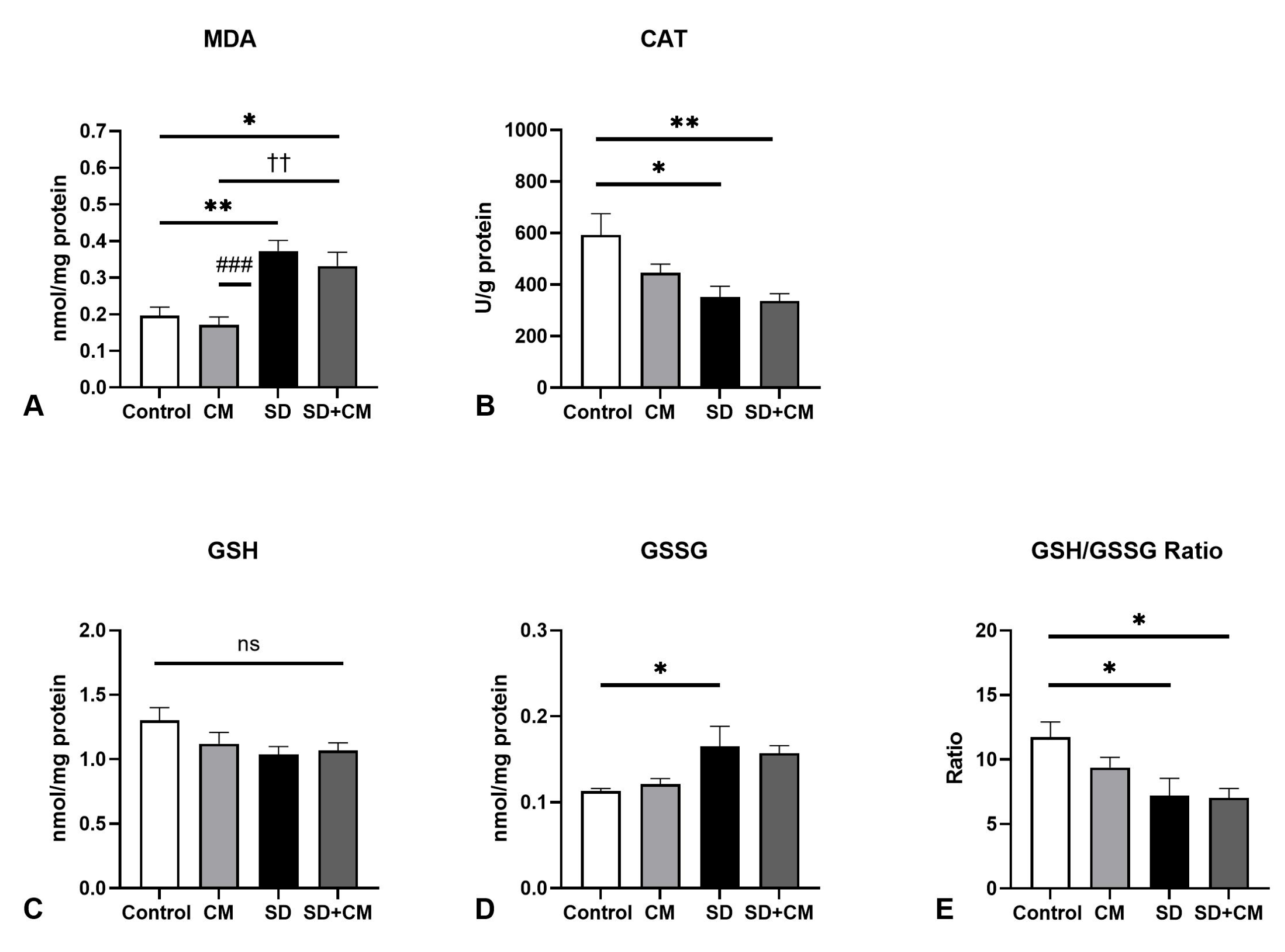
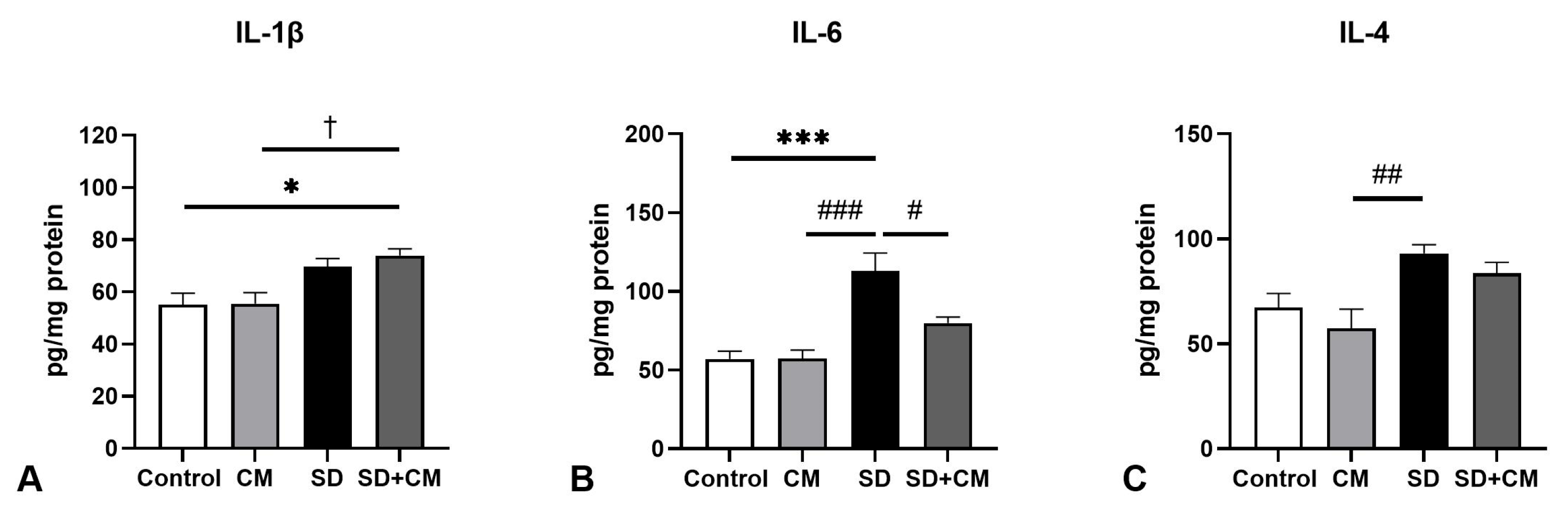
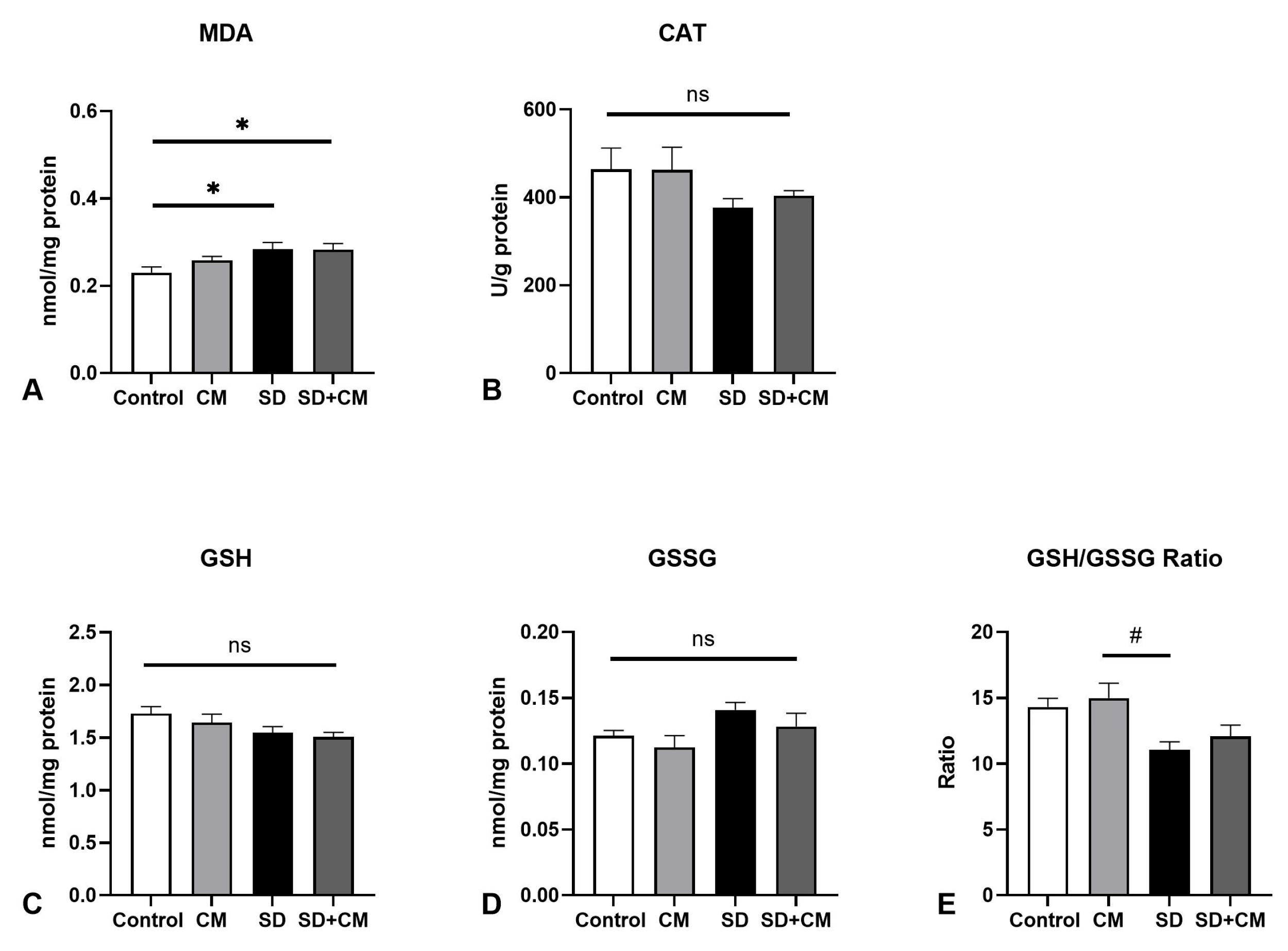
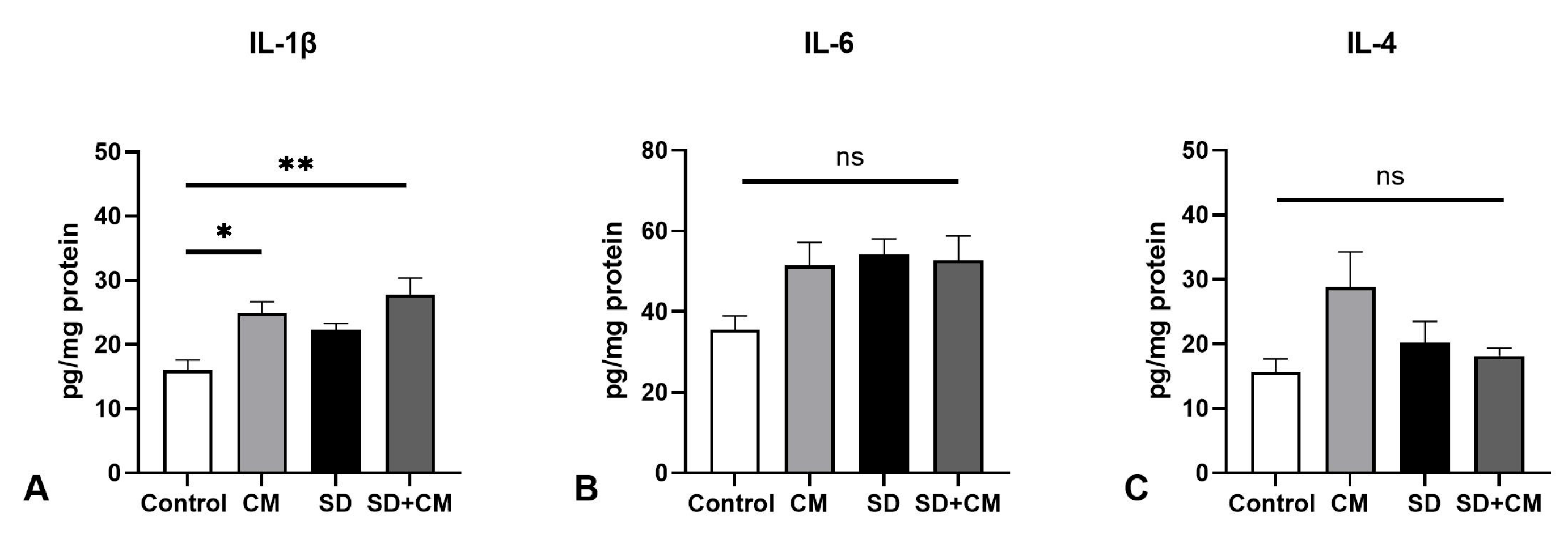
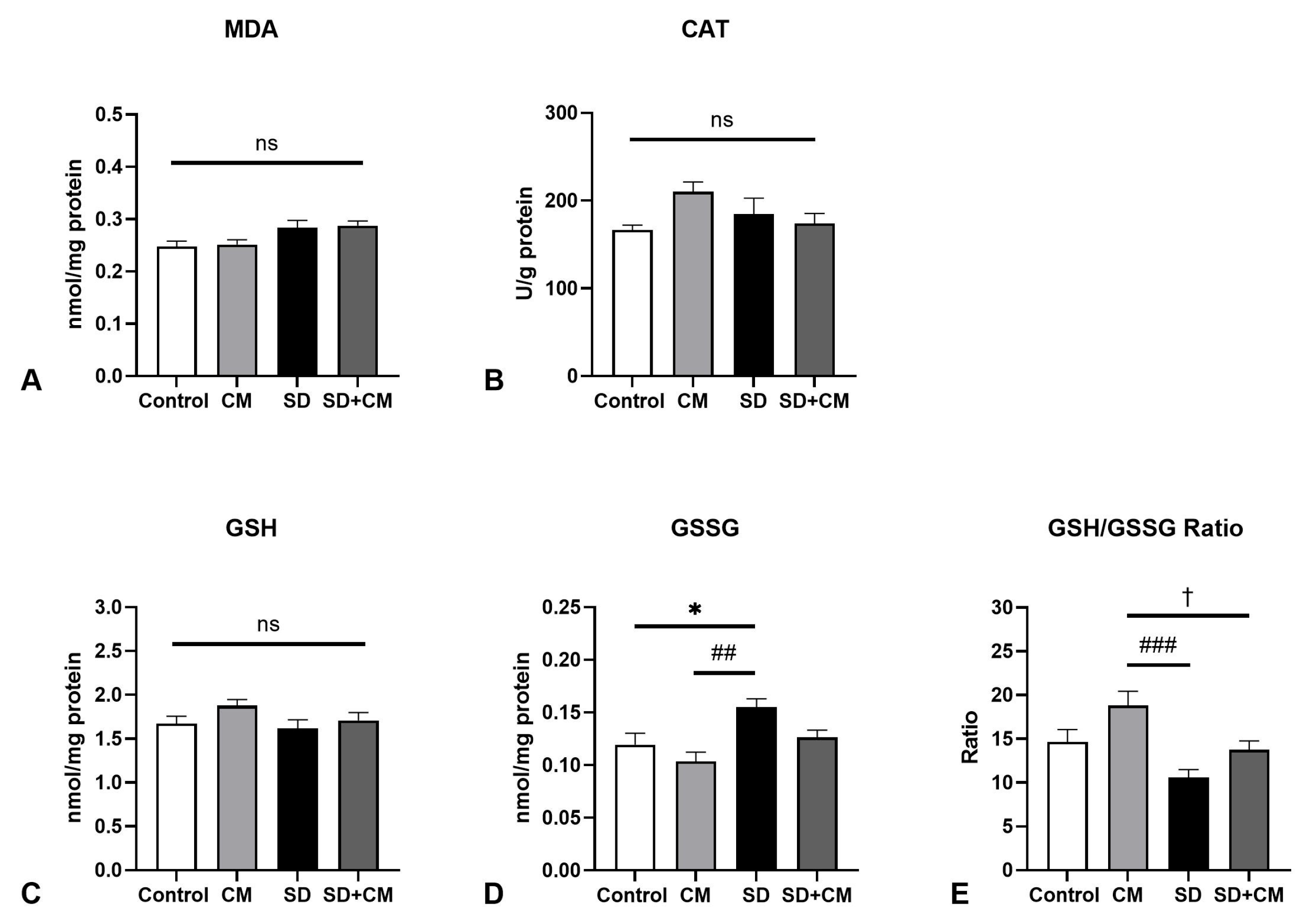
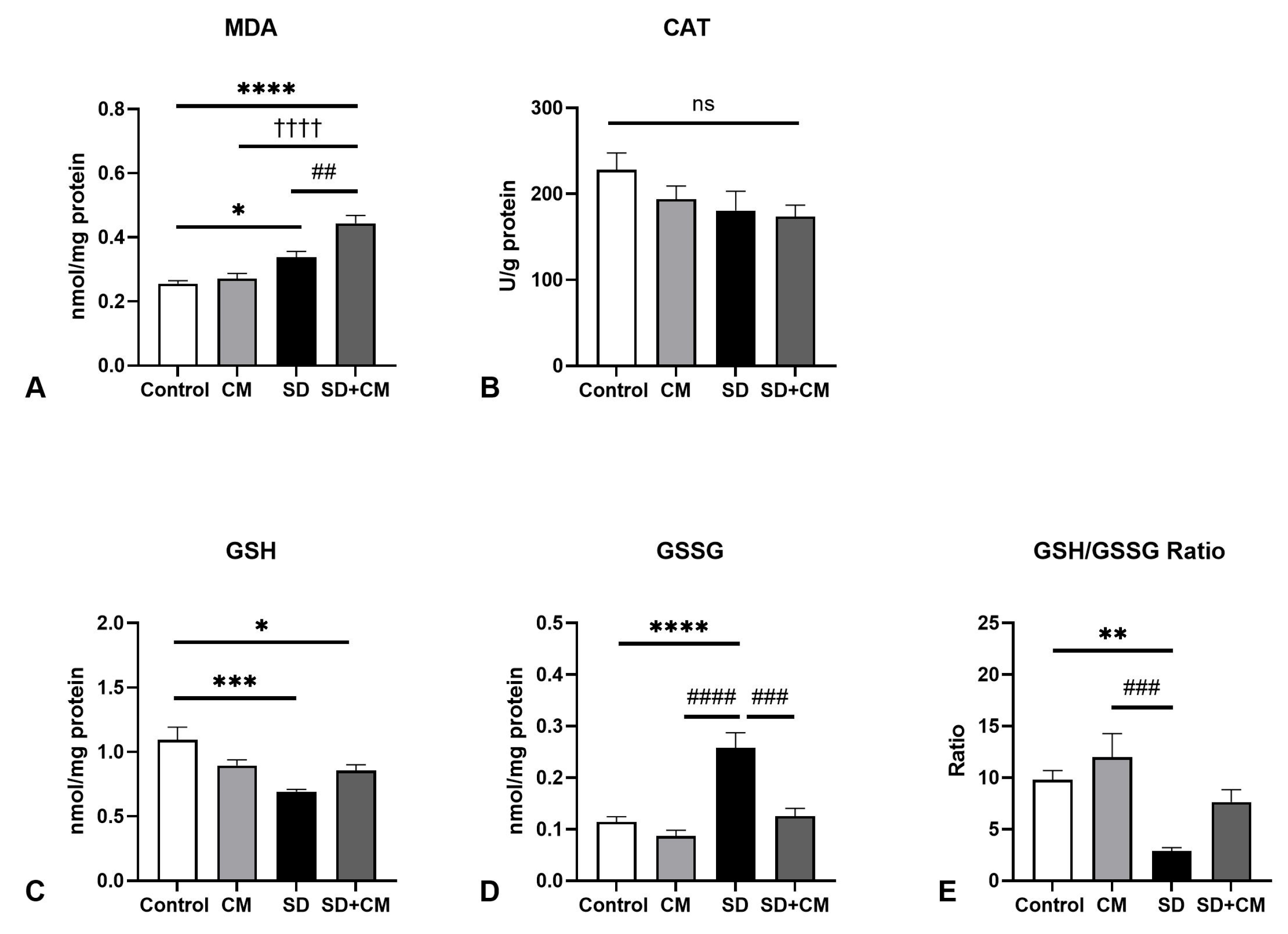
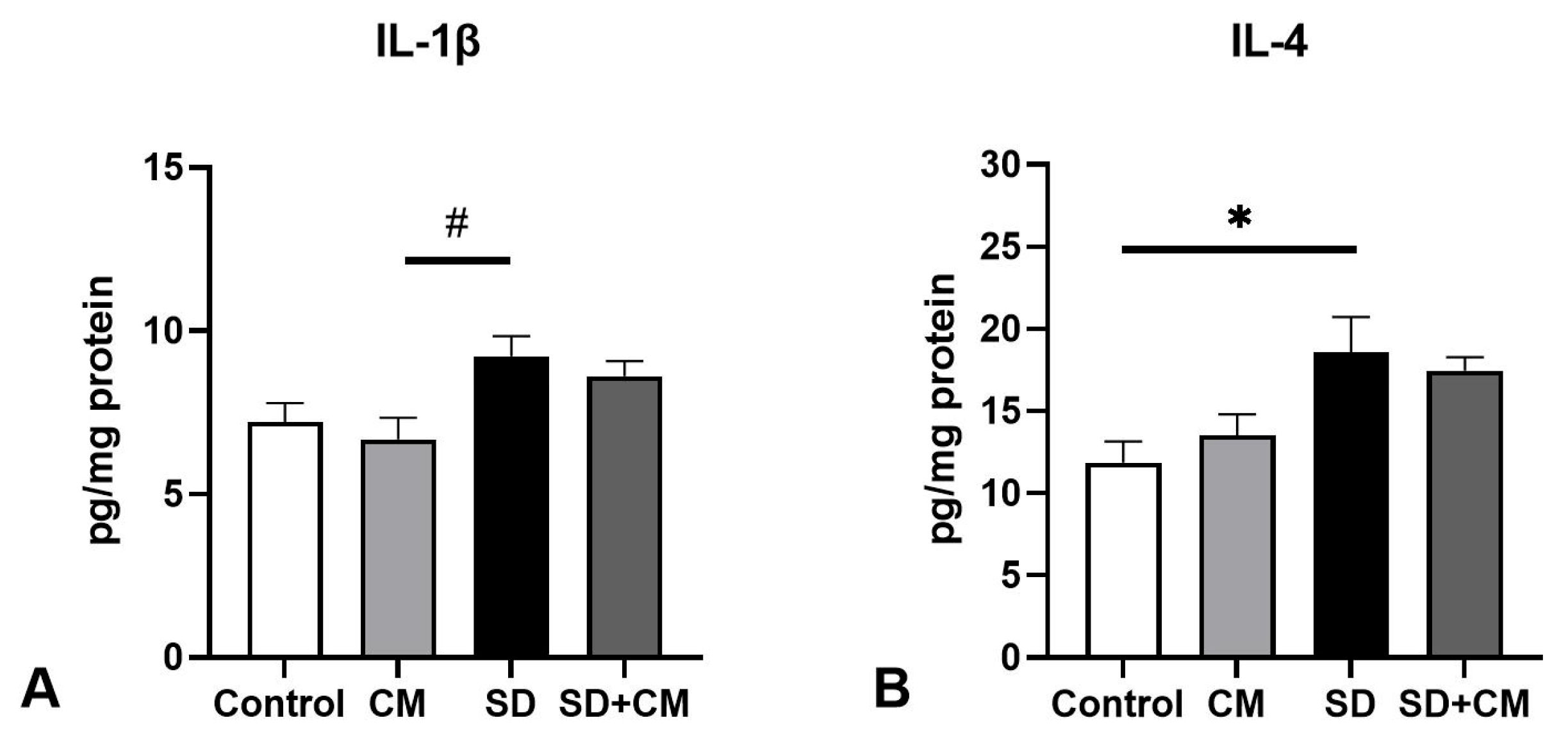
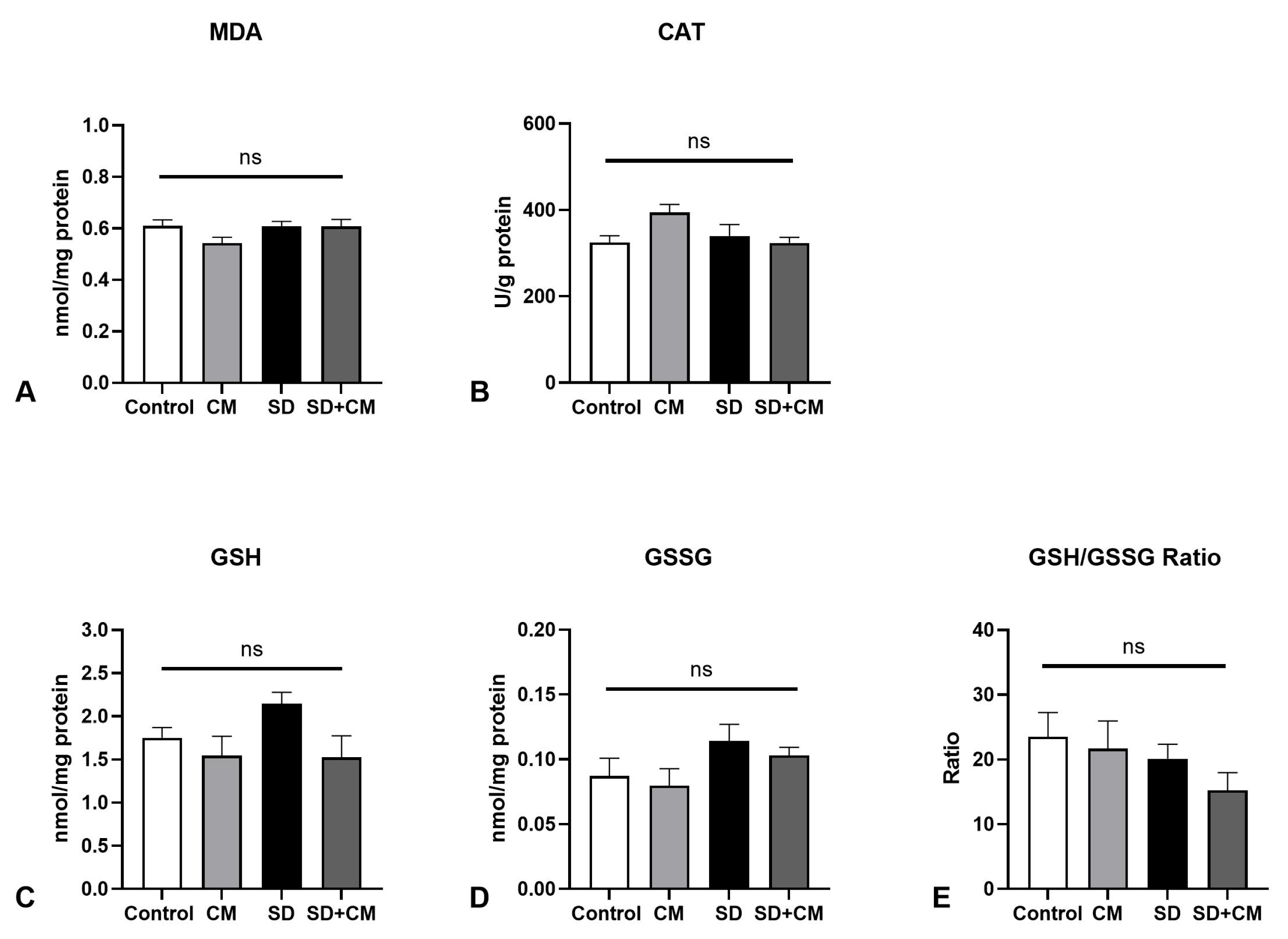
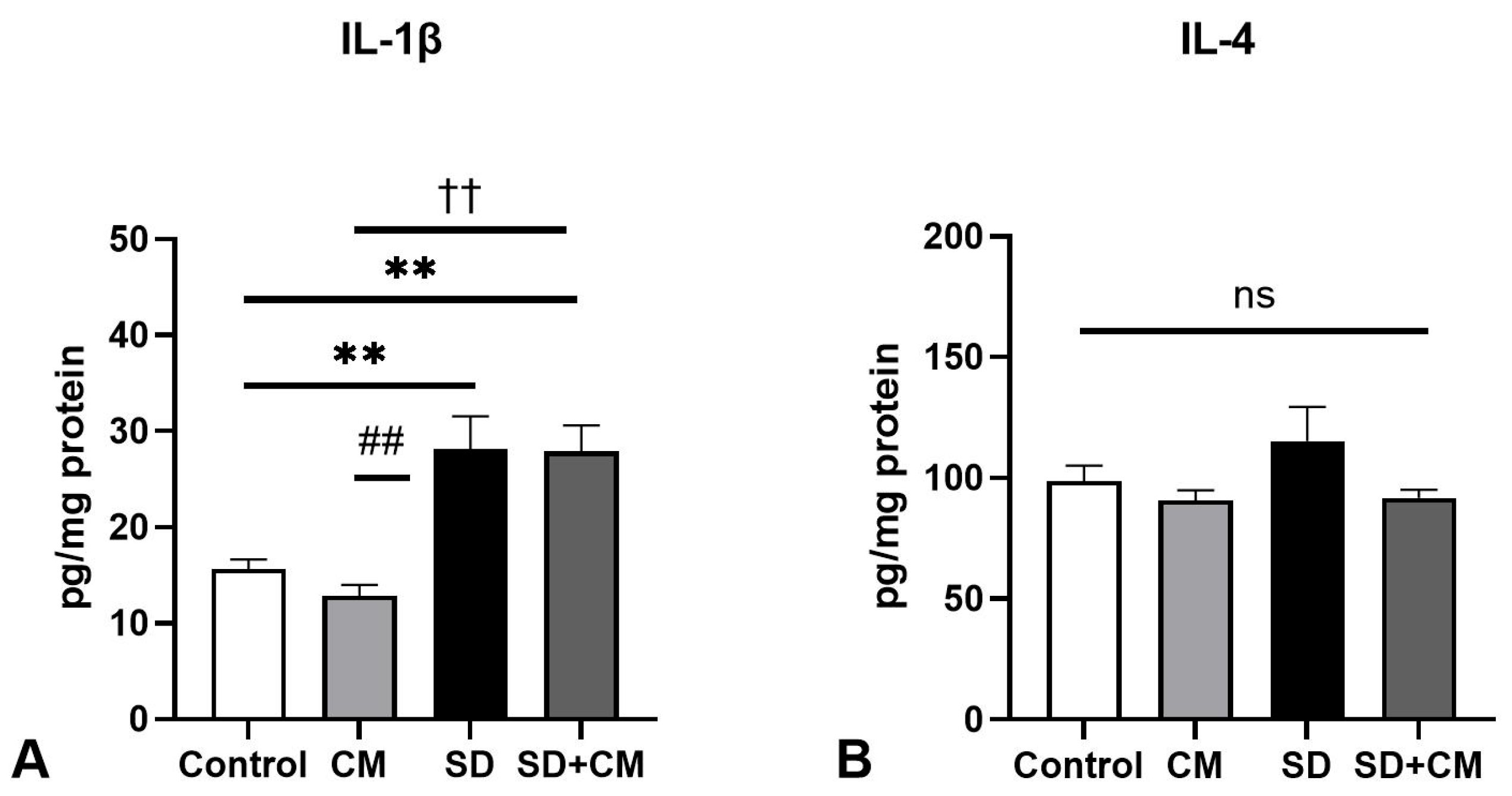
3.2. Oxidative Stress Parameters and Cytokine Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Itani, O.; Jike, M.; Watanabe, N.; Kaneita, Y. Short Sleep Duration and Health Outcomes: A Systematic Review, Meta-Analysis, and Meta-Regression. Sleep Med. 2017, 32, 246–256. [Google Scholar] [CrossRef]
- CDC FastStats: Sleep in Adults. Available online: https://www.cdc.gov/sleep/data-research/facts-stats/adults-sleep-facts-and-stats.html#:~:text=The%20recommended%20amount%20of%20sleep,Have%20short%20sleep%20duration (accessed on 26 September 2024).
- Neculicioiu, V.S.; Colosi, I.A.; Costache, C.; Sevastre-Berghian, A.; Clichici, S. Time to Sleep?—A Review of the Impact of the COVID-19 Pandemic on Sleep and Mental Health. Int. J. Environ. Res. Public Health 2022, 19, 3497. [Google Scholar] [CrossRef]
- Naiman, R. Dreamless: The Silent Epidemic of REM Sleep Loss. Ann. N. Y. Acad. Sci. 2017, 1406, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Gul, M.; Alzweiri, M.; Ishaqat, A.; ALSalamat, H.A.; Bashatwah, R.M. Reactive Oxygen Species: The Dual Role in Physiological and Pathological Conditions of the Human Body. Eurasian J. Med. 2018, 50, 193–201. [Google Scholar] [CrossRef]
- Hobson, J.A. Sleep Is of the Brain, by the Brain and for the Brain. Nature 2005, 437, 1254–1256. [Google Scholar] [CrossRef]
- Neculicioiu, V.S.; Colosi, I.A.; Costache, C.; Toc, D.A.; Sevastre-Berghian, A.; Colosi, H.A.; Clichici, S. Sleep Deprivation-Induced Oxidative Stress in Rat Models: A Scoping Systematic Review. Antioxidants 2023, 12, 1600. [Google Scholar] [CrossRef]
- Villafuerte, G.; Miguel-Puga, A.; Rodríguez, E.M.; Machado, S.; Manjarrez, E.; Arias-Carrión, O. Sleep Deprivation and Oxidative Stress in Animal Models: A Systematic Review. Oxid. Med. Cell. Longev. 2015, 2015, 234952. [Google Scholar] [CrossRef]
- Shah, R.; Shah, V.K.; Emin, M.; Gao, S.; Sampogna, R.V.; Aggarwal, B.; Chang, A.; St-Onge, M.-P.; Malik, V.; Wang, J.; et al. Mild Sleep Restriction Increases Endothelial Oxidative Stress in Female Persons. Sci. Rep. 2023, 13, 15360. [Google Scholar] [CrossRef]
- Trivedi, M.S.; Holger, D.; Bui, A.T.; Craddock, T.J.A.; Tartar, J.L. Short-Term Sleep Deprivation Leads to Decreased Systemic Redox Metabolites and Altered Epigenetic Status. PLoS ONE 2017, 12, e0181978. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xie, Y.; Li, Y.; Fan, X.; Xing, F.; Mao, Y.; Xing, N.; Wang, J.; Yang, J.; Wang, Z.; et al. Sleep Deprivation and Recovery Sleep Affect Healthy Male Resident’s Pain Sensitivity and Oxidative Stress Markers: The Medial Prefrontal Cortex May Play a Role in Sleep Deprivation Model. Front. Mol. Neurosci. 2022, 15, 937468. [Google Scholar] [CrossRef]
- Anis Syahirah, M.S.; Che Badariah, A.A.; Idris, L.; Rosfaiizah, S.; Liza, N. Impact of Rapid Eye Movement Sleep Deprivation on Pain Behaviour and Oxidative Stress in the Thalamus: Role of Tualang Honey Supplementation. Malays. J. Med. Sci. 2022, 29, 69–79. [Google Scholar] [CrossRef]
- Suganya, K.; Kayalvizhi, E.; Yuvaraj, R.; Chandrasekar, M.; Kavitha, U.; Konakanchi Suresh, K. Effect of Withania Somnifera on the Antioxidant and Neurotransmitter Status in Sleep Deprivation Induced Wistar Rats. Bioinformation 2020, 16, 631–637. [Google Scholar] [CrossRef]
- Coluk, Y.; Peker, E.G.G.; Yildirmak, S.; Keskin, A.; Yildirim, G. Exploring the Protective Role of Green Tea Extract against Cardiovascular Alterations Induced by Chronic REM Sleep Deprivation via Modulation of Inflammation and Oxidative Stress. BMC Complement. Med. Ther. 2024, 24, 351. [Google Scholar] [CrossRef]
- Nishi, S.K.; Khoury, N.; Valle Hita, C.; Zurbau, A.; Salas-Salvadó, J.; Babio, N. Vegetable and Fruit Intake Variety and Cardiovascular Health and Mortality: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2023, 15, 351. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and Vegetable Intake and the Risk of Cardiovascular Disease, Total Cancer and All-Cause Mortality—A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary Intake and Blood Concentrations of Antioxidants and the Risk of Cardiovascular Disease, Total Cancer, and All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef]
- Mishra, S.; Stierman, B.; Gahche, J.J.; Potischman, N. Dietary Supplement Use among Adults: United States, 2017–2018; National Center for Health Statistics: Hyattsville, MD, USA, 2021. [Google Scholar]
- Li, S.; Fasipe, B.; Laher, I. Potential Harms of Supplementation with High Doses of Antioxidants in Athletes. J. Exerc. Sci. Fit. 2022, 20, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Kristoffersen, A.E.; Stub, T.; Nilsen, J.V.; Nordberg, J.H.; Broderstad, A.R.; Wider, B.; Bjelland, M. Exploring Dietary Changes and Supplement Use among Cancer Patients in Norway: Prevalence, Motivations, Disclosure, Information, and Perceived Risks and Benefits: A Cross Sectional Study. BMC Nutr. 2024, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Sarangarajan, R.; Meera, S.; Rukkumani, R.; Sankar, P.; Anuradha, G. Antioxidants: Friend or Foe? Asian Pac. J. Trop. Med. 2017, 10, 1111–1116. [Google Scholar] [CrossRef]
- Tyuryaeva, I.; Lyublinskaya, O. Expected and Unexpected Effects of Pharmacological Antioxidants. Int. J. Mol. Sci. 2023, 24, 9303. [Google Scholar] [CrossRef]
- Villanueva, C.; Kross, R.D. Antioxidant-Induced Stress. Int. J. Mol. Sci. 2012, 13, 2091–2109. [Google Scholar] [CrossRef]
- Neculicioiu, V.S.; Colosi, I.; Sevastre-Berghian, A.; Toc, D.A.; Colosi, H.A.; David, L.; Muntean, M.; Moldovan, R.; Vlase, A.-M.; Toma, V.A.; et al. Molecular Effects of Cornelian Cherry Fruit (Cornus mas L.) Extract on Sleep Deprivation-Induced Oxidative Stress, Cytokine Dysregulation, and Behavioural Changes in Wistar Rats. Curr. Issues Mol. Biol. 2025, 47, 399. [Google Scholar] [CrossRef]
- Machado, R.B.; Hipólide, D.C.; Benedito-Silva, A.A.; Tufik, S. Sleep Deprivation Induced by the Modified Multiple Platform Technique: Quantification of Sleep Loss and Recovery. Brain Res. 2004, 1004, 45–51. [Google Scholar] [CrossRef]
- Ashley, N.T.; Sams, D.W.; Brown, A.C.; Dumaine, J.E. Novel Environment Influences the Effect of Paradoxical Sleep Deprivation upon Brain and Peripheral Cytokine Gene Expression. Neurosci. Lett. 2016, 615, 55–59. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Conti, M.; Morand, P.C.; Levillain, P.; Lemonnier, A. Improved Fluorometric Determination of Malonaldehyde. Clin. Chem. 1991, 37, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.B.; Butterfield, D.A. Measurement of Oxidized/Reduced Glutathione Ratio. Methods Mol. Biol. 2010, 648, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Pippenger, C.E.; Browne, R.W.; Armstrong, D. Regulatory Antioxidant Enzymes. Methods Mol. Biol. 1998, 108, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, F.; Liu, Y.; Ma, J.; Yu, X.; Wu, X.; Zhang, W.; Li, D.; Chen, D.; Dai, N.; et al. Modified Si-Ni-San Decoction Ameliorates Central Fatigue by Improving Mitochondrial Biogenesis in the Rat Hippocampus. Evid. Based Complement. Alternat. Med. 2018, 2018, 9452127. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Ji, G.; Shen, Y.; Zhao, N.; Liang, Y.; Wang, Z.; Liu, M.; Lin, L. Autophagy Triggered by Oxidative Stress Appears to Be Mediated by the AKT/MTOR Signaling Pathway in the Liver of Sleep-Deprived Rats. Oxid. Med. Cell. Longev. 2020, 2020, 6181630. [Google Scholar] [CrossRef]
- Hernández Santiago, K.; López-López, A.L.; Sánchez-Muñoz, F.; Cortés Altamirano, J.L.; Alfaro-Rodríguez, A.; Bonilla-Jaime, H. Sleep Deprivation Induces Oxidative Stress in the Liver and Pancreas in Young and Aging Rats. Heliyon 2021, 7, e06466. [Google Scholar] [CrossRef]
- Renn, T.-Y.; Yang, C.-P.; Wu, U.-I.; Chen, L.-Y.; Mai, F.-D.; Tikhonova, M.A.; Amstislavskaya, T.G.; Liao, W.-C.; Lin, C.-T.; Liu, Y.-C.; et al. Water Composed of Reduced Hydrogen Bonds Activated by Localized Surface Plasmon Resonance Effectively Enhances Anti-Viral and Anti-Oxidative Activities of Melatonin. Chem. Eng. J. 2022, 427, 131626. [Google Scholar] [CrossRef]
- Chen, H.-C.; Cheng, C.-Y.; Chen, L.-Y.; Chang, C.-C.; Yang, C.-P.; Mai, F.-D.; Liao, W.-C.; Chang, H.-M.; Liu, Y.-C. Plasmon-Activated Water Effectively Relieves Hepatic Oxidative Damage Resulting from Chronic Sleep Deprivation. RSC Adv. 2018, 8, 9618–9626. [Google Scholar] [CrossRef]
- Lungato, L.; Marques, M.S.; Pereira, V.G.; Hix, S.; Gazarini, M.L.; Tufik, S.; D’Almeida, V. Sleep Deprivation Alters Gene Expression and Antioxidant Enzyme Activity in Mice Splenocytes. Scand. J. Immunol. 2013, 77, 195–199. [Google Scholar] [CrossRef]
- Lungato, L.; Nogueira-Pedro, A.; Carvalho Dias, C.; Paredes-Gamero, E.J.; Tufik, S.; D’Almeida, V. Effects of Sleep Deprivation on Mice Bone Marrow and Spleen B Lymphopoiesis: Sleep Deprivation Affects b Lymphopoiesis. J. Cell. Physiol. 2016, 231, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Nawi, A.; Eu, K.L.; Faris, A.N.A.; Wan Ahmad, W.A.N.; Noordin, L. Lipid Peroxidation in the Descending Thoracic Aorta of Rats Deprived of REM Sleep Using the Inverted Flowerpot Technique. Exp. Physiol. 2020, 105, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gan, Z.; Li, Y.; Zhao, W.; Li, H.; Zheng, J.-P.; Ke, Y. REM Sleep Deprivation Induces Endothelial Dysfunction and Hypertension in Middle-Aged Rats: Roles of the ENOS/NO/CGMP Pathway and Supplementation with L-Arginine. PLoS ONE 2017, 12, e0182746. [Google Scholar] [CrossRef]
- Periasamy, S.; Hsu, D.-Z.; Fu, Y.-H.; Liu, M.-Y. Sleep Deprivation-Induced Multi-Organ Injury: Role of Oxidative Stress and Inflammation. EXCLI J. 2015, 14, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Roubalová, L.; Vošahlíková, M.; Slaninová, J.; Kaufman, J.; Alda, M.; Svoboda, P. Tissue-Specific Protective Properties of Lithium: Comparison of Rat Kidney, Erythrocytes and Brain. Naunyn. Schmiedeberg’s Arch. Pharmacol. 2021, 394, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, A.; Ji, L.L.; Cirelli, C. Sleep Deprivation and Cellular Responses to Oxidative Stress. Sleep 2004, 27, 27–35. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, D.; Wu, X.; Yue, F.; Yang, H.; Hu, K. Rapamycin Ameliorates Chronic Intermittent Hypoxia and Sleep Deprivation-Induced Renal Damage via the Mammalian Target of Rapamycin (MTOR)/NOD-like Receptor Protein 3 (NLRP3) Signaling Pathway. Bioengineered 2022, 13, 5537–5550. [Google Scholar] [CrossRef]
- Everson, C.A.; Henchen, C.J.; Szabo, A.; Hogg, N. Cell Injury and Repair Resulting from Sleep Loss and Sleep Recovery in Laboratory Rats. Sleep 2014, 37, 1929–1940. [Google Scholar] [CrossRef]
- Mourad, I.M.; Fahmy, H. Induction of Oxidative Stress by Paradoxical Sleep Deprivation in Different Body Organs of Rats. Int. J. Pharma Bio Sci. 2017, 8, 420–427. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef]
- Davis, C.J.; Krueger, J.M. Sleep and Cytokines. Sleep Med. Clin. 2012, 7, 517–527. [Google Scholar] [CrossRef]
- Aboul Ezz, H.S.; Noor, A.E.; Mourad, I.M.; Fahmy, H.; Khadrawy, Y.A. Neurochemical Effects of Sleep Deprivation in the Hippocampus of the Pilocarpine-Induced Rat Model of Epilepsy. Iran. J. Basic Med. Sci. 2021, 24, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, S.; Singh, H.; Kaur, T.; Kaur, G. Low-Grade Neuroinflammation Due to Chronic Sleep Deprivation Results in Anxiety and Learning and Memory Impairments. Mol. Cell. Biochem. 2018, 449, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Kaur, G. Acute Sleep Deprivation-Induced Anxiety and Disruption of Hypothalamic Cell Survival and Plasticity: A Mechanistic Study of Protection by Butanol Extract of Tinospora Cordifolia. Neurochem. Res. 2022, 47, 1692–1706. [Google Scholar] [CrossRef]
- Mishra, I.; Pullum, K.B.; Thayer, D.C.; Plummer, E.R.; Conkright, B.W.; Morris, A.J.; O’Hara, B.F.; Demas, G.E.; Ashley, N.T. Chemical Sympathectomy Reduces Peripheral Inflammatory Responses to Acute and Chronic Sleep Fragmentation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R781–R789. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kar, S.K. REM Sleep Deprivation of Rats Induces Acute Phase Response in Liver. Biochem. Biophys. Res. Commun. 2011, 410, 242–246. [Google Scholar] [CrossRef]
- Hamed, M.A.; Akhigbe, T.M.; Akhigbe, R.E.; Aremu, A.O.; Oyedokun, P.A.; Gbadamosi, J.A.; Anifowose, P.E.; Adewole, M.A.; Aboyeji, O.O.; Yisau, H.O.; et al. Glutamine Restores Testicular Glutathione-Dependent Antioxidant Defense and Upregulates NO/CGMP Signaling in Sleep Deprivation-Induced Reproductive Dysfunction in Rats. Biomed. Pharmacother. 2022, 148, 112765. [Google Scholar] [CrossRef] [PubMed]
- Sang, D.; Lin, K.; Yang, Y.; Ran, G.; Li, B.; Chen, C.; Li, Q.; Ma, Y.; Lu, L.; Cui, X.-Y.; et al. Prolonged Sleep Deprivation Induces a Cytokine-Storm-like Syndrome in Mammals. Cell 2023, 186, 5500–5516.e21. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef]
- Pandey, A.; Kar, S.K. Rapid Eye Movement Sleep Deprivation of Rat Generates ROS in the Hepatocytes and Makes Them More Susceptible to Oxidative Stress. Sleep Sci. 2018, 11, 245–253. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, K.; Xi, Z.; Ma, Y.; Shao, C.; Wang, W.; Tang, Y.-D. Short Sleep Duration and the Risk of Nonalcoholic Fatty Liver Disease/Metabolic Associated Fatty Liver Disease: A Systematic Review and Meta-Analysis. Sleep Breath. 2023, 27, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Zhao, L.; Petrick, J.L.; Liao, L.M.; Huang, T.; Hakim, A.; Yang, W.; Campbell, P.T.; Giovannucci, E.; McGlynn, K.A.; et al. Daytime Napping, Nighttime Sleeping Duration, and Risk of Hepatocellular Carcinoma and Liver Disease-Related Mortality. JHEP Rep. 2023, 5, 100819. [Google Scholar] [CrossRef]
- Taha, M.; Rady, H.Y.; Olama, N.K. Effect of Sleep Deprivation on the Liver, Kidney and Heart: Histological and Immunohistochemical Study. Int. J. Sci. Rep. 2018, 4, 172. [Google Scholar] [CrossRef]
- Si, Q.; Sun, W.; Liang, B.; Chen, B.; Meng, J.; Xie, D.; Feng, L.; Jiang, P. Systematic Metabolic Profiling of Mice with Sleep-Deprivation. Adv. Biol. 2024, 8, e2300413. [Google Scholar] [CrossRef]
- Argeri, R.; Nishi, E.E.; Volpini, R.A.; Palma, B.D.; Tufik, S.; Gomes, G.N. Sleep Restriction during Pregnancy and Its Effects on Blood Pressure and Renal Function among Female Offspring. Physiol. Rep. 2016, 4, e12888. [Google Scholar] [CrossRef]
- Koh, J.H.; Yeo, B.S.Y.; Tan, T.W.E.; See, M.Y.S.; Ng, A.C.W.; Loh, S.R.H.; Gooley, J.; Tan, C.S.; Toh, S.T. The Association of Sleep Duration with the Risk of Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Clin. Kidney J. 2024, 17, sfae177. [Google Scholar] [CrossRef] [PubMed]
- Siervo, G.E.M.L.; Ogo, F.M.; Staurengo-Ferrari, L.; Anselmo-Franci, J.A.; Cunha, F.Q.; Cecchini, R.; Guarnier, F.A.; Verri, W.A., Jr.; Fernandes, G.S.A. Sleep Restriction during Peripuberty Unbalances Sexual Hormones and Testicular Cytokines in Rats. Biol. Reprod. 2019, 100, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Kim, J.K.; Shin, H.J.; Park, E.-J.; Kim, I.S.; Jo, E.-K. Updated Insights into the Molecular Networks for NLRP3 Inflammasome Activation. Cell. Mol. Immunol. 2025, 22, 563–596. [Google Scholar] [CrossRef] [PubMed]
- Hayrabedyan, S.; Todorova, K.; Jabeen, A.; Metodieva, G.; Toshkov, S.; Metodiev, M.V.; Mincheff, M.; Fernández, N. Sertoli Cells Have a Functional NALP3 Inflammasome That Can Modulate Autophagy and Cytokine Production. Sci. Rep. 2016, 6, 18896. [Google Scholar] [CrossRef]
- Tavalaee, M.; Rahmani, M.; Drevet, J.R.; Nasr-Esfahani, M.H. The NLRP3 Inflammasome: Molecular Activation and Regulation in Spermatogenesis and Male Infertility; a Systematic Review. Basic Clin. Androl. 2022, 32, 8. [Google Scholar] [CrossRef]
- Zhong, O.; Liao, B.; Wang, J.; Liu, K.; Lei, X.; Hu, L. Effects of Sleep Disorders and Circadian Rhythm Changes on Male Reproductive Health: A Systematic Review and Meta-Analysis. Front. Physiol. 2022, 13, 913369. [Google Scholar] [CrossRef]
- Ashley, N.T.; Weil, Z.M.; Nelson, R.J. Inflammation: Mechanisms, Costs, and Natural Variation. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 385–406. [Google Scholar] [CrossRef]
- Sotler, R.; Poljšak, B.; Dahmane, R.; Jukić, T.; Pavan Jukić, D.; Rotim, C.; Trebše, P.; Starc, A. Prooxidant Activities of Antioxidants and Their Impact on Health. Acta Clin. Croat. 2019, 58, 726–736. [Google Scholar] [CrossRef]
- Tubaş, F.; Per, S.; Taşdemir, A.; Bayram, A.K.; Yıldırım, M.; Uzun, A.; Saraymen, R.; Gümüş, H.; Elmalı, F.; Per, H. Effects of Cornus mas L. and Morus rubra L. Extracts on Penicillin-Induced Epileptiform Activity: An Electrophysiological and Biochemical Study. Acta Neurobiol. Exp. 2017, 77, 45–56. [Google Scholar] [CrossRef]
- Moldovan, B.; Filip, A.; Clichici, S.; Suharoschi, R.; Bolfa, P.; David, L. Antioxidant Activity of Cornelian Cherry (Cornus mas L.) Fruits Extract and the in Vivo Evaluation of Its Anti-Inflammatory Effects. J. Funct. Foods 2016, 26, 77–87. [Google Scholar] [CrossRef]
- Filip, G.A.; Moldovan, B.; Baldea, I.; Olteanu, D.; Suharoschi, R.; Decea, N.; Cismaru, C.M.; Gal, E.; Cenariu, M.; Clichici, S.; et al. UV-Light Mediated Green Synthesis of Silver and Gold Nanoparticles Using Cornelian Cherry Fruit Extract and Their Comparative Effects in Experimental Inflammation. J. Photochem. Photobiol. B 2019, 191, 26–37. [Google Scholar] [CrossRef]
- Saei, H.; Hatami, H.; Azarmi, M.; Dehghan, G. Hepatoprotective Effect of Cornus Mas Fruits Extract on Serum Biomarkers in Methotrexate-Induced Liver Injury in Male Rats. Pharmacologyonline 2016, 1, 91–98. [Google Scholar]
- Bayram, H.M.; Iliaz, R.; Gunes, F.E. Effects of Cornus mas L. on Anthropometric and Biochemical Parameters among Metabolic Associated Fatty Liver Disease Patients: Randomized Clinical Trial. J. Ethnopharmacol. 2024, 318, 117068. [Google Scholar] [CrossRef] [PubMed]
- Klongová, L.; Kovár, M.; Navrátilová, A.; Fialkova, V.; Požgajová, M. Cornus mas L. Extract-Mediated Modulations of the Redox State Induce Cytotoxicity in Schizosaccharomyces Pombe. Appl. Sci. 2024, 14, 4049. [Google Scholar] [CrossRef]
- Miláčková, I.; Meščanová, M.; Ševčíková, V.; Mučaji, P. Water Leaves Extracts of Cornus mas and Cornus kousa as Aldose Reductase Inhibitors: The Potential Therapeutic Agents. Chem. Pap. 2017, 71, 2335–2341. [Google Scholar] [CrossRef]
- Moldovan, R.; Mitrea, D.-R.; Florea, A.; Chiş, I.-C.; Suciu, Ş.; David, L.; Moldovan, B.E.; Mureşan, L.E.; Lenghel, M.; Ungur, R.A.; et al. Effects of Gold Nanoparticles Functionalized with Bioactive Compounds from Cornus mas Fruit on Aorta Ultrastructural and Biochemical Changes in Rats on a Hyperlipid Diet—A Preliminary Study. Antioxidants 2022, 11, 1343. [Google Scholar] [CrossRef] [PubMed]
- Cladis, D.P.; Li, S.; Reddivari, L.; Cox, A.; Ferruzzi, M.G.; Weaver, C.M. A 90 Day Oral Toxicity Study of Blueberry Polyphenols in Ovariectomized Sprague-Dawley Rats. Food Chem. Toxicol. 2020, 139, 111254. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Yu, M.; Chen, C.; Xu, L.; Cao, Y.; Qi, R. Grape-Seed Polyphenols Play a Protective Role in Elastase-Induced Abdominal Aortic Aneurysm in Mice. Sci. Rep. 2017, 7, 9402. [Google Scholar] [CrossRef]
- Li, N.; Lv, T.; Pan, J.; Liu, C.; Sun, J.; Lan, Y.; Wang, A.; Li, Y.; Wang, Y.; Lu, Y. Comparative Tissue Distribution of 6 Major Polyphenolic Compounds in Normal and Myocardial Ischemia Model Rats after Oral Administration of the Polygonum orientale L. Extract. Nat. Prod. Commun. 2020, 15, 1934578X2092944. [Google Scholar] [CrossRef]
- Sun, J.; Fang, D.; Wang, Z.; Liu, Y. Sleep Deprivation and Gut Microbiota Dysbiosis: Current Understandings and Implications. Int. J. Mol. Sci. 2023, 24, 9603. [Google Scholar] [CrossRef] [PubMed]
- Pala, B.; Pennazzi, L.; Nardoianni, G.; Fogacci, F.; Cicero, A.F.G.; Di Renzo, L.; Barbato, E.; Tocci, G. Gut Microbiota Dysbiosis and Sleep Disorders: Culprit in Cardiovascular Diseases. J. Clin. Med. 2024, 13, 3254. [Google Scholar] [CrossRef] [PubMed]
- Matenchuk, B.A.; Mandhane, P.J.; Kozyrskyj, A.L. Sleep, Circadian Rhythm, and Gut Microbiota. Sleep Med. Rev. 2020, 53, 101340. [Google Scholar] [CrossRef]
- Vaccaro, A.; Kaplan Dor, Y.; Nambara, K.; Pollina, E.A.; Lin, C.; Greenberg, M.E.; Rogulja, D. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell 2020, 181, 1307–1328.e15. [Google Scholar] [CrossRef]
- Pisano, C.; Benedetto, U.; Ruvolo, G.; Balistreri, C.R. Oxidative Stress in the Pathogenesis of Aorta Diseases as a Source of Potential Biomarkers and Therapeutic Targets, with a Particular Focus on Ascending Aorta Aneurysms. Antioxidants 2022, 11, 182. [Google Scholar] [CrossRef]
- Cladis, D.P.; Simpson, A.M.R.; Cooper, K.J.; Nakatsu, C.H.; Ferruzzi, M.G.; Weaver, C.M. Blueberry Polyphenols Alter Gut Microbiota & Phenolic Metabolism in Rats. Food Funct. 2021, 12, 2442–2456. [Google Scholar] [CrossRef]
- McEwen, B.S.; Karatsoreos, I.N. Sleep Deprivation and Circadian Disruption: Stress, Allostasis, and Allostatic Load. Sleep Med. Clin. 2015, 10, 1–10. [Google Scholar] [CrossRef]
- Gadacha, W.; Ben-Attia, M.; Bonnefont-Rousselot, D.; Aouani, E.; Ghanem-Boughanmi, N.; Touitou, Y. Resveratrol Opposite Effects on Rat Tissue Lipoperoxidation: Pro-Oxidant during Day-Time and Antioxidant at Night. Redox Rep. 2009, 14, 154–158. [Google Scholar] [CrossRef]
- Blanton, C.; Ghimire, B.; Khajeh Pour, S.; Aghazadeh-Habashi, A. Circadian Modulation of the Antioxidant Effect of Grape Consumption: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 6502. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, G.; Zhao, T.; Wang, S.; Sun, B.; Zheng, L.; Zhao, M. The Memory Improving Effects of Round Scad (Decapterus maruadsi) Hydrolysates on Sleep Deprivation-Induced Memory Deficits in Rats via Antioxidant and Neurotrophic Pathways. Food Funct. 2019, 10, 7733–7744. [Google Scholar] [CrossRef] [PubMed]
- Suchecki, D.; Lobo, L.L.; Hipólide, D.C.; Tufik, S. Increased ACTH and Corticosterone Secretion Induced by Different Methods of Paradoxical Sleep Deprivation. J. Sleep Res. 1998, 7, 276–281. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neculicioiu, V.S.; Chiș, I.C.; Colosi, I.A.; Sevastre-Berghian, A.; David, L.; Muntean, M.; Vlase, A.-M.; Moldovan, R.; Decea, R.M.; Costache, C.; et al. Cornelian Cherry (Cornus mas) Fruit Extract Administration in Sleep Deprived Wistar Rats—Friend or Foe? Biology 2025, 14, 1341. https://doi.org/10.3390/biology14101341
Neculicioiu VS, Chiș IC, Colosi IA, Sevastre-Berghian A, David L, Muntean M, Vlase A-M, Moldovan R, Decea RM, Costache C, et al. Cornelian Cherry (Cornus mas) Fruit Extract Administration in Sleep Deprived Wistar Rats—Friend or Foe? Biology. 2025; 14(10):1341. https://doi.org/10.3390/biology14101341
Chicago/Turabian StyleNeculicioiu, Vlad Sever, Irina Camelia Chiș, Ioana Alina Colosi, Alexandra Sevastre-Berghian, Luminita David, Mara Muntean, Ana-Maria Vlase, Remus Moldovan, Roxana Maria Decea, Carmen Costache, and et al. 2025. "Cornelian Cherry (Cornus mas) Fruit Extract Administration in Sleep Deprived Wistar Rats—Friend or Foe?" Biology 14, no. 10: 1341. https://doi.org/10.3390/biology14101341
APA StyleNeculicioiu, V. S., Chiș, I. C., Colosi, I. A., Sevastre-Berghian, A., David, L., Muntean, M., Vlase, A.-M., Moldovan, R., Decea, R. M., Costache, C., Colosi, H. A., Toc, D. A., Suciu, Ş. M., & Clichici, S. (2025). Cornelian Cherry (Cornus mas) Fruit Extract Administration in Sleep Deprived Wistar Rats—Friend or Foe? Biology, 14(10), 1341. https://doi.org/10.3390/biology14101341









